Abstract
Introduction
Super-enhancer-associated lncRNAs play important roles in the occurrence and development of malignant tumors, including hepatocellular carcinoma (HCC).
Objectives
The current work aimed to identify and characterize super-enhancer-associated lncRNAs in the pathogenesis of HCC.
Methods
H3K27ac ChIP-seq data from HepG2 cell line and two HCC tissues were used to identify super-enhancer-associated lncRNAs in HCC. JQ-1 treatment and CRISPR-dCas9 system were performed to confirm super-enhancer activity. Quantitative real-time PCR (qPCR), ChIP-qPCR, and dual-luciferase reporter system assay demonstrated the regulation of E2F1 on super-enhancer. Functional loss experiment was used to identify the function of LINC01004.
Results
In this study, we identified and characterized LINC01004, a novel super-enhancer-associated lncRNA, as a crucial oncogene in HCC. LINC01004 was upregulated in liver cancer tissues and was associated with poor patient prognosis. Moreover, LINC01004 promoted cell proliferation and metastasis of HCC. The binding of E2F1 to the super-enhancer could promote the transcription of LINC01004, while the inhibition of super-enhancer activity decreased LINC01004 expression.
Conclusion
This finding might provide mechanistic insights into the molecular mechanisms underlying hepatocarcinogenesis and the biological function of super-enhancer. LINC01004 can serve as a potential therapeutic target for HCC patient.
Graphical abstract

Similar content being viewed by others
Introduction
Primary liver cancer is the sixth most common malignancy, which also ranks the third most common cause of cancer-related death [1]. Hepatocellular carcinoma (HCC) is the main histological subtype of primary liver cancer (comprising 75–85% of cases) [1]. HCC is also a devastating disease with limited therapeutic approaches and relatively low 5-year survival rate [2]. Although recent diagnosis of HCC has been greatly improved, a large portion of patients are still diagnosed in advanced stage [3]. Therefore, an urgent need exists to understand the pathogenesis of HCC, detail the illustration of genetic landscape during hepatocarcinogenesis, and discover the novel tumor biomarkers.
Abnormal transcription of oncogenes has been proved to be an important reason for hepatocarcinogenesis [4]. Recent advance indicates that some oncogenes transcription is frequently driven by enhancer which is an active transcription regulatory element [5, 6]. Super-enhancer (SE) is an exceptionally large cluster of enhancers that synergistically drives gene transcriptions [7]. Super-enhancers have high levels of histone-3-lysine-27 acetylation (H3K27Ac) and histone-3-lysine-4 methylation modification, and was densely occupied by various transcription factors [7, 8]. Emerging evidence indicates that cancer cells use SEs to drive transcription of oncogenes, ranging from protein coding genes to non-coding RNAs [9,10,11].
Long non-coding RNAs (lncRNAs) are transcripts over 200 nucleotides, which are involved in many key biological processes including malignancy [12,13,14]. Many tumor-associated lncRNAs are also regulated by super-enhancers [15,16,17,18,19]. In HCC, HCCL5 was found to be a novel super-enhancer-associated lncRNA, which was upregulated during epithelial to mesenchymal transition (EMT) and facilitated the EMT process. Simultaneously, the binding of ZEB1 to the SE promoted the expression of HCCL5 [20]. LncRNA-DAW was another super-enhancer-associated lncRNA in HCC. LncRNA-DAW was driven by a liver-specific SE and promoted tumor growth. Mechanistically, lncRNA-DAW antagonized the suppressive effect of EZH2 on Wnt2 expression, leading to the activation of the Wnt/β-catenin pathway [21]. It will be of value to identify and characterize such super-enhancer-associated lncRNAs in the pathogenesis of HCC.
In this study, we used H3K27ac ChIP-seq data from HCC cells and tissues to identify SEs, and discovered 25 super-enhancer-associated lncRNAs. LINC01004 was identified as one of the novel lncRNAs driven by SE. LINC01004 was frequently upregulated in liver cancer tissues and was associated with poor patient prognosis. The binding of E2F1 to the SE could promote the transcription of LINC01004. However, the inhibition of SE activity could decrease LINC01004 expression. Functionally, knockdown of LINC01004 inhibited HCC cell proliferation and metastasis. Together, this study characterized LINC01004 as a novel super-enhancer-driven lncRNA regulated by E2F1 promoting HCC cell proliferation and metastasis. Our data also suggest that LINC01004 may serve as a novel prognostic biomarker and therapeutic target in HCC.
Materials and methods
Experimental animals and ethic
Mice used in this research were 5-week-old male nude mice. The mice were kept in SPF standard animal house with plenty of food and water. The xenograft model was established by subcutaneous injection of tumor cells. The methods were carried out in accordance with the approved guidelines. This study was approved by Ethics Committee of Shandong University School of Clinical Medicine (SDULCLL2022-2-2).
Identification of super-enhancers and associated lncRNAs
The H3K27ac ChIP-seq data of hepatocellular carcinoma were obtained from ENCODE project (ENCSR000AMO) and GEO database (GSE112221). Super-enhancers (SEs) were identified by ROSE (https://bitbucket.org/youngcomputation/rose). Closely spaced peaks (except those within 2 kb of TSS) within a range of 12.5 kb were merged together, followed by the measurement of input and H3K27Ac signals. These merged peaks were ranked by H3K27Ac signal and then classified into SEs or TEs. SEs were assigned to the nearest genes. SE-associated lncRNAs were screened from the nearest genes.
Bioinformatics analysis
Prognostic information of lncRNAs was obtained from TCGA (The Cancer Genome Atlas) and analyze by GEPIA (http://gepia.cancer-pku.cn/). Expression pattern of LINC01004 in hepatocellular carcinoma was analyzed by using GEO database (GSE67260, GSE55092, GSE58043, GSE62232, and GSE6764). E2F1 ChIP-seq and ATAC-seq data in HepG2 cells were obtained from ENCODE project (ENCSR717ZZW and ENCSR042AWH). ChIP-Seq and ATAC-seq data analysis were performed as described previously. The AQUAS pipeline (https://github.com/kundajelab/chipseq_pipeline) was used to processed ChIP-Seq and ATAC-seq data. Reads was filtered by fastqc (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to remove duplicate reads and low quality bases. Then reads were aligned to the reference human genome (hg38). MACS2 (https://pypi.python.org/pypi/MACS2) was used for peak calling.
Quantitative real-time PCR (qRT-PCR) for gene expression
LncRNAs and mRNAs were reverse transcribed using RevertAid First Strand cDNA Synthesis Kit (Thermo, Wuhan, China) in accordance with the manufacturer’s instructions. The qRT-PCR were performed using a standard UltraSYBR Mixture (CWBIO, Beijing, China) in the Roche LightCycler 480 system (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Human β-actin gene was used as endogenous control genes for qRT-PCR. The qRT-PCR data were analyzed using the 2−∆∆CT method, as previously described [22].
Western blot
Cells were split by radio immunoprecipitation assay (RIPA) buffer (Beyotime, Jiangsu, China) and supplemented with 0.01% of phenylmethanesulfonyl fluoride (PMSF) (Beyotime, Jiangsu, China). The protein was separated by sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis, and then was transferred to a polyvinylidene Fluoride (PVDF) membrane (Millipore, Boston, USA). Next, the protein was incubated with the corresponding primary antibody and secondary antibody. Antibodies included Cas9 (1: 1000, Proteintech, Wuhan, China), E2F1 (1: 1000, Proteintech, Wuhan, China), and β-actin (1: 1000, Proteintech, Wuhan, China).
Cell culture and transfection
The human hepatocellular carcinoma cells (HepG2 and SK-Hep-1 cell lines) were maintained at 37 °C in humidified 5% CO2 atmosphere containing 5% CO2 in Dulbecco’s modified Eagle medium (Hyclone, Logan, USA) supplemented with 10% fetal bovine serum (FBS) (CLARK, Richmond, USA). Cells were plated and grown until they were reached 70–80% confluent. pCMVHA E2F1 was a gift from Kristian Helin [23] (Addgene plasmid # 24,225, https://www.addgene.org/24225/, RRID:Addgene_24225). pLV hUbC-dCas9 KRAB-T2A-GFP was a gift from Charles Gersbach (Addgene plasmid # 67,620, https://www.addgene.org/67620/, RRID:Addgene_67620) [24]. Then plasmids and siRNAs (GenePharma, Suzhou, China) were transfected using lipofectamine 3000 (Invitrogen, Carlsbad, USA).
si-NC: sence 5′- UUCUCCGAACGUGUCACGUTT-3′,
antisence 5′- ACGUGACACGUUCGGAGAATT-3′;
si-LINC01004-1: sence 5′- GGUUCAAGGUAUAAGCUAAACTT-3′;
antisence 5′- UUAGCUUAUACCUUGAACCUATT-3′;
si-LINC01004-2: sence 5′- GGGAAUAUGUUGUGUUCUAAUTT-3′;
antisence 5′- UAGAACACAACAUAUUCCCGATT-3′;
Chromatin immunoprecipitation (ChIP)
Chromatin Immunoprecipitation was performed as described previously [25]. Briefly, Cells were initially cross-linked with 1% formaldehyde, and followed by nuclear extraction. Chromatin/DNA complex was sheared in a Bioruptor Sonicator (Diagenode, Denville, NJ). Sonicated lysates were cleared and incubated overnight at 4 °C with magnetic beads coupled with E2F1 antibody (Proteintech, Wuhan, China). Precipitated immune-complex was washed, and DNA was eluted and analyzed by qRT-PCR.
Luciferase reporter assays
Enhancers were cloned into firefly luciferase reporter vector pGL3-Promoter (Promega, Madison, WI). The indicated cells were transfected using lipofectamine 3000 (Invitrogen, Carlsbad, USA) and A Renilla luciferase plasmid was co-transfected as a normalization control. pGL3-basic plasmid was used as negative control. Luciferase reporter activity was measured using the DualLuciferase Reporter Assay System (Promega).
Cell counting and colony-formation assay
In cell counting assays, 5*104 cells were seeded onto 12-well plates, and cultured for 3 days. Cell counting assays were performed at 24 h, 48 h, and 72 h after inoculation. In colony-formation assay, 2000 cells were seeded onto 6-well plates, and cultured for 2 weeks. Cells were fixed with methanol and stained with crystal violet.
Transwell migration assay
Cell migration assay was performed in Boyden Chamber. Briefly, 1*105 cells in fetal bovine serum–free medium were seeded onto the membrane with 8-mm pores of the top chamber (Thermo Fisher Scientific), with the bottom chamber containing regular medium with 20% fetal bovine serum. After 24 h (SK-Hep-1 cell) or 48 h (HepG2 cell), the membranes were washed, fixed, and stained with crystal violet, and migrated cells were quantified.
Wound-healing and transwell assays
For wound-healing assays, a wound was scratched by a 10 μl pipette tip when the cell layer of SK-Hep-1 or HepG2 cells reached about 90% confluence. Cells were continued cultured at 37 °C with 5% CO2, and the average extent of wound closure was quantified.
CRSIPR-dCas9
gRNAs were designed by E-CRISPR (http://www.e-crisp.org/E-CRISP/). The gRNA sequences are shown in Additional file 2: Figure S2. Then the gRNAs were inserted into the pLV hU6-sgRNA hUbC-dCas9-KRAB-T2a-GFP vector (addgene, #71,237). The recombinant plasmids were transfected with Lipofectamine 3000 (Thermo Fisher, L3000001).
Statistics
The difference between two groups was calculated using Student’s t test. One-way ANOVA analysis with Dunnett’s test was used for multiple comparisons. The significance of expression association between different genes was calculated using Spearman’s correlation. A P value of less than 0.05 was used as the criterion of statistical significance. All analyses were performed with SPSS software package (Version 16.0, SPSS Inc.) or GraphPad Prism (Version 5, GraphPad Software, Inc.).
Results
Identification of super-enhancer-associated lncRNAs in HCC
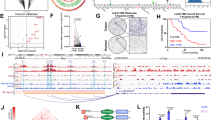
To identify SEs in HCC, H3K27ac ChIP-seq data from HepG2 cell line (ENCODE, ENCSR000AMO) (HepG2 group) and two HCC tissues (GEO, GSE112221) (HCC1 group, GSM3061126 and HCC3 group, GSM3061128) were analyzed (Fig. 1A). As shown in Fig. 1B, we identified 3,651, 332, and 1039 SEs, respectively, in HCC1 tissue, HCC3 tissue, and HepG2 cell line by using ROSE software. The SEs were assigned to the nearest genes. According to the genome annotation, 920, 585, and 2016 SE-associated non-coding RNAs (ncRNAs) were obtained, respectively (Fig. 1B). There were 41 SE-associated ncRNAs were obtained in the intersection of the three groups (Fig. 1C), in which there were 25 lncRNAs (Fig. 1D). Then The Cancer Genome Atlas Program (TCGA) was used to analyze the correlation between these 25 SE-associated lncRNAs and the prognosis of HCC patients. As shown in Fig. 1E, there was 7 lncRNAs (LINC01004, LINC00673, MIR4435-2HG, A2M-AS1, MALAT1, PCBP1-AS1, and LINC00152) significantly negatively correlated with HCC patients’ prognosis, in which LINC00673 [26], MIR4435-2HG [27], MALAT1 [28], PCBP1-AS1 [29], and LINC00152 [30] have been reported to promote the progression of hepatocellular carcinoma, demonstrating the important oncogenic function of SE-associated lncRNAs. The roles of LINC01004 and A2M-AS1 in cancer were still unknown. In this study, LINC01004 was choose for the following study.
Identification of SE-associated lncRNAs in HCC. A Schematic diagram of SE-associated lncRNAs screening. B Statistics of SEs and SE-associated genes in HCC. C Venn diagram of SE-associated ncRNAs from HCC1, HCC3, and HepG2 groups. D Distribution of different types of SE-associated ncRNAs. E Correlation between SE-associated lncRNAs and prognosis of HCC patients. OS means overall survival; DFS means disease-free survival
Feature identification of LINC01004
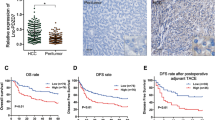
LINC01004 was a long intergenic non-protein coding RNA on chromosome 7, whose biological function remained to be further studied. Online software CPC (http://cpc.gao-lab.org/) was used to confirm the coding potential of LINC01004, and showed that LINC01004 had no coding potential (Table 1). The IGV results of H3K27ac ChIP-seq signals in Fig. 2A demonstrated there was a SE element within LINC01004. Then, we analyzed the H3K4me1(GSM3061121 and GSM3061123), and H3K4me3 (GSM3061131 and GSM3061133) ChIP-seq signals near LINC01004 SE element, and the results showed that H3K4me1 signal was located in the SE region, while H3K4me3 signal was mainly located in the promoter region (Additional file 1: Figure S1). According to the prognosis data from TCGA Program, HCC patients with high LINC01004 expression had significantly poorer disease-free survival (DFS) than patients with low LINC01004 expression (Fig. 2B). Then we analyzed the expression pattern of LINC01004 in HCC tumor and normal tissues in multiple GEO datasets (GSE67260, GSE55092, GSE58043, and GSE62232). As shown in Fig. 2C, the expression of LINC01004 in tumor tissues was significantly higher than that in normal tissues. Moreover, we also found that the expression of LINC01004 in tumor tissues with high stage was also significantly higher than that with low stage in GSE6764 dataset (Fig. 2D). These results suggest that LINC01004 was a highly expressed SE-associated lncRNA in HCC tumors.
Feature identification of LINC01004. A The IGV results of H3K27ac ChIP-seq signals from HCC1, HCC3, and HepG2 groups. The red box represents SE. B Disease free survival analysis of HCC patients with high LINC01004 expression and low LINC01004 expression from TCGA program. C The expression pattern of LINC01004 in HCC tumor and normal tissues in GSE67260, GSE55092, GSE58043, and GSE62232 datasets. D The expression pattern of LINC01004 in HCC tumor tissues with high stage and low stage in GSE6764 dataset
Inhibition of super-enhancer activity can decrease LINC01004 expression
BRD4 can interact with hyperacetylated histone regions on chromosome and accumulate on SE elements to promote gene transcription [31]. JQ-1 was a BET-bromodomain inhibitor, which can lead to preferential loss of BRD4 at SEs and consequent transcription elongation defects that preferentially impacted genes with SEs [32] (Fig. 3A). To confirm the regulation of SE on LINC01004, hepatoma cell lines SK-Hep-1 and HepG2 were treated with 10 μM JQ-1 for 48 h. As shown in Fig. 3B, the JQ-1 treatment significantly inhibited LINC01004 expression. Moreover, we designed three gRNAs targeting SE element of LINC01004 and used CRISPR-dCas9 system to inhibit the SE activity (Fig. 3C and Additional file 2: S2). Fluorescence and Western blot results in Fig. 3D and E showed that dCas9 protein was successfully transfected into the HepG2 cells. qRT-PCR results in Fig. 3F demonstrated that gRNA-1 and gRNA-2 can decrease LINC01004 expression without effecting nearby genes of LINC01004 (Additional file 3: Figure S3). The above results indicated that the inhibition of SE activity can decrease LINC01004 expression.
Inhibition of SE activity can decrease LINC01004 expression. A Schematic diagram of JQ-1 inhibiting SE activity. B The expression of LINC01004 in SK-Hep-1 and HepG2 cells after JQ-1(10 μM) treatment for 48 h. **represents P < 0.01. C Schematic diagram of gRNAs design and CRISPR-dCas9. D GFP fluorescence results of HepG2 cells after gRNA-dCas9 vectors transfection for 48 h. E Western blot results of HepG2 cells after gRNA-dCas9 vectors transfection for 48 h. F The expression of LINC01004 in HepG2 cells after gRNA-dCas9 vectors transfection for 48 h. **represents P < 0.01
E2F1 combined with LINC01004 super-enhancer to promote its expression
To identify the transcription factors combined with LINC01004 SE element, online software JASPAR (https://jaspar.genereg.net/) was performed. As shown in Fig. 4A, there was an E2F1 binding motif in LINC01004 SE element. Moreover, E2F1 ChIP-seq and ATAC-seq results of HepG2 cells from ENCODE program demonstrated the interaction between E2F1 and LINC01004 SE element. Our E2F1 ChIP-qPCR results also confirmed that E2F1 combined with LINC01004 SE (Fig. 4B). E2F1 was significantly overexpressed in HCC tumor tissues and negatively correlated with the prognosis of patients (Additional file 4: Figure S4). The expression of E2F1 and LINC01004 in tumor tissues of TCGA HCC patients was significantly positively correlated (Fig. 4C). Moreover, the expression of E2F1 and BRD4 was also significantly positively correlated (Additional file 5: Figure S5). These results implied that E2F1 can bind to LINC01004 SE to promote its expression. To verify our hypothesis, we transfected E2F1 overexpression vector into HepG2 cells and discovered that E2F1 significantly promoted LINC01004 expression (Fig. 4D and E). Then the LINC01004 SE element was insert into the pGL3-promoter vector (named as pGL3-enhancer) (Fig. 4F). As shown in Fig. 4G, dual-luciferase reporter system assay results indicated that LINC01004 SE were active and E2F1 can promote its activity. When the E2F1 binding site in LINC01004 SE was mutated, E2F1 could not affect the luciferase activity. The above results demonstrated that E2F1 combined with LINC01004 SE to promote its expression.
E2F1 combined with LINC01004 SE to promote its expression. A The IGV results of H3K27ac ChIP-seq, E2F1 ChIP-seq, and ATAC-seq. B E2F1 ChIP-qPCR results of HepG2 cells. IgG antibody was used as negative control. C Correlation analysis of E2F1 and LINC01004 in tumor tissues of TCGA HCC patients. D qRT-PCR results of E2F1 and LINC01004 in HepG2 cells. E Western blot results of E2F1. F Schematic diagram of pGL3-enhancer vectors. G Dual-luciferase reporter system assay of pGL3-enhancer vector. pGL3-basic vector was used as negative control
Knockdown of LINC01004 inhibited HCC cell proliferation and metastasis in vitro
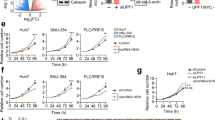
To identify the function of LINC01004 in HCC, siRNAs was used to decline the expression of LINC01004. As shown in Fig. 5A, LINC01004 expression levels was extremely significantly inhibited after the transfection of siRNAs in HepG2 and SK-Hep-1 cells. Then cell counting assay showed that knockdown of LINC01004 by siRNAs inhibited HepG2 and SK-Hep-1 cell proliferation (Fig. 5B). Similarly, knockdown of LINC01004 also inhibited HCC cell colony formation (Fig. 5C). Moreover, cell transwell assay suggested that knockdown of LINC01004 decreased the metastasis ability of HCC cells (Fig. 5D). The wound-healing assays demonstrated that knockdown of LINC01089 impaired the motility of SK-Hep-1 cells (Fig. 5E and F). These results indicated that knockdown of LINC01004 inhibited HCC cell proliferation and metastasis in vitro.
Knockdown of LINC01004 inhibited HCC cell proliferation and metastasis in vitro. A qRT-PCR results of LINC01004 to detect siRNAs knockdown efficiency. ** represents P < 0.01. B Cell counting assay of SK-Hep-1 and HepG2 cells after knockdown of LINC01004. C Colony-formation assay of SK-Hep-1 and HepG2 cells after knockdown of LINC01004. D Cell transwell assay of SK-Hep-1 and HepG2 cells after knockdown of LINC01004. E Wound-healing assays of SK-Hep-1 cells after knockdown of LINC01004. F Quantification result of wound-healing assays. *represents P < 0.05. **represents P < 0.01
Knockdown of LINC01004 inhibited HCC cell proliferation and metastasis in vivo
To identify the function of LINC01004 in vivo, generated LINC01004-knockdown stable HCC cell lines (SK-Hep-1 and Huh7). We then evaluated the oncogenicity capability of LINC01004 in vivo. We found that the growth of the LINC01004-knockdown SK-Hep-1 or Huh7 xenografts in mice was significantly retarded compared with that of control xenografts after 5 weeks (Fig. 6A and B). Evidently increased tumor weights were also observed in the LINC01004-knockout group compared to the control group (Fig. 6A and B). Ki67 protein levels in xenografts of the LINC01004-knockdown group were lower than those in control xenografts (Fig. 6C). Moreover, we used intraperitoneal injection mouse model to confirm the roles of LINC01004 in cell metastasis in vivo. As shown in Fig. 6D, knockdown of LINC01004 can remarkably suppress liver metastasis of HCC cells in vivo. These results indicated that knockdown of LINC01004 inhibited HCC cell proliferation and metastasis in vitro.
Knockdown of LINC01004 inhibited HCC cell proliferation and metastasis in vivo. A Tumor volume and weight of SK-Hep-1 xenografts in mice. B Tumor volume and weight of Huh7 xenografts in mice. C Ki67 immunohistochemical results of xenografts. D Luciferase activity of intraperitoneal injection mouse model
Discussion
Super-enhancers, as active regulatory elements, are capable of driving much higher levels of transcription, and exhibit much stronger lineage- and tissue-specificity compared with typical enhancers [33]. The activity of SEs is related to histone modification, and it is generally considered that the active SE is marked by H3K27ac and H3K4me1, accompanied by lack of H3K4me3 [8]. The ChIP-seq technique is usually used for epigenetics research, and has also been used to analyze genome-wide histone modifications. In the present study, we used H3K27ac ChIP-seq data from HepG2 cell line and HCC tissues to identify SEs in HCC. Moreover, we obtained 25 SE-associated lncRNAs, of which 7 lncRNAs were significantly negatively correlated with HCC patient prognosis. LINC00673 [26], MIR4435-2HG [27], MALAT1 [28], PCBP1-AS1 [29], and LINC00152 [30] have been reported to promote the progression of hepatocellular carcinoma, demonstrating the important oncogenic function of SE-associated lncRNAs.
Particularly, we identify an uncharacterized SE-associated lncRNA, LINC01004, as a novel oncogenic factor promoting HCC cells proliferation and metastasis in vitro and in vivo. LINC01004 was significantly negatively correlated with HCC patient DFS, and the expression of LINC01004 was upregulated in HCC tumor tissue. However, the molecular mechanism of its oncogenic function were still unclear, which need to be further studied. The abnormally high expression of LINC01004 in tumor tissue may be driven by SE. BET inhibition including the use of specific chemical BET inhibitors like JQ-1 can significantly inhibit expression of SE-driven genes [34], including LINC01004 expression. CRISPR-dCas9 system was also a potential way to inhibit SE activity [35]. These results will provide new ideas for the treatment of HCC driven by SEs.
There was exceptionally high degree of enrichment for the binding of transcriptional factors and coactivators on SEs [36, 37]. In this study, E2F1 was found to be a transcriptional factor combined with LINC01004 SE, and promote LINC01004 expression. E2F1, a member of E2F family, play important roles in the regulation of many cellular processes including cell proliferation and apoptosis in HCC [38]. E2F1 is highly expressed in HCC to promote HCC progression through a variety of pathways [39,40,41]. The high expression of E2F1 is one of the important reasons for the high expression of LINC01004.
In conclusion, this study investigated novel super-enhancer-associated lncRNAs and revealed the oncogenic role of a previously unknown lncRNA in liver cancer. LINC01004 was a liver-enriched lncRNA and it was frequently upregulated in liver cancer tissues. Subsequent functional studies demonstrated that knockdown of LINC01004 significantly inhibited tumor cell proliferation and metastasis. E2F1 combined with SEs to promote LINC01004 expression. Inhibition of SE activity could significantly decrease LINC01004 expression. This finding might provide mechanistic insights into the molecular mechanisms underlying hepatocarcinogenesis and the biological function of SE, and LINC01004 can serve as a potential therapeutic target for HCC patient.
Availability of data and materials
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
Bradner JE, Hnisz D, Young RA. Transcriptional addiction in cancer. Cell. 2017;168(4):629–43.
Ko JY, Oh S, Yoo KH. Functional enhancers as master regulators of tissue-specific gene regulation and cancer development. Mol Cells. 2017;40(3):169–77.
Buecker C, Wysocka J. Enhancers as information integration hubs in development: lessons from genomics. Trends Genet. 2012;28(6):276–84.
Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155(4):934–47.
Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470(7333):279–83.
Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM, Abraham BJ, Sharma B, Yeung C, Altabef A, et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell. 2014;159(5):1126–39.
Thandapani P. Super-enhancers in cancer. Pharmacol Ther. 2019;199:129–38.
Wang Y, Nie H, He X, Liao Z, Zhou Y, Zhou J, Ou C. The emerging role of super enhancer-derived noncoding RNAs in human cancer. Theranostics. 2020;10(24):11049–62.
Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965–81.
Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–7.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407.
Wang X, Zhang R, Wu S, Shen L, Ke M, Ouyang Y, Lin M, Lyu Y, Sun B, Zheng Z, et al. Super-enhancer LncRNA LINC00162 promotes progression of bladder cancer. iScience. 2020;23(12):101857.
Lin X, Spindler TJ, de Souza Fonseca MA, Corona RI, Seo JH, Dezem FS, Li L, Lee JM, Long HW, Sellers TA, et al. Super-enhancer-associated LncRNA UCA1 interacts directly with AMOT to activate YAP target genes in epithelial ovarian cancer. iScience. 2019;17:242–55.
Xie JJ, Jiang YY, Jiang Y, Li CQ, Lim MC, An O, Mayakonda A, Ding LW, Long L, Sun C, et al. Super-enhancer-driven long non-coding RNA LINC01503, regulated by TP63, is over-expressed and oncogenic in squamous cell carcinoma. Gastroenterology. 2018;154(8):2137-2151 e2131.
Yan L, Chen H, Tang L, Jiang P, Yan F. Super-enhancer-associated long noncoding RNA AC005592.2 promotes tumor progression by regulating OLFM4 in colorectal cancer. BMC Cancer. 2021;21(1):187.
Bian E, Chen X, Cheng L, Cheng M, Chen Z, Yue X, Zhang Z, Chen J, Sun L, Huang K, et al. Super-enhancer-associated TMEM44-AS1 aggravated glioma progression by forming a positive feedback loop with Myc. J Exp Clin Cancer Res. 2021;40(1):337.
Peng L, Jiang B, Yuan X, Qiu Y, Peng J, Huang Y, Zhang C, Zhang Y, Lin Z, Li J, et al. Super-enhancer-associated long noncoding RNA HCCL5 is activated by ZEB1 and promotes the malignancy of hepatocellular carcinoma. Cancer Res. 2019;79(3):572–84.
Liang W, Shi C, Hong W, Li P, Zhou X, Fu W, Lin L, Zhang J. Super-enhancer-driven lncRNA-DAW promotes liver cancer cell proliferation through activation of Wnt/beta-catenin pathway. Mol Ther Nucleic Acids. 2021;26:1351–63.
Ren Q, Xu ZL, Wang XW, Zhao XF, Wang JX. Clip domain serine protease and its homolog respond to Vibrio challenge in Chinese white shrimp. Fenneropenaeus chinensis Fish Shellfish Immunol. 2009;26(5):787–98.
Lukas J, Petersen BO, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16(3):1047–57.
Kabadi AM, Ousterout DG, Hilton IB, Gersbach CA. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 2014;42(19): e147.
Jiang YY, Lin DC, Mayakonda A, Hazawa M, Ding LW, Chien WW, Xu L, Chen Y, Xiao JF, Senapedis W, et al. Targeting super-enhancer-associated oncogenes in oesophageal squamous cell carcinoma. Gut. 2017;66(8):1358–68.
Zhang LG, Zhou XK, Zhou RJ, Lv HZ, Li WP. Long non-coding RNA LINC00673 promotes hepatocellular carcinoma progression and metastasis through negatively regulating miR-205. Am J Cancer Res. 2017;7(12):2536–44.
Kong Q, Liang C, Jin Y, Pan Y, Tong D, Kong Q, Zhou J. The lncRNA MIR4435-2HG is upregulated in hepatocellular carcinoma and promotes cancer cell proliferation by upregulating miRNA-487a. Cell Mol Biol Lett. 2019;24:26.
Malakar P, Stein I, Saragovi A, Winkler R, Stern-Ginossar N, Berger M, Pikarsky E, Karni R. Long noncoding RNA MALAT1 regulates cancer glucose metabolism by enhancing mTOR-mediated translation of TCF7L2. Can Res. 2019;79(10):2480–93.
Luo T, Gao Y, Zhangyuan G, Xu X, Xue C, Jin L, Zhang W, Zhu C, Sun B, Qin X. lncRNA PCBP1-AS1 aggravates the progression of hepatocellular carcinoma via regulating PCBP1/PRL-3/AKT pathway. Cancer Manag Res. 2020;12:5395–408.
Deng X, Zhao XF, Liang XQ, Chen R, Pan YF, Liang J. Linc00152 promotes cancer progression in hepatitis B virus-associated hepatocellular carcinoma. Biomed Pharmacother Biomed Pharmacother. 2017;90:100–8.
Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018. https://doi.org/10.1126/science.aar3958.
Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320–34.
Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A phase separation model for transcriptional control. Cell. 2017;169(1):13–23.
Borck PC, Guo LW, Plutzky J. BET epigenetic reader proteins in cardiovascular transcriptional programs. Circ Res. 2020;126(9):1190–208.
Li K, Liu Y, Cao H, Zhang Y, Gu Z, Liu X, Yu A, Kaphle P, Dickerson KE, Ni M, et al. Interrogation of enhancer function by enhancer-targeting CRISPR epigenetic editing. Nat Commun. 2020;11(1):485.
Pott S, Lieb JD. What are super-enhancers? Nat Genet. 2015;47(1):8–12.
Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153(2):307–19.
Farra R, Grassi G, Tonon F, Abrami M, Grassi M, Pozzato G, Fiotti N, Forte G, Dapas B. The role of the transcription factor E2F1 in hepatocellular carcinoma. Curr Drug Deliv. 2017;14(2):272–81.
Yu Y, Zhao D, Li K, Cai Y, Xu P, Li R, Li J, Chen X, Chen P, Cui G. E2F1 mediated DDX11 transcriptional activation promotes hepatocellular carcinoma progression through PI3K/AKT/mTOR pathway. Cell Death Dis. 2020;11(4):273.
Su SG, Li QL, Zhang MF, Zhang PW, Shen H, Zhang CZ. An E2F1/DDX11/EZH2 positive feedback loop promotes cell proliferation in hepatocellular carcinoma. Front Oncol. 2020;10: 593293.
Chen Q, Wang L, Jiang M, Huang J, Jiang Z, Feng H, Ji Z. E2F1 interactive with BRCA1 pathway induces HCC two different small molecule metabolism or cell cycle regulation via mitochondrion or CD4+T to cytosol. J Cell Physiol. 2018;233(2):1213–21.
Funding
This study was supported by the Natural Science Foundation of Shandong Province (ZR2022QC073), the Agricultural Animal Breeding Project of Shandong Province (No. 2020LZGC012), the Shandong Swine Industry Technology System Innovation (SDAIT-08-03), Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (13200214443117).
Author information
Authors and Affiliations
Contributions
JL and TS conceived and designed this study. JL, YW, XZ, and TS performed the experiments. JL, JW, and TS conducted the data analysis and prepared figures and tables. JL and TS wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by Ethics Committee of Shandong University School of Clinical Medicine (SDULCLL2022-2–2).
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Figure S1: H3K4me1 and H3K4me3 ChIP-seq signals in HCC1 and HCC3 (GSE112221).
Additional file 2
. Figure S2: gRNAs design by E-CRISPR. (A) The sequence and SAE-Score of gRNAs. (B) The location of gRNA targets on genome.
Additional file 3. Figure S3
: Expression levels of KMT2E and LOC101927902 in HepG2 cells after gRNA-dCas9 vectors transfection for 48 hours.
Additional file 4
. Figure S4. Expression pattern and prognostic correlation of E2F1 in HCC. (A) Expression pattern of E2F1 in HCC. Left: data from TCGA; Right: data from TGCA and GTEx. (B) Prognostic correlation of E2F1 in HCC. Left: Overall survival (OS); Right: Disease free survival.
Additional file 5
. Figure S5: Correlation analysis of E2F1 and BRD4 in tumor tissues of TCGA HCC patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Wang, J., Wang, Y. et al. E2F1 combined with LINC01004 super-enhancer to promote hepatocellular carcinoma cell proliferation and metastasis. Clin Epigenet 15, 17 (2023). https://doi.org/10.1186/s13148-023-01428-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-023-01428-6