Abstract
Introduction
Plasma glial fibrillary acidic protein (GFAP) is a marker of astroglial activation and astrocytosis. We assessed the ability of plasma GFAP to detect Alzheimer’s disease (AD) pathology in the form of AD-related amyloid-β (Aβ) pathology and conversion to AD dementia in a mild cognitive impairment (MCI) cohort.
Method
One hundred sixty MCI patients were followed for 4.7 years (average). AD pathology was defined using cerebrospinal fluid (CSF) Aβ42/40 and Aβ42/total tau (T-tau). Plasma GFAP was measured at baseline and follow-up using Simoa technology.
Results
Baseline plasma GFAP could detect abnormal CSF Aβ42/40 and CSF Aβ42/T-tau with an AUC of 0.79 (95% CI 0.72–0.86) and 0.80 (95% CI 0.72–0.86), respectively. When also including APOE ε4 status as a predictor, the accuracy of the model to detect abnormal CSF Aβ42/40 status improved (AUC = 0.86, p = 0.02). Plasma GFAP predicted subsequent conversion to AD dementia with an AUC of 0.84 (95% CI 0.77–0.91), which was not significantly improved when adding APOE ε4 or age as predictors to the model. Longitudinal GFAP slopes for Aβ-positive and MCI who progressed to dementia (AD or other) were significantly steeper than those for Aβ-negative (p = 0.007) and stable MCI (p < 0.0001), respectively.
Conclusion
Plasma GFAP can detect AD pathology in patients with MCI and predict conversion to AD dementia.
Similar content being viewed by others
Introduction
By 2050, more than 150 million people worldwide are estimated to be affected by dementia, with Alzheimer’s disease (AD) causing up to 70% of all cases [1]. AD dementia is still largely a clinical diagnosis, although amyloid-β (Aβ) in brain starts decades before the symptoms appear [2]. Aβ plaques cause astrogliosis, i.e., functional and morphological changes in the surrounding astrocytes, which are glial cells involved in brain signaling, modulation of synapses, transport of nutrients, homeostasis, and structural support [3, 4]. Animal and cell studies have shown the presence of astrogliosis around Aβ plaques and the involvement of reactive astrocytes in Aβ production and toxicity [5,6,7,8]. Studies conducted with PET tracers targeting astrocytes have shown that astrogliosis is an early feature in the pathological cascade of AD, which decreases over the course of disease as opposed to the increase of Aβ plaque load [9,10,11].
Glial fibrillary acidic protein (GFAP) is expressed in the cytoskeleton of astrocytes and has been found significantly increased in CSF in AD and other neurodegenerative diseases compared to healthy controls [12,13,14,15]. GFAP has also been recently measured in plasma and serum, where it was found increased in different neurological conditions, including AD [16,17,18,19,20,21,22,23,24]. Different studies have shown that higher concentrations of plasma GFAP were associated to amyloid-PET positivity and worse outcomes in global cognition [22, 24,25,26,27]. Even though previous studies suggest that blood GFAP levels are elevated in AD and can identify an amyloid-PET positive status, only one study has measured GFAP in cognitively normal subjects followed over time for conversion to dementia (of any kind) [27]. Higher baseline GFAP, measured in serum, was associated to increased risk of dementia, but no significant difference was seen in the change over time of GFAP levels between the cognitively normal and dementia groups [27]. No studies have been done in patients with mild cognitive impairment (MCI) investigating whether plasma GFAP can predict future conversion to AD dementia, specifically.
In this study, our aim was to evaluate plasma GFAP as a potential plasma biomarker of AD in MCI patients, and assess its association with AD-related Aβ pathology and conversion to AD dementia. Early identification of patients that are more likely to have worse cognitive outcome could positively affect their clinical management and help fast-track the diagnostic process; moreover, these patients could be selected for inclusion in clinical trials with disease-modifying drugs currently under development. The study included 160 subjects with a baseline clinical diagnosis of MCI, followed for an average of 4.7 years. CSF and plasma samples were collected at baseline and follow-up. Patients were divided according to clinical diagnosis into the groups stable MCI (those who did not progress to AD dementia or other dementias), MCI-AD (those who progressed to dementia due to AD), and MCI-other (those who progressed to dementia due to other non-AD diseases). Each diagnostic groups was further stratified in Aβ-positive and Aβ-negative based on CSF Aβ42/40 and Aβ42/total tau (T-tau) ratios.
Materials and methods
MCI clinical cohort
The present study includes 160 patients referred to the Memory Clinic at Skåne University Hospital, Malmö. CSF data from the cohort have previously been published [28]. At the clinical baseline visit, physicians with an expertise in cognitive disorders performed a thorough physical, neurological, and psychiatric examination, as well as a clinical interview focusing on cognitive symptoms and ADL function. Furthermore, analysis of APOE genotype was performed.
Patients with MCI at baseline had to fulfill the criteria by Petersen, including (1) memory complaint, preferably corroborated by an informant; (2) objective memory impairment adjusted for age and education, as judged by the physician; (3) preservation of general cognitive functioning, as determined by the clinician’s judgment based on a structured interview with the patient and a Mini Mental Status Examination (MMSE) score greater than or equal to 24; (4) zero or minimal impairment of daily life activities; and (5) not fulfilling the DSM-IIIR criteria for dementia [29]. Patients with other causes of cognitive impairment, including subdural hematoma, brain tumor, CNS infection, schizophrenia, major depressive episode, and current alcohol abuse were excluded. However, MCI subjects were allowed to show signs of white matter changes or silent brain infarcts, because these changes are frequent in elderly subjects with or without cognitive deficits. Similarly, MCI patients with mild to moderate depressive symptoms and low plasma concentrations of vitamin B12 or folate were not excluded. The included patients with MCI at baseline subsequently developed a certain type of dementia or remained cognitively stable for an average of 4.7 years. The patients with MCI who received a diagnosis of AD during clinical follow-up were required to meet the DSM-IIIR criteria for dementia and the criteria of probable AD defined by NINCDS-ADRDA [30]. Subjects who during follow-up were diagnosed as having vascular dementia (VaD) fulfilled the DSM-IIIR criteria of dementia and the requirements for probable VaD by NINDS-AIREN [31]. The consensus criteria by McKeith and coworkers were applied when diagnosing dementia with Lewy bodies (DLB) [32]. Subjects with progressive supranuclear palsy (PSP) fulfilled the criteria by Litvan et al. [33] and Höglinger et al. [34]. The clinical diagnoses of all patients were reviewed by a consensus group consisting of three medical doctors with special interest in cognitive disorders. The study was approved by the Ethics Committee at the University of Lund and the patients and/or their relatives gave their informed consent (for research). Patients were divided according to clinical diagnosis into the groups stable MCI (those who did not progress to AD dementia or other dementias, n = 79), MCI-AD (those who progressed to dementia due to AD, n = 47), and MCI-other (those who progressed to dementia due to other diseases: n = 34, of which VaD = 25, DLB = 4, PSP = 3, other neurological diseases = 2).
Plasma and CSF sampling
Blood and CSF samples were collected in the morning during the same visit, with participants non-fasting. Blood was collected in six EDTA-plasma tubes and centrifuged (2000g, + 4 °C) for 10 min. Following centrifugation, plasma was aliquoted into 1.5-ml polypropylene tubes (1 ml plasma in each tube) and stored at − 80 °C within 30–60 min of collection. CSF was collected by lumbar puncture and stored at − 80 °C in polypropylene tubes following the Alzheimer’s Association flow chart for lumbar puncture and CSF sample processing [35].
Plasma and CSF analysis
Plasma GFAP was measured with Simoa GFAP Discovery kits for SR-X (Quanterix®, Billerica, MA, USA) according to the manufacturer’s instructions. The levels of CSF total tau (T-tau) and tau phosphorylated at Thr181 (P-tau181) were determined using xMAP technology as previously described [36]. CSF Aβ40 and Aβ42 levels were analyzed by electrochemiluminescence technology (Meso Scale Discovery, Gaithersburg, Maryland, USA), using the MS6000 Human Abeta 3-Plex Ultra-Sensitive Kit, following the manufacturer’s recommendation. An Aβ-positive subject was defined as having a CSF Aβ42/40 value below 0.07. The cut-off was previously calculated by Youden index as the best to separate AD patients from cognitively healthy controls [28]. Groups were also divided in CSF Aβ42/T-tau-positive and Aβ42/T-tau-negative groups using a cut-off previously calculated in the same cohort using Youden index [28]. A cut-off of 7.3 was the best to separate AD patients from healthy controls.
Statistical analysis
Analyses were performed using SPSS v.24 (IBM) and R v.3.5.3. Data were visualized using either Prism 8.4 (Graphpad) or R. Group differences were assessed in univariate general linear models, adjusted for age and sex and post hoc least significant difference (LSD) tests for pairwise group comparisons. Biomarkers values were LOG10 transformed prior to this analysis. Diagnostic accuracies were assessed with receiver operating characteristic (ROC) curve analysis and binary logistic regression models. Akaike information criterion (AIC) was calculated for each logistic regression model. Sensitivities and specificities for the biomarkers were calculated at Youden index thresholds. Linear mixed-effect model with random intercept adjusted for age and sex was used to determine biomarker slopes over time and differences between groups. Spearman r was used to measure correlations between biomarkers. P values < 0.05 were considered significant.
Results
Group sizes, age, and gender distribution for subgroups are shown in Table 1. Of these 160 patients with MCI, 159 had GFAP measurements at baseline and one case had only the follow-up measurement of GFAP. One hundred and ten subjects had both baseline and follow-up measurements. The average length of the follow-up was 4.7 years.
GFAP baseline values in Aβ-negative MCI subjects correlated significantly with age (r = 0.57, p < 0.0001) and CSF Aβ42/40 (r = − 0.33, p = 0.003) (Table 2). In Aβ-positive MCI patients, GFAP also correlated with CSF T-tau (r = 0.29, p = 0.01) and CSF P-tau181 (r = 0.28, p = 0.01) (Table 2).
Plasma GFAP for detection of AD pathology
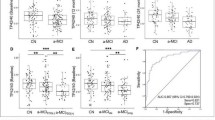
We first analyzed the whole cohort comparing plasma GFAP in groups defined according to their CSF Aβ42/40 and Aβ42/T-tau status. Plasma GFAP levels at baseline were significantly different between CSF Aβ42/40-positive and CSFAβ42/40-negative groups as well as between CSF Aβ42/T-tau-positive and CSF Aβ42/T-tau-negative groups (both p < 0.0001) (Fig. 1a, b). We then compared those patients who progressed to dementia due to AD (MCI-AD) to those who remained stable (stable MCI) and those who progressed to dementia due to other diseases (MCI-other). The last two groups were subdivided in Aβ-positive or Aβ-negative according to the presence or absence of biomarker evidence of brain amyloidosis based on CSF Aβ42/40. The MCI-AD group had significantly higher plasma GFAP concentrations than Aβ-negative cognitively stable MCI and Aβ-negative MCI-other (both p < 0.0001) (Fig. 1c). Aβ-positive stable MCI and Aβ-positive MCI-other had significantly higher concentration of plasma GFAP compared to stable MCI Aβ-negative cases (p = 0.01 both) (Fig. 1c). The Aβ-positive MCI-other group had significantly higher GFAP levels than the Aβ-negative MCI-other group (p < 0.004) (Fig. 1c). No significant differences were present between the different Aβ-positive diagnostic groups or the different Aβ-negative diagnostic groups.
a Plasma GFAP in the Aβ-positive (Aβ+, CSF Aβ42/Aβ40 < 0.07) and Aβ-negative (Aβ−, CSF Aβ42/Aβ40 > 0.07) groups. b Plasma GFAP in the Aβ42/tau positive (Aβ42/T-tau+, CSF Aβ42/T-tau < 7.3) and Aβ42/tau negative (Aβ42/tau−, CSF Aβ42/T-tau > 7.3) groups. c Plasma GFAP in the stable MCI, MCI-AD and MCI-other groups stratified by Aβ status. Line across represents median, box represents interquartile range (IQR), bars represent min and max value (within ± 1.5 IQR). P values were calculated with univariate linear model and least significant differences (LSD) post hoc tests, with adjustments for age and sex
Next plasma GFAP baseline measurements were tested alone or when combined with age and/or APOE ε4 status when predicting the CSF Aβ status. Receiver operating characteristics (ROC) curve analysis showed the greatest area under the curve (AUC) for GFAP combined with APOE ε4 status (AUC = 0.86) (Fig. 2a, Table 3). This combination had a significantly higher AUC than GFAP by itself (p = 0.02). Adding age to the model with GFAP and APOE ε4 status did not further improve the accuracy (AUC = 0.86) (Table 3). Binary logistic regression with Aβ-positive status as outcome showed that plasma GFAP combined with APOE ε4 status was the best predictor, with the lowest Akaike information criterion (AIC, 152; Δ AIC = − 24) (Table 2).
The same approach was used for the identification of CSF Aβ42/T-tau-positive status. Plasma GFAP combined with APOE e4 status (with or without age) had the greatest AUC (0.84 for both) (Fig. 2b, Table 4). GFAP alone had the same AUC as in combination with age (0.80) (Fig. 2b, Table 4). Binary logistic regression with Aβ42/T-tau-positive status as outcome showed that GFAP combined with APOE was the best predictor, with the lowest AIC (157, Δ AIC = − 15) (Table 4). AUCs for combinations of GFAP, APOE ε4, and age were not significantly superior to the AUC of GFAP by itself (differences between AUCs not significant).
GFAP cut-off for Aβ-positive (> 44.7 pg/mL) had a sensitivity and of 73% and 75%, respectively. GFAP cut-off for Aβ42/T-tau-positive (> 44.9 pg/mL) had a sensitivity of 69% and a specificity of 75%.
Plasma GFAP can predict subsequent of conversion to AD dementia
Plasma GFAP baseline measurements were tested alone or combined with age and/or presence of at least one APOE ε4 allele for accuracy in prediction of conversion to AD dementia at follow-up (MCI-AD). ROC curve analysis showed the greatest AUC for plasma GFAP combined with APOE (AUC = 0.86) (Fig. 2c, Table 5). Adding age to the model did not improve the accuracy (AUC = 0.86). Binary logistic regression with MCI-AD status as outcome showed GFAP combined with APOE ε4 and age as the best predictor having the lowest AIC (136, Δ AIC = − 12) (Table 5). AUCs for combinations of GFAP, APOE ε4 and age were not significantly superior to the AUC of GFAP by itself (differences between AUCs not significant).
GFAP cut-off for an MCI-AD status (> 54.1 pg/mL) had a sensitivity and specificity of 72% and 85%, respectively.
Longitudinal changes in plasma GFAP
Slopes for plasma GFAP adjusted for age and gender showed a significant longitudinal increase in Aβ-negative (β = 2.02, p < 0.0001), with a larger increase in the Aβ-positive group compared to Aβ-negative (β = 2.06, p = 0.01 compared to Aβ-negative) (Fig. 3a).
Longitudinal slopes for plasma GFAP from linear mixed-effects model adjusted for age and sex. X-axis represents time between baseline and follow-up plasma sampling; y-axis represents plasma concentrations in pg/mL. Plasma GFAP measurements were stratified by Aβ-positive (Aβ+) status defined by CSF Aβ 42/40 < 0.07 (a). Slopes for Aβ-positive subjects were significantly steeper than Aβ-negative (p = 0.007). In b, plasma GFAP measurements were stratified by clinical diagnosis at follow-up and Aβ status. GFAP slopes in MCI-AD and Aβ-negative MCI-other subjects were significantly steeper compared to stable MCI Aβ-negative (p < 0.0001 both) and stable MCI Aβ-positive (p = 0.049, p = 0.037, respectively)
When looking at changes over time for different cognitive groups, plasma GFAP showed a significantly higher longitudinal increase in MCI-AD and Aβ-negative MCI-other compared to Aβ-negative stable MCI (p < 0.0001 both) and Aβ-positive stable MCI (p = 0.049 and p = 0.034, respectively) (Fig. 3b). No significant difference was seen between Aβ-positive MCI-other and stable MCI groups and MCI-AD and MCI-other groups.
Discussion
In this study, we assessed the potential of plasma GFAP as a diagnostic and prognostic biomarker for AD in a longitudinal MCI cohort. We observed that high GFAP concentration measured at baseline was a relatively strong indicator of AD pathology and could accurately predict future development of AD dementia. Differences at baseline seemed to be associated with (and possibly driven by) the Aβ status; higher concentrations at baseline were in fact observed in every Aβ-positive subgroup compared to Aβ-negative ones. When looking at AUCs as measures of accuracy, the presence of at least one APOE ε4 allele moderately increased the accuracy of GFAP in detecting AD pathology, but not the accuracy in predicting conversion to AD dementia (no significant difference between AUCs), although the model was overall improved (Δ AIC = − 8). This suggests that plasma GFAP is an accurate biomarker for AD diagnosis and progression.
All cognitive groups showed a mild to moderate increasing trend over time for plasma GFAP (Fig. 3b). However, the MCI-AD and Aβ-negative MCI-other group showed a significantly steeper trajectory compared to the groups that remained cognitively stable. It is possible that GFAP generally increases over time because of its significant correlation with age in both Aβ-negative and Aβ-positive groups (Aβ-: r = 0.57; Aβ+: r = 0.46); however, this could also suggest that higher concentrations of plasma GFAP are associated to general worsening in clinical symptoms, as the steepness of the increase in GFAP was greater in all groups that evolved to dementia. The lack of significance in the difference between the Aβ-positive MCI-other group compared to the stable MCI ones is probably due to the small size of the first group (n = 9). This hypothesis is also reinforced by recent studies on CSF and plasma that show an inverse correlation between concentrations of GFAP and cognition [22, 27, 37]. However, one study from Verberk and colleagues [27], following cognitively normal subjects over time for evolution to dementia (AD and non-AD combined), showed that the dementia group had no steeper trend of increase over time compared to the cognitively healthy group.
There are a series of considerations to take into account when comparing the two studies. The studies have comparable follow-up times (average 4.7 years versus median 3.6 years) and sample sizes of patients with follow-up measurements (160 versus 92 subjects). Both studies also show that patients later evolving to dementia have higher baseline GFAP concentrations. However, in Verberk et al., only 9% of patients in the longitudinal cohort developed dementia (six AD dementia, one progressive supranuclear palsy, one primary progressive aphasia), probably also due to the relatively young age of the population (average 61 years) and low percentage (20%) of CSF Aβ-positive subjects. This might have affected the power of the statistical analysis when comparing the longitudinal slopes between the dementia and cognitively healthy groups. It should also be considered that, in Verberk et al., GFAP was measured in serum, as opposed to plasma in the present study. So far, only one study has compared plasma and serum concentrations of GFAP in a traumatic brain injury cohort, with good correlation but significantly higher concentrations of GFAP in plasma [19]. Extensive head-to-head comparisons are yet to be done between the two different methods of analysis and it cannot be excluded that the presence of clotting agents in the sample might affect the results.
In our study, the finding of a strong association with Aβ is not surprising, given the fact that AD (which is defined by Aβ deposition in plaques) is associated with astrogliosis and release of GFAP from astrocytes [3]. However, the results from previous studies with amyloid-PET imaging suggest that as the Aβ load increases in brain along the course of disease, astrogliosis decreases [9, 11]. A similar dynamic association was observed in a recent cross-sectional study on plasma GFAP, where linear positive associations between brain Aβ load measured with amyloid-PET were observed in subjects at earlier stages of disease and diverged in more severe disease stages [25]. Our cohort had an adequate follow-up time (4.7 years on average); however, we cannot exclude that at a longer follow-up a similar association could be observed. Although GFAP is not specific for AD, the high accuracy showed in detecting AD pathology and conversion to AD dementia suggest that plasma GFAP could be a useful indicator of the astroglial activation component of the multifaceted pathology in AD. In our cohort, plasma GFAP also showed a significant negative correlation to CSF Aβ42/40 in Aβ-negative subjects (Table 2), indicating that lower values of CSF Aβ42/40 (still in the normal range) are associated with higher plasma GFAP. This also suggests that plasma GFAP could be very early marker in the pre-dementia phase, like that of our patients at baseline. The strong association with Aβ status also suggests that plasma GFAP could add to and complement the information from plasma Aβ, as immunoassay measurements of Aβ42 and Aβ40 (and their ratio) still require further optimization [38,39,40].
Limitations
One limitation of our study was the lack of a cognitively healthy control group. However, we did comparisons between the AD group and the stable MCI Aβ-negative, which had a non-progressing type of cognitive impairment and no objectifiable evidence of underlying neurodegenerative disease. Another limitation is the lack of PET imaging data for determination of Aβ status; we have, however, defined Aβ-positive patients based on the CSF Aβ42/40 ratio, which has consistently shown a strong association with brain Aβ load evaluated by PET or at neuropathology [41, 42].
Conclusions
In conclusion, our results show that plasma GFAP is associated to AD-type pathology and can accurately predict clinical progression to AD dementia, making it a potential candidate to add to the blood-based biomarker panel for AD.
Availability of data and materials
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Ethical Review Board of Sweden and Region Skåne, which should be regulated in a material transfer agreement.
Abbreviations
- Aβ:
-
Amyloid β
- AD:
-
Alzheimer’s disease
- AIC:
-
Akaike information criterion
- AUC:
-
Area under the curve
- CBS:
-
Corticobasal syndrome
- CSF:
-
Cerebrospinal fluid
- DLB:
-
Lewy body dementia
- MCI:
-
Mild cognitive impairment
- PSP:
-
Progressive supranuclear palsy
- P-tau181:
-
Phosphorylated tau
- ROC:
-
Receiver operating characteristic
- Simoa:
-
Single molecule array
- T-tau:
-
Total tau
- VaD:
-
Vascular dementia
References
World Health Organization W. Global Dementia Observatory, Global action plan on the public health response to dementia 2017–2025 2017
Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11(12):1048–56. https://doi.org/10.1016/S1474-4422(12)70228-4.
Osborn LM, Kamphuis W, Wadman WJ, Hol EM. Astrogliosis: an integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2016;144:121–41. https://doi.org/10.1016/j.pneurobio.2016.01.001.
Mathur R, Ince PG, Minett T, Garwood CJ, Shaw PJ, Matthews FE, Brayne C, Simpson JE, Wharton SB, on behalf of the MRC Cognitive Function and Ageing Neuropathology Study Group. A reduced astrocyte response to beta-amyloid plaques in the ageing brain associates with cognitive impairment. Plos One. 2015;10(2):e0118463. https://doi.org/10.1371/journal.pone.0118463.
Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W. Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2011;2(6):e167-e.
DaRocha-Souto B, Scotton TC, Coma M, Serrano-Pozo A, Hashimoto T, Sereno L, et al. Brain oligomeric beta-amyloid but not total amyloid plaque burden correlates with neuronal loss and astrocyte inflammatory response in amyloid precursor protein/tau transgenic mice. J Neuropathol Exp Neurol. 2011;70(5):360–76. https://doi.org/10.1097/NEN.0b013e318217a118.
Munger EL, Edler MK, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, Sherwood CC, Raghanti MA. Astrocytic changes with aging and Alzheimer's disease-type pathology in chimpanzees. J Comp Neurol. 2019;527(7):1179–95. https://doi.org/10.1002/cne.24610.
Sollvander S, Nikitidou E, Brolin R, Soderberg L, Sehlin D, Lannfelt L, et al. Accumulation of amyloid-beta by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol Neurodegener. 2016;11(1):38. https://doi.org/10.1186/s13024-016-0098-z.
Carter SF, Schöll M, Almkvist O, Wall A, Engler H, Långström B, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nuclear Med. 2012;53(1):37–46. https://doi.org/10.2967/jnumed.110.087031.
Schöll M, Carter SF, Westman E, Rodriguez-Vieitez E, Almkvist O, Thordardottir S, Wall A, Graff C, Långström B, Nordberg A. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci Rep. 2015;5(1):16404. https://doi.org/10.1038/srep16404.
Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, Almkvist O, Farid K, Schöll M, Chiotis K, Thordardottir S, Graff C, Wall A, Långström B, Nordberg A. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain. 2016;139(Pt 3):922–36. https://doi.org/10.1093/brain/awv404.
Abu-Rumeileh S, Steinacker P, Polischi B, Mammana A, Bartoletti-Stella A, Oeckl P, Baiardi S, Zenesini C, Huss A, Cortelli P, Capellari S, Otto M, Parchi P. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther. 2019;12(1):2. https://doi.org/10.1186/s13195-019-0562-4.
Ishiki A, Kamada M, Kawamura Y, Terao C, Shimoda F, Tomita N, Arai H, Furukawa K. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J Neurochem. 2016;136(2):258–61. https://doi.org/10.1111/jnc.13399.
Jesse S, Steinacker P, Cepek L, von Arnim CA, Tumani H, Lehnert S, et al. Glial fibrillary acidic protein and protein S-100B: different concentration pattern of glial proteins in cerebrospinal fluid of patients with Alzheimer’s disease and Creutzfeldt-Jakob disease. J Alzheimers Dis. 2009;17(3):541–51. https://doi.org/10.3233/JAD-2009-1075.
Wallin A, Blennow K, Rosengren LE. Glial fibrillary acidic protein in the cerebrospinal fluid of patients with dementia. Dementia. 1996;7(5):267–72. https://doi.org/10.1159/000106891.
Chen CH, Cheng YW, Chen YF, Tang SC, Jeng JS. Plasma neurofilament light chain and glial fibrillary acidic protein predict stroke in CADASIL. J Neuroinflammation. 2020;17(1):124. https://doi.org/10.1186/s12974-020-01813-5.
Elahi FM, Casaletto KB, La Joie R, Walters SM, Harvey D, Wolf A, et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimers Dement. 2020;16(4):681–95. https://doi.org/10.1016/j.jalz.2019.09.004.
Heller C, Foiani MS, Moore K, Convery R, Bocchetta M, Neason M, Cash DM, Thomas D, Greaves CV, Woollacott IOC, Shafei R, van Swieten JC, Moreno F, Sanchez-Valle R, Borroni B, Laforce Jr R, Masellis M, Tartaglia MC, Graff C, Galimberti D, Rowe JB, Finger E, Synofzik M, Vandenberghe R, de Mendonca A, Tagliavini F, Santana I, Ducharme S, Butler CR, Gerhard A, Levin J, Danek A, Frisoni G, Sorbi S, Otto M, Heslegrave AJ, Zetterberg H, Rohrer JD. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2020;91(3):263–70. https://doi.org/10.1136/jnnp-2019-321954.
Huebschmann NA, Luoto TM, Karr JE, Berghem K, Blennow K, Zetterberg H, Ashton NJ, Simrén J, Posti JP, Gill JM, Iverson GL. Comparing glial fibrillary acidic protein (GFAP) in serum and plasma following mild traumatic brain injury in older adults. Front Neurol. 2020;11:1054. https://doi.org/10.3389/fneur.2020.01054.
Mayer CA, Brunkhorst R, Niessner M, Pfeilschifter W, Steinmetz H, Foerch C. Blood levels of glial fibrillary acidic protein (GFAP) in patients with neurological diseases. Plos One. 2013;8(4):e62101-e.
Oeckl P, Halbgebauer S, Anderl-Straub S, Steinacker P, Huss AM, Neugebauer H, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer’s disease and correlates with cognitive impairment. J Alzheimers Dis. 2019;67(2):481–8. https://doi.org/10.3233/JAD-180325.
Rajan KB, Aggarwal NT, McAninch EA, Weuve J, Barnes LL, Wilson RS, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020.
van Ballegoij WJC, van de Stadt SIW, Huffnagel IC, Kemp S, Willemse EAJ, Teunissen CE, et al. Plasma NfL and GFAP as biomarkers of spinal cord degeneration in adrenoleukodystrophy. Ann Clin Transl Neurol. 2020;7(11):2127–36. https://doi.org/10.1002/acn3.51188.
Verberk IMW, Thijssen E, Koelewijn J, Mauroo K, Vanbrabant J, de Wilde A, Zwan MD, Verfaillie SCJ, Ossenkoppele R, Barkhof F, van Berckel BNM, Scheltens P, van der Flier WM, Stoops E, Vanderstichele HM, Teunissen CE. Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020;12(1):118. https://doi.org/10.1186/s13195-020-00682-7.
Asken BM, Elahi FM, La Joie R, Strom A, Staffaroni AM, Lindbergh CA, et al. Plasma glial fibrillary acidic protein levels differ along the spectra of amyloid burden and clinical disease stage. J Alzheimers Dis. 2020;78(1):265–76. https://doi.org/10.3233/JAD-200755.
Chatterjee P, Pedrini S, Stoops E, Goozee K, Villemagne VL, Asih PR, Verberk IMW, Dave P, Taddei K, Sohrabi HR, Zetterberg H, Blennow K, Teunissen CE, Vanderstichele HM, Martins RN. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27. https://doi.org/10.1038/s41398-020-01137-1.
Verberk IMW, Laarhuis MB, van den Bosch KA, Ebenau JL, van Leeuwenstijn M, Prins ND, et al. Serum markers glial fibrillary acidic protein and neurofilament light for prognosis and monitoring in cognitively normal older people: a prospective memory clinic-based cohort study. Lancet Healthy Longevity.
Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: a clinical follow-up study of 4.7 years. J Alzheimers Dis. 2010;21(4):1119–28. https://doi.org/10.3233/JAD-2010-100207.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. https://doi.org/10.1212/WNL.34.7.939.
Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–60. https://doi.org/10.1212/WNL.43.2.250.
McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902–5. https://doi.org/10.1212/WNL.53.5.902.
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. https://doi.org/10.1212/WNL.47.1.1.
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853–64. https://doi.org/10.1002/mds.26987.
Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–44. https://doi.org/10.1038/nrneurol.2010.4.
Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51(2):336–45. https://doi.org/10.1373/clinchem.2004.039347.
Teitsdottir UD, Jonsdottir MK, Lund SH, Darreh-Shori T, Snaedal J, Petersen PH. Association of glial and neuronal degeneration markers with Alzheimer’s disease cerebrospinal fluid profile and cognitive functions. Alzheimers Res Ther. 2020;12(1):92. https://doi.org/10.1186/s13195-020-00657-8.
Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, Song L, Hanlon D, Tan Hehir CA, Baker D, Blennow K, Hansson O. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6(1):26801. https://doi.org/10.1038/srep26801.
Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, Scheltens P, Teunissen CE, van der Flier WM. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84(5):648–58. https://doi.org/10.1002/ana.25334.
Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–84. https://doi.org/10.1016/S1474-4422(16)00070-3.
Janelidze S, Pannee J, Mikulskis A, Chiao P, Zetterberg H, Blennow K, Hansson O. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol. 2017;74(12):1492–501. https://doi.org/10.1001/jamaneurol.2017.2814.
Shaw LM, Arias J, Blennow K, Galasko D, Molinuevo JL, Salloway S, Schindler S, Carrillo MC, Hendrix JA, Ross A, Illes J, Ramus C, Fifer S. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018;14(11):1505–21. https://doi.org/10.1016/j.jalz.2018.07.220.
Acknowledgements
Not applicable.
Funding
Work at the authors’ research center was supported by the Swedish Research Council, the Knut and Alice Wallenberg Foundation, the Marianne and Marcus Wallenberg Foundation, the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation, the Swedish Brain Foundation, The Parkinson foundation of Sweden, The Parkinson Research Foundation, the Skåne University Hospital Foundation, the Wallenberg Center for Molecular Medicine, The Medical Faculty at Lund University, Region Skåne, the Swedish Medical Association, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Bundy Academy, and the Swedish federal government under the ALF agreement. Open Access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
CC analyzed the samples, performed the data analysis, and wrote the manuscript. SJ analyzed the samples and supervised the statistical analysis. JH selected and diagnosed the patients included in the study. HZ and KB were responsible for the CSF biomarkers analyses. NMC performed the statistical analysis. OH designed the study. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee at the University of Lund and the patients and/or their relatives gave their informed consent (for research).
Consent for publication
Not applicable.
Competing interests
HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS) (outside submitted work). OH has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, Biogen, Cerveau, and Roche. The other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cicognola, C., Janelidze, S., Hertze, J. et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alz Res Therapy 13, 68 (2021). https://doi.org/10.1186/s13195-021-00804-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-021-00804-9