Abstract
Purpose
To characterize and optimize the productivity of melanin using an extremotolerant actinobacterium, Dietzia schimae NM3, for the first time.
Methods
An extracellular brown pigment produced by D. schimae NM3 in the nutrient broth and cheese whey medium by adding L-tyrosine. The extracted melanin was analyzed by UV-visible, HPLC, and FTIR assays. The radical scavenging activity (by DPPH) and sun protection factor (SPF) of the extracted melanin were measured. The melanin cytotoxicity was assayed by MTT and chromate biosorption was measured by atomic absorption spectroscopy. Finally, melanin production by D. schimae NM3 was optimized by response surface methodology (RSM) using Box-Behnken design in the whey medium.
Result
The purified melanin showed similar peak to the standard melanin (SIGMA) at 3.5 min in HPLC, and C=O bands, NH2, CH, C-N, and aromatic groups by FTIR. The radical scavenging activity (by DPPH) and SPF of the extracted melanin were obtained 188.9% and 20.22, respectively. Using MTT assay, the melanin revealed non-toxic effect on the normal human fibroblast (HFB) cell culture. The melanin yield 790 mg l−1, and tyrosinase activity 3400 U ml−1 were obtained in the medium contained whey powder [5% (w v−1)], L-tyrosine 2.5 g l−1, CuSO4 0.013 g l−1, and pH 10.5, incubated at 32 °C for 3 days. The ANOVA results indicated significant P-value, model F-value, and probability, with insignificant lack of fit. After optimization with mono-factors, the nutrient broth came up with melanin yield as 1.2 g l−1 and tyrosinase activity as 4040 U ml−1.
Conclusion
This is the first report of melanin production by D. schimae NM3 and this natural melanin showed valuable biological properties such as high antioxidant activity and radioprotection (SPF) and the biocompatibility to human cell line.
Similar content being viewed by others
Introduction
Dietzia genus is an actinobacterium consuming many compounds, also tyrosine is the sole carbon source for producing melanin pigment (Koerner et al. 2009). This bacterium is in fact capable to biodegrade aniline, alkane, and hydrocarbons in the soil polluted with crude oil and aromatic compounds (SMT et al. 2013).
Today, the consumers are concerned about the chemical synthetic compounds of such pigments due to their side effects, which can be related to their complex chemical structures compared to the natural ones. The microbial cells factories have been considered for their easy and fast production. The natural pigments from microbial sources are used in food, cosmetics, and the industries without causing side effects or environmental contamination (Sun et al. 2016).
Melanin pigment is a dark- or black-brown irregular negatively charged hydrophilic complex polymer. It is produced by oxidative polymerization of phenolic or indolic compounds in various organisms (Plonka and Grabacka 2006). Melanins are resistant to heat, free radicals, radiation energy, and oxidizing agents. Generally speaking, melanin can protect microorganism against ionizing radiation (Cordero et al. 2017). Melanin possesses anti-UV radiation property by absorbing the electromagnetic spectrum and preventing optical damage in the living organisms. As shown, melanin can synthesize in response to the antifungal drugs (Kurian and Bhat 2014). Melanin granules are insoluble in water and common organic solvents (such as hexane, chloroform, ethanol, methanol, or acetone) (Varga et al. 2016). The melanin produced by Pseudomonas balearica has been characterized by spectroscopic and chemical properties (Zerrad et al. 2014). The carboxyl, phenolic, hydroxyl, and amine groups on melanin pigments provide numerous potential binding sites for interacting with other molecules (Hoa et al. 2017). For this reason, melanins have some important properties including antitumor, antimicrobial, and antivenin activities. Moreover, these molecules can protect the cells from DNA damage induced by hydrogen peroxide, indicating the antioxidant activity of melanin (El-Naggar and El-Ewasy 2017). A broad range of bacteria such as Pseudomonads, Aeromonas, Azotobacter, Mycobacterium, Micrococcus, Bacillus, Legionella, and Streptomyces can produce melanin (Nosanchuk and Casadevall 2003). It is critical to optimize the growth conditions for microorganisms, particularly the physiochemical and nutritional parameters. An optimization method using “one factor at a time” is difficult to run and time-consuming (El-Naggar and El-Ewasy 2017). Response surface methodology (RSM) is a set of mathematical methods with several independent variables (Surwase et al. 2012).
Recently, several studies have focused on the species capable to be used as different substances instead of hydrocarbons so that to produce melanin at high purities. So far, there has been no report on melanin pigment production by radioresistant Dietzia strains. The current research mainly targets to produce and characterize melanin pigment from Dietzia schimae NM3 by L-tyrosine in the nutrient broth. In addition, in the present study, we proposed an inexpensive culture medium to optimally bioproduce the melanin by D. schimae NM3 using economical cheese whey medium.
Materials and methods
Microbial strain and inoculum preparation
D. schimae NM3 (GeneBank accession no. KP207685) that was isolated and identified previously in our lab was used for melanin pigment production in this study for the first time. To prepare the work inoculum, one fresh colony of D. schimae NM3 was incubated in tryptone glucose yeast extract (TGY) agar medium at 30 °C for 48 h (Zamanian and Etemadifar 2017).
Melanin production and purification
The nutrient broth containing 2 g l−1 L-tyrosine was inoculated by 5% (v v−1) of the D. schimae culture (108 CFU ml−1) and incubated at 35 °C with 160 rpm shaking for 4 days to observe dark melanin pigment (Wang et al. 2015). The growth of D. schimae NM3 was assayed by measuring the optical density (OD600nm) spectrophotometrically (Milton Roy Spectronic 21D). Extracellular melanin production was assayed by colorimetry (A400nm) after medium filtration by 0.45 μ membrane filter at different intervals during the incubation period (El-Naggar and El-Ewasy 2017).
Melanin extraction
In order to extract melanin, the darkly pigmented medium was centrifuged at 3500 rpm for 15 min (by refrigerating Eppendorf 5810R) and the bacterial cells (pelleted) separated from the supernatant (El-Naggar and El-Ewasy 2017). The supernatant was acidified by 3 mol l−1 HCl to reach pH 3, left at room temperature for 24 h, and then centrifuged at 3500 rpm for 15 min to separate crude pigment granules (melanin). The pigment granules were washed by water, and the pellet was boiled in water bath for 5 min to avoid melanoidins formation, then was dried in the oven (Tarangini and Mishra 2013). To purify the melanin, it was dissolved in dimethyl sulfoxide (DMSO) by shaking slowly in hot water until it was completely dissolved, then centrifuged so that the impurities got settled. The top liquid was kept in the plate and put in the oven to dry (Jakoby 1971).
Tyrosinase assay
The fresh colonies of D. schimae NM3 were inoculated in NB medium (pH 7.0) containing L-tyrosine and incubated at 35 °C on a rotary shaker at 160 rpm. The cells were harvested by centrifugation at 5000 rpm for 5 min at 4 °C and disrupted by ultrasonic (280 kHz) for ten times at 1-min intervals on ice. For tyrosinase assay, 0.1 ml of crude intracellular enzymes, 1.0 ml of 0.5 mol l−1 phosphate buffer (pH 6.5), 1.0 ml of 0.001 mol l−1 L-tyrosine, and 0.9 ml of reagent water were added to a test tube. After 4–5 min waiting for the mixture to get deoxygenated and the temperature to get balanced (done inside ice), the absorbance was recorded at 280 nm using UV-Vis spectrophotometer. The enzyme activity was calculated by Eq. (1) (Ingle and Khobragade 2013; Roy et al. 2014).
Analytical methods
UV-visible spectroscopy
The purified melanin was dissolved in 0.1 mol l−1 NaOH, sonicated for 5 min, and its UV-visible absorbance was recorded at the spectrum range 200–400 nm using Specord Plus UV-VIS spectrophotometer (Wang et al. 2015).
FTIR analysis
For FTIR analysis, the melanin-containing KBr pellets were prepared following the method developed by Sun et al. (2016). The melanin was analyzed with FTIR (Nicolet AVATAR360) at wave numbers 4000–500 cm−1. The FTIR instrument’s resolution was 4.0 cm−1, the optical path difference (OPD) velocity as 0.20 cm s−1, and the data collection interval of 1.0 s. The actual spectra were measured as the absorbance ratio. The background spectra were measured by the clean KBr pellet containing no sample material (Sun et al. 2016).
HPLC analysis
The pure melanin and the standard melanin (Sigma) were dissolved in 0.5 mol l−1 DMSO solution prior to HPLC analysis. The quantitative method was done as stated by Sun et al., and the detection wavelength was set at 280 nm and the column temperature was set at 25 °C (Sun et al. 2016).
In vitro cytotoxicity assay (MTT Assay)
Human fibroblast cell line (HFB) was used for in vitro studies. The cells were cultured in T-flask containing RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin/streptomycin) solution and incubated in a humidified incubator at 37 °C under 5% CO2 atmosphere. The cells were harvested using 0.05% trypsin and centrifuged for 4 min at 1000 rpm. Then, the cells were dispersed and inoculated in a 96-well plate at a density of 104 cells per well and incubated for 24 h under the same conditions to allow the cells to adhere.
Following the cells being exposed to microbial melanin at different concentrations (31, 62.5, 125, 250, and 500 μg ml−1) for 72 h, the medium was completely discarded, and 100 μL of (MTT) solution (0.5 mg ml−1 in medium) was added to each well and the plates were re-incubated at 37 °C for 4 h. After that, the absorbance was measured at 570 nm. The cell viability was determined as the ratio of absorbance values from each treatment and the control. Upon being exposed to different concentrations of melanin, the cell morphology was investigated by optical microscope (Arun et al. 2015). The percentage of the killed cells was calculated by Eq. (2).
Sun protection factor measurement
Sun protection factor (SPF) value was assayed in ethanol solution containing 100 mg pigment by measuring the absorbance in the spectra of the 280–400 nm range. The NM3 strain extracted melanin’s SPF value was determined by Mansur mathematical equation (Eq. 3) (Huang et al. 2011).
DPPH-based radical scavenging assay
Radical scavenging activity was assayed according to the modified method of Brand-Williams et al. The crude extracted microbial melanin and the ascorbic acid as standard antioxidant were supplied at different concentrations (2.5, 5, 10 mg l−1). Then, 1 ml of each concentration of melanin and ascorbic acid were added to 3 ml out of 0.1 m mol DPPH (2,2-diphenyl-1-picrylhydrazyl) solution. After that, the tubes were incubated in the dark at 37 °C for 30 min, and the absorbance was monitored at 516 nm by spectrophotometer (Milton Roy Spectronic 21D). The scavenging effect was measured using Eq. 4 (Zerrad et al. 2014).
Metal biosorption activity
Melanin solution was supplied at different concentrations (0, 25, 50, 100 mg l−1). The metal solution of K2Cr2O4 (2 m mol) was prepared and mixed with melanin, and the activated carbon was used as the standard sorbent in this test. The initial pH 4.0 was kept fixed for all tubes test; then, the solution was shaken fast at room temperature for 1 h. The solution was filtered with 0.45 μ membrane filter and then analyzed by atomic absorption spectroscopy to measure metal biosorption (Chen et al. 2009).
All the results indicated as means ± standard deviation (SD) of three independent experiments.
Melanin production in whey medium
D. schimae culture suspension (108 CFU ml−1) was inoculated as 5% (v v−1) into the whey medium contained 5% whey powder, and 0.25% L-tyrosine and incubated at 35 °C with 160 rpm shaking for 96 h. After different times intervals the bacterial growth (A600nm), sugar utilization (by DNS method), L-tyrosine consumption (A492nm), and melanin production (A400nm) were determined spectrophotometrically (Ganesh Kumar et al. 2013).
Optimizing melanin pigment production using Box-Behnken design-based RSM
At first, the optimization was performed with one factor at a time (temperature, light, shaking rate, culture medium, pH, L-tyrosine, ion catalysts, salts) to enhance melanin production. As the results indicate, four important factors were selected for RSM optimization of the melanin production by Dietzia. The 27 designed runs are shown in Table 2. According to the results of one factor at a time optimization in the previous experiments by RSM statistical method in actinobacteria, four factors (L-tyrosine, pH, CuSO4, and temperature) were selected as the most effective factors involved in the melanin production and the bacterial growth (Dholakiya et al. 2017). Expert design software 11.1.2.0 was applied for the runs’ design type (Box-Behnken) and design model (quadratic) (El-Batal et al. 2017).
The model’s statistical analysis was represented as ANOVA (Surwase et al. 2013). The multivariate model is presented as (Eq. 5).
Finally, the optimized factors of whey medium were used in the nutrient broth. After the incubation period, melanin yield and tyrosinase activity were measured.
Results
D. schimae NM3 strain is not capable to transform L-tyrosine into melanin in the presence of high carbohydrate and nitrogen nutrients available in the enriched media such as TGY and TSA. In the nutrient agar with L-tyrosine as a substrate, the dark pigmented melanin was observed after 3–4 days (Fig. S1). Bacterial growth and melanin production are depicted in Fig. S2. The bacteria were growing using L-tyrosine by producing various mediator metabolites transformed into melanin at the stationary phase (52–64 h). L-tyrosine begins to decrease after 18 h and the next peak indicates the next metabolite’s formation. These reactions continue until the reduction of tyrosine peak and the melanin peak formation.
It is worth mentioning that the melanin production was at its highest level (450 mg l−1) after 52–64 h. The tyrosinase activity was obtained as 2010 U ml−1 at this time. After melanin production optimization using some important factors, i.e., pH 9, temperature (32 °C), and tyrosine (2 g l−1), the nutrient broth got to 1.2 g l−1 level.
Melanin characterization
The photographs were taken by an optical microscope (Fig. 1a, b) to represent melanin layer in the cell wall (melanin ghost) after acid (KOH) treatment (MHS et al. 2013). The scan electron microscopy image of melanin with big structure in the cell wall is illustrated in Fig. 1c and d.
The photograph of melanin of D. schimae by light microscopy without staining in stationary phase after melanin extraction by acid treatment (a), the bacterial cell walls with external melanin ghosts (b), and electron micrograph of purified melanin granules by scan electron microscopy (SEM), with magnification × 500 (c) and × 5000 (d)
D. schimae NM3 extracted melanin revealed absorbance in the spectrum of 200–300 nm range by UV-visible spectrophotometry (Fig. S3), which was like standard melanin. The standard melanin’s HPLC chromatogram included a single symmetrical elution peak, and the retention times were 3.5 min, which were like the NM3 melanin’s peak pattern (Fig. 2a, b).
To perceive the melanin’s structure and its functional groups, FTIR analysis was performed on the purified melanin from D. schimae and the standard melanin. The similar spectral pattern suggested that both melanin pigments had identical structure (Fig. 2c). To determine the structure and the present excess bands in the melanin product of D. schimae, IR Pal V.2.0 was applied and then compared with the standard melanin (Table S2). However, the purified melanin from NM3 and the standard melanin demonstrated some peaks which may be caused by some fragments produced by the destruction in the acid precipitation step.
Sunscreen protection factor (SPF)
The UV-Abs-derived results at different wavelengths for SPF determination are listed at Table 1. The D. schimae derived melanin’s SPF value was measured as 20.22.
Radical scavenging assay by DPPH
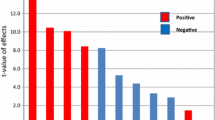
The antioxidant properties of melanin particles are given in Fig. 3. The results exhibited high amounts of radical scavengers (188.9%) and reduction of DPPH in 10 mg l−1 melanin from NM3 strain. In addition, the electron donating ability of the extracted melanin was obtained higher than that of the commercial antioxidant (vitamin C). The results denoted that within the min time required by the melanin particle to divulge, its max DPPH reduction and the scavenging activity depend on pigment’s dose.
Biosorption of Cr6+
The adsorption efficiency of Cr6+ by melanin was examined by dose dependence. Based on the results in Fig. 4, melanin could remove Cr6+ with adsorption efficiency 33.3%, 22.9%, and 17.7% in the presence of melanin concentrations at 25, 50, and 100 mg ml−1, respectively, while under the same conditions, the activated carbon at the concentrations of 25, 50, and 100 mg ml−1 have the adsorption efficiencies of 24%, 8%, and 0%, respectively. In general, the adsorption capacity of melanin was higher than that of the activated carbon.
MTT assay
The results of cell cultures in the presence of purified melanin from D. schimae, in comparison with the control cell without melanin, were shown in Fig. 5. As the results exhibited, the melanin (at the concentrations up to 250 μg ml−1) lacked toxicity on the normal cells, which indicates the bacterial melanin’s biocompatibility. These results could also confirm that no significant toxicity was observed due to the impurities. No tangible changes were seen in the morphology of the cells exposed to melanin compared with the control cells (Fig. S4).
Melanin production in whey medium
Dietzia NM3 produced melanin pigment in whey medium contained L-tyrosine (Fig. 6). The obtained results showed this bacterium utilized whey sugar and grew to OD=3; simultaneously, it produced melanin by L-tyrosine consumption. After melanin production in whey medium, we performed the optimization experiment. Without whey, any growth did not occur in the medium, and in the whey medium without L-tyrosine, pigment was not produced.
RSM-based optimization of melanin production by D. schimae NM3
D. schimae NM3 was inoculated in whey medium with L-tyrosine as substrate. The dark brown pigment melanin was diffused after 3 days in whey broth medium as an inexpensive medium. In large-scale fermentation (1 l) of whey medium as an inexpensive medium, the max melanin production was gained as 790 mg l−1. Through this method, it is merely viable to gain the proper concentration for each factor separately. The resulted model can be observed in Table 2, in which the melanin yield (Y) is a function of the independent variables’ value. This table has three replicates at the central point. The results for predicting the response are given as the final equation from Box-Behnken experiment (Eq. 6).
As spotted in Eq. (6), among the abovementioned factors, the pH is negative. Their negative effect on the response function suggests that higher values of this parameter lead to lower melanin yield. CuSO4 possesses the max effect out of these four parameters.
Equation (6) was statistically evaluated through F-test and the ANOVA of surface response quadratic model depicted in Table 2. F-value is 78.84, being typically very high, which indicates the model Box-Behnken as fully meaningful. P-value < 0.0001 refers to the model being significant.
Lack of fit (0.0919) estimation can be a good reason for the accuracy of the model data. As the model analysis derived results indicated (Table 3), Std.Dev was achieved as 29.55, which confirms the model’s accuracy. This parameter value for melanin yield of 790 mg l−1 level denotes the accuracy of the model. The closer R-squared to 1, the better relationship exists between the lab (adjusted R2 = 0.97) and the estimated results (predicted R2 = 0.938).
The 3-D graphs show the overall impact of the factors’ relationship. In Fig. 7a, two factors of temperature at 32 °C and cooper 0.013 g l−1 are constant, and two factors of acidity and tyrosine are measured by their interaction. The blue color means the lowest and red means the highest product. The interactions between L-tyrosine and pH improved the melanin yield by increasing the pH from 9 to 11 and decreasing L-tyrosine to 2.2–2.8 g l−1.
The interactions between copper and temperature are given in Fig. 7b. Two factors of acidity at 10.5 and L-tyrosine 2.5 g l−1 are constant, and two factors of temperature and cooper were measured by their interactions. It was indicated that by the temperature rise to 32 °C and Cu to 0.013 g l−1, the melanin production increased.
The interactions between copper and pH (Fig. 7c) indicated the melanin production increase in the pH range of about 9 to 11 with the copper concentration decrease to 0.013 g l−1. The interactions between L-tyrosine and CuSO4 (Fig. 7d) signal the melanin production increase which depends on L-tyrosine 2.5 g l−1 than copper. The pH and temperature (Fig. 7e) denoted that the temperature and acidity factors exert an equal effect on the melanin production. The interaction of two factors, i.e., temperature and L-tyrosine (Fig. 7f) exhibited the factors’ effect on the melanin production at constant acidity. According to Fig. 7f, the temperature increase did not have effect on the melanin production, but tyrosine increases up to 2.5 g l−1 exerted significant effect on the melanin yield.
The optimal whey medium compositions obtained 5% (w/v) whey, 2.5 g l−1 L-tyrosine, 0.013 g l−1 CuSO4, and pH 10.5, at temperature 32 °C by max yield of 790 mg l−1 melanin pigment production.
The melanin yield and tyrosinase activity were achieved as 1.2 g l−1 and 4040 U ml−1, respectively, using the nutrient broth with the optimized factors in the whey medium, except the whey powder.
Discussion
The present study is the first report in Dietzia schimae to produce melanin pigment instead of carotenoids in low-nutrient medium that contained L-tyrosine. This UV-resistant actinobacterium was isolated previously from a desert soil sample in Iran (Zamanian and Etemadifar 2017).
The tyrosinase enzyme in the Dietzia family has been reported as a pollutant removal and the culture medium component has been stated as important on the their tyrosinase enzyme’s activity to produce melanin (Babu et al. 2015).
Melanization results in protection and adaptation for the organisms against chemical and mechanical stressors such as temperature, radiation, humidity, and toxicity because of various pollutants. The microbial melanins provide a biocompatible compound for bioremediation and radioprotection technologies (Cordero et al. 2017).
With respect to high antioxidant activity and biocompatibility of UV-resistant extremophilic bacterial pigments, in our research, we surveyed some UV-resistant bacteria in which we found melanin production in extremophilic Dietzia NM3 for the first time. This strain was capable to activate tyrosinases enzyme by L-tyrosine in low-nutrient culture media.
D. schimae NM3 changed metabolic pathway (Shikimate) in the presence of L-tyrosine and the melanin pigment production got started in the early stages of the bacterial growth phase by Mason-Raper pathway to appear as dark pigment in broth culture media. NM3 strain could grow in the cheap culture medium such as whey broth to produce dark pigment. Whey was used as a carbon and energy source to grow and produce biomass by Dietzia NM3, but without L-tyrosine it could not produce melanin. So Dietzia grew in whey medium and after biomass production, it used L-tyrosine to produce melanin.
Melanin production in bacteria requires diverse conditions for each strain. For example, the DMZ-3 strain is capable to use tyrosine in the presence of starch in agar cultures, while in other strains, the melanin was not produced. Streptomyces strain DAS139 produced less melanin in the culture medium with glucose and L-tyrosine, while it produces high melanin in the culture medium containing glycerol and starch (Dastager et al. 2006; Madhusudhan et al. 2014).
The antioxidant level of NM3 strain melanin (188.9%) was higher than that of the ascorbic acid as the standard. In other bacteria, the antioxidant activity was 30% and 44.7%, much lower than the melanin from NM3; however, the antioxidant activity was reported to account for more than 60% in other research cases (El-Naggar and El-Ewasy 2017; Mishra and Singh 2015). Melanin can potentially stabilize harmful unpaired electrons such as those from ROS and show high antioxidant (DPPH) and sun protection factor (SPF) (Geis et al. 1984). Some factors capable to change the SPF values of sunscreens include different solvents, the concentration, and the type of emulsion used in the formulation (Dutra et al. 2004). Melanins have been used commercially in cosmetics’ materials against ultraviolet damage, and also in foodstuff (Brenner and Hearing 2008). Melanin possesses high photo stability and strong UV-absorption than the metabolites of Porphyra-334, palythine and mycosporine-like amino acids reported before (Solano 2020). Melanin has physiological and photoprotective properties that could absorb wide broad UV-visible spectrum. Melanin is resistant to degradation and have the ability to remove up to 90% of the absorbed energy from the sunlight radiation such as heat (Solano 2014). At present, the main commercial application of melanin is as a dye in lenses of sunglasses. In this case, the natural origin of the pigment and the capacity to reduce high energy visible light are highlighted as an advantage (https://espeyewear.com/) (Martínez et al. 2019).
Metal biosorption potential in some bacterial melanin is up to 97% at 120 min (Chen et al. 2009). In this study, the saturated biosorption was seen up to 50% within 60 min, the reason behind which is the functional groups surrounding melanin’s structure. After 1 h, due to biosorption, Vibrio parahaemolyticus melanin reduced metal from 8.0 mg ml−1 to 0.35 mg ml−1 (Abbas et al. 2014; Hoa et al. 2017).
The melanin from Streptomyces DMZ-3 strain at concentration of 64 μg mL−1 inhibited cancer cells growing after 24 h (Madhusudhan et al. 2014). Melanin concentration of 100 μg mL−1 from Streptomyces glaucescens NEAE-H has been shown to inhibit the growth of fibroblast cancer cells as 81.3% (El-Naggar and El-Ewasy 2017). This high inhibitory activity is due to the large structure of its melanin and may be due in part to the solvent. The extracted melanin produced by Dietzia NM3 strain could dissolve in distilled water, after addition of small amount of DMSO was dissolved in distilled water. This melanin in concentration of 500 μg mL−1 showed low inhibition effect (16%) on fibroblast normal cells growth. However, at concentrations lower than 250 μg mL−1, it was not toxic to normal cells and was biocompatible. One of the advantages of this melanin is biocompatibility even in high concentrations.
Box-Behnken design for a bioproduct optimization has fewer design points and less expensive than central composite designs with the same number of factors. Box-Behnken designs do not have axial points. Thus, all design points fall within their safe operating zone. Box-Behnken designs factors are not set at their high levels at the same time (Stamenković et al. 2018). Hence, Box-Behnken design is easy to predict the lower and upper limits at 3 level point, and this method was used for the melanin optimization in this study.
The optimized melanin production by Auricularia auricula demonstrated 519.54 mg l−1 yield (Kurian and Bhat 2014), the optimized melanin production by Streptomyces glaucescens NEAE-H was obtained as 316.50 μg ml−1 by Placket–Burman’s design (El-Naggar and El-Ewasy 2017). In this study, according to the RSM results, the max yield of melanin by Dietzia NM3 was gained as 790 mg l−1 under the optimal whey medium and culture conditions. These results confirmed that the developed model being valid in the present study. According to the results, the model’s quality was adequately good and might describe the real relationship among the medium components.
Compared to other studies, the HPLC peak pattern of melanin from D. schimae NM3 showed proper extraction with appropriate column chromatography and similarity with the synthesized standard melanin in terms of the functional group and chemical elements (Sun et al. 2016). In some studies, the brown melanin synthesized with L-tyrosine has a different peak time than the melanin naturally produced by microorganisms in the culture medium. For instance, the melanin produced by Cryptococcus neoformans exhibited a peak time within 9 min (Frases et al. 2007).
According to the classification method, various absorption peaks showing bands and groups in the FTIR spectra represent the traits of D. schimae NM3 extracted melanin being of eumelanin type (Sun et al. 2016). Eumelanin structure in FTIR revealed the groups -OH and -NH2, C=C, –COO-, C=O in the 1600-3419 region in Auricularia auricular (Sun et al. 2016) and Bacillus thuringiensis (Sansinenea et al. 2015). Dietzia showed low carbon structure with -OH and -NH2 and the functional groups and peaks, representing them similar to the standard (Kurian and Bhat 2014).
Study impact
Dietzia is a drought- and ultraviolet-resistant actinobacterium, whose melanin pigment was not reported. In this study, the potential of this bacterium to produce a dark brown melanin pigment using L-tyrosine was exhibited. Also, for the growth of Dietzia to produce the brown melanin, a simple and cheap culture medium of whey (recycled materials of dairy factories) was used in this study. To produce melanin by activating the tyrosinase enzyme, it is imperative to optimize various physiological and dietary conditions employed for production by RSM procedure. The melanin exhibited various biological properties such as the ability to scavenge free radicals and to protect against the sun ultraviolet rays. These properties make this pigment a good choice to be used in cosmetics and pharmaceutical applications. The melanin from Dietzia NM3 also possesses the capability to remove chromium ions by adsorption, which could be an option to remove metals without any environmental pollution or the risk of damaging the skin surfaces of the living organisms.
Availability of data and materials
All data generated and analyzed during this study are included in this article.
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3:74–102
Arun G, Eyini M, Gunasekaran P (2015) Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J Exp Biol 53(6):380–387
Babu SS, Mohandass C, Vijayaraj A, Dhale MA (2015) Detoxification and color removal of Congo Red by a novel Dietzia sp.(DTS26)–a microcosm approach. Ecotoxicol Environ Saf 114:52–60
Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84(3):539–549. https://doi.org/10.1111/j.1751-1097.2007.00226.x
Chen S, Xue C, Wang J, Feng H, Wang Y, Ma Q, Wang D (2009) Adsorption of Pb(II) and Cd(II) by squid Ommastrephes bartrami melanin. Bioinorg Chem Appl 2009:901563. https://doi.org/10.1155/2009/901563
Cordero RJ, Vij R, Casadevall A (2017) Microbial melanins for radioprotection and bioremediation. Microb Biotechnol 10:1186
Dastager S et al (2006) Seperation, identification and analysis of pigment (melanin) production in Streptomyces. Afr J Biotechnol 5:1131–1134
Dholakiya RN, Kumar MA, Mody KH (2017) Production and characterization of melanin from Streptomyces cavourensis strain RD8 using response surface optimization. Environ Pollution Protect 2:168–178
Dutra EA, ERM K-H, MIRM S (2004) Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Revista Brasileira de Ciências Farmacêuticas 381:385–340. https://doi.org/10.1590/S1516-9332200400030001
El-Batal AI, El-Sayyad GS, El-Ghamery A, Gobara M (2017) Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J Clust Sci 37(1):63
El-Naggar NE-A, El-Ewasy SM (2017) Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens. NEAE-H Sci Rep 7:42129
Frases S, Salazar A, Dadachova E, Casadevall A (2007) Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl Environ Microbiol 73(2):615–621. https://doi.org/10.1128/AEM.01947-06
Ganesh Kumar C, Sahu N, Narender Reddy G, RBN P, Nagesh N, Kamal A (2013) Production of melanin pigment from Pseudomonas stutzeri isolated from red seaweed H ypnea musciformis. Lett Appl Microbiol 57:295–302
Geis PA, Wheeler MH, Szaniszlo PJ (1984) Pentaketide metabolites of melanin synthesis in the dematiaceous fungus Wangiella dermatitidis. Arch Microbiol 137(4):324–328. https://doi.org/10.1007/BF00410729
Hoa PT, Thuy LB, Thang ND (2017) Natural melanin as a potential biomaterial for elimination of heavy metals and bacteria from aqueous solution. Nat Sci Technol 32:2588–1140
Huang S, Pan Y, Gan D, Ouyang X, Tang S, Ekunwe SI, Wang H (2011) Antioxidant activities and UV-protective properties of melanin from the berry of Cinnamomum burmannii and Osmanthus fragrans. Med Chem Res 20:475–481
Ingle SS, Khobragade C (2013) Production and purification of the tyrosinase enzyme from soil bacteria Helix. 6:436–440
Jakoby WB (1971) [23] Crystallization as a purification technique. In: Methods in enzymology, 22. Elsevier, pp 248-252
Koerner RJ, Goodfellow M, Jones AL (2009) The genus Dietzia: a new home for some known and emerging opportunist pathogens FEMS. Immunol Med Microbiol 55(3):296–305. https://doi.org/10.1111/j.1574-695X.2008.00513.x
Kurian N, Bhat SG (2014) Bacterial melanins. Microbial Bioprod 1:97–110
Madhusudhan DN, Mazhari BB, Dastager SG, Agsar D (2014) Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. Biomed Res Int 2014:306895. https://doi.org/10.1155/2014/306895
Martínez LM, Martinez A, Gosset G (2019) Production of melanins with recombinant microorganisms. Front Bioeng Biotechnol 7:285. https://doi.org/10.3389/fbioe.2019.00285
MHS R, Ramesh T, Subramanian J, Kalaiselvam M (2013) Production and characterization of melanin pigment from halophilic black yeast Hortaea werneckii. Int J Pharma Res Rev 2:9–17
Mishra S, Singh HB (2015) Silver nanoparticles mediated altered gene expression of melanin biosynthesis genes in Bipolaris sorokiniana. Microbiol Res 172:16–18. https://doi.org/10.1016/j.micres.2015.01.006
Nosanchuk JD, Casadevall A (2003) The contribution of melanin to microbial pathogenesis. Cell Microbiol 5(4):203–223. https://doi.org/10.1046/j.1462-5814.2003.00268.x
Plonka PM, Grabacka M (2006) Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim Pol 53(3):429–443. https://doi.org/10.18388/abp.2006_3314
Roy S, Das I, Munjal M, Karthik L, Kumar G, Kumar S, KVB R (2014) Isolation and characterization of tyrosinase produced by marine actinobacteria and its application in the removal of phenol from aqueous environment. Front Biol 9:306–316
Sansinenea E, Salazar F, Ramirez M, Ortiz A (2015) An ultra-violet tolerant wild-type strain of melanin-producing Bacillus thuringiensis. Jundishapur J Microbiol 8(6):e20910. https://doi.org/10.5812/jjm.20910v2
SMT G, Razavi SH, Mousavi SM (2013) Comparison of antioxidant and free radical scavenging activities of biocolorant synthesized by Dietzia natronolimnaea HS-1 cells grown in batch, fed-batch and continuous cultures. Ind Crop Prod 49:10–16
Solano F (2014) Melanins: skin pigments and much more—types, structural models, biological functions, and formation routes. New J Sci 18:2014
Solano F (2020) Photoprotection and skin pigmentation: melanin-related molecules and some other new agents obtained from natural sources. Molecules 25:1537
Stamenković OS, Kostić MD, Radosavljević DB, Veljković VB (2018) Comparison of box-behnken, face central composite and full factorial designs in optimization of hempseed oil extraction by n-hexane: a case study. Periodica Polytechnica Chem Eng 62:359–367
Sun S, Zhang X, Sun S, Zhang L, Shan S, Zhu H (2016) Production of natural melanin by Auricularia auricula and study on its molecular structure. Food Chem 190:801–807. https://doi.org/10.1016/j.foodchem.2015.06.042
Surwase SN, Jadhav SB, Phugare SS, Jadhav JP (2013) Optimization of melanin production by Brevundimonas sp. SGJ using response surface methodology 3. Biotech 3:187–194
Surwase SN, Patil SA, Jadhav SB, Jadhav JP (2012) Optimization of l-DOPA production by Brevundimonas sp. SGJ using response surface methodology. Microb Biotechnol 5(6):731–737. https://doi.org/10.1111/j.1751-7915.2012.00363.x
Tarangini K, Mishra S (2013) Production, characterization and analysis of melanin from isolated marine Pseudomonas sp. using vegetable waste. Res J Eng Sci 2278:9472
Varga M, Berkesi O, Darula Z, May NV, Palágyi A (2016) Structural characterization of allomelanin from black oat. Phytochemistry 130:313–320. https://doi.org/10.1016/j.phytochem.2016.07.002
Wang H, Qiao Y, Chai B, Qiu C, Chen X (2015) Identification and molecular characterization of the homogentisate pathway responsible for pyomelanin production, the major melanin constituents in Aeromonas media. WS PLoS One 10:e0120923. https://doi.org/10.1371/journal.pone.0120923
Zamanian SN, Etemadifar Z (2017) Radical scavengering of pigments from novel strains of Dietzia schimae and Microbacterium esteraromaticum. Prog Biol Sci 6:159–170
Zerrad A, Anissi J, Ghanam J, Sendide K, El Hassouni M (2014) Antioxidant and antimicrobial activities of melanin produced by a Pseudomonas Balearica strain. J Biotechnol Lett 5:87 ISSN:0976-7045
Acknowledgement
We sincerely appreciate the University of Isfahan for financially supporting this research which covers the part of Ph.D. thesis. We are also grateful to Dr. Abbasi for assisting us in the cell culture.
Funding
No funding available.
Author information
Authors and Affiliations
Contributions
SE performed and analyzed the experimental research and wrote the manuscript. ZE planned the study and edited the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Normalized product function for calculating SPF. Table S2. FTIR analysis of the extracted melanin structure. Figure S1. Colonies of Dietzia schimae NM3 on NA medium with L-tyrosine (A), and dark pigmentation on NB medium with L- tyrosine (B), after 72 hours. Figure S2. Melanin production of Dietzia schimae NM3 in broth media (4 days incubation). Growth of bacteria (A600nm), L-tyrosine reduction (A492nm) and melanin production (A400nm). Figure S3. The UV-VIS spectra of synthetic melanin (SIGMA) solution as standard (B1), and melanin extracted from Dietzia schimae NM3 (D1) solution in 5% NaOH pH 9. Figure S4. HFB cell line after 72 hours treatment by melanin (125, and 500 μg/ml) from Dietzia schimae NM3, and without melanin (blank) (×40).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eskandari, S., Etemadifar, Z. Biocompatibility and radioprotection by newly characterized melanin pigment and its production from Dietzia schimae NM3 in optimized whey medium by response surface methodology. Ann Microbiol 71, 17 (2021). https://doi.org/10.1186/s13213-021-01628-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-021-01628-6