Abstract
Background
Advanced biological aging, as measured by epigenetic aging indices, is associated with early mortality and morbidity. Associations between maternal epigenetic aging indices in pregnancy and pregnancy outcomes, namely gestational length and birthweight, have not been assessed. The purpose of this study was to examine whether epigenetic age during pregnancy was associated with gestational length and birthweight.
Results
The sample consisted of 77 women from the Los Angeles, CA, area enrolled in the Healthy Babies Before Birth study. Whole blood samples for DNA methylation assay were obtained during the second trimester (15.6 ± 2.15 weeks gestation). Epigenetic age indices GrimAge acceleration (GrimAgeAccel), DNAm PAI-1, DNAm ADM, and DNAm cystatin C were calculated. Gestational length and birthweight were obtained from medical chart review. Covariates were maternal sociodemographic variables, gestational age at blood sample collection, and pre-pregnancy body mass index. In separate covariate-adjusted linear regression models, higher early second trimester GrimAgeAccel, b(SE) = − .171 (.056), p = .004; DNAm PAI-1, b(SE) = − 1.95 × 10−4 (8.5 × 10−5), p = .004; DNAm ADM, b(SE) = − .033 (.011), p = .003; and DNAm cystatin C, b(SE) = 2.10 × 10−5 (8.0 × 10−5), p = .012, were each associated with shorter gestational length. Higher GrimAgeAccel, b(SE) = − 75.2 (19.7), p < .001; DNAm PAI-1, b(SE) = − .079(.031), p = .013; DNAm ADM, b(SE) = − 13.8 (3.87), p = .001; and DNAm cystatin C, b(SE) = − .010 (.003), p = .001, were also associated with lower birthweight, independent of gestational length.
Discussion
Higher maternal prenatal GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C were associated with shorter gestational length and lower birthweight. These findings suggest that biological age, as measured by these epigenetic indices, could indicate risk for adverse pregnancy outcomes.
Similar content being viewed by others
Background
Adverse pregnancy outcomes, such as preterm birth (< 37 weeks gestation) and low birthweight (< 2500 g), affect approximately 10% and 8.3% of pregnancies in the USA, respectively [1, 2]. Adverse pregnancy outcomes are leading causes of neonatal death [3] and have long-term implications for child and fetal development, including poorer cognitive and physiological outcomes [4, 5]. Shorter gestation and lower birthweight are also costly on a societal level, for example, resulting in millions of dollars in healthcare costs, and loses in future educational attainment and earning potential [6, 7]. Understanding the factors associated with risk for adverse pregnancy outcomes is a research priority.
Epigenetic age indices are proposed to capture unique epigenetic signatures of biological aging. As an indicator of biological age, they are highly correlated with chronological age [8, 9] but are also associated with disease morbidity and death, e.g., cancer [10, 11] and cardiovascular disease [12], and early mortality [13,14,15], pointing to the utility of epigenetic age as a biomarker of biological aging [16]. In the pregnancy context, older maternal chronological age is associated with higher rates of adverse birth outcomes, including shortened gestational length and lower birthweight [17,18,19,20,21], suggesting that biological aging might be involved in risk. However minimal research has tested whether maternal biological aging, as opposed to chronological aging, is also a prognostic marker.
The few studies that exist suggest that prenatal markers of maternal biological age, e.g., telomere length, an indicator of cellular aging, could be associated with pregnancy outcomes. With respect to shorter gestational length, lower maternal peripheral blood telomerase activity and shortened leukocyte telomere length during pregnancy (key elements of biological aging) were associated with shorter gestational length [22, 23]. And with respect to lower birthweight, one study reported that shortened leukocyte telomere length was not associated with risk for having a small-for-gestational-age infant [24]. Although a few studies suggest that maternal variation in distinct methylation profiles are associated with gestational length [25,26,27], epigenetic estimates of maternal biological age as prognostic of birth outcomes have not been tested, although accelerated placenta epigenetic aging, as indexed by the Horvath clock, has been associated with lower birthweight [28]. Indeed, no studies to date have specifically considered whether maternal epigenetic biological age is associated with offspring birthweight. Given that epigenetic age acceleration is robustly associated with morbidity and mortality in non-pregnant adults, examining the association between epigenetic age and adverse pregnancy outcomes is an important research direction [29].
The purpose of this study is to test whether maternal epigenetic age acceleration during pregnancy is associated with major birth outcomes gestational length and birthweight. It was hypothesized that greater epigenetic age acceleration would be associated with shorter gestational length and lower birthweight. Exploratory analyses tested whether epigenetic age acceleration indices are more strongly associated with gestational length, independent of birthweight, or birthweight, independent of gestational length.
Results
Study characteristics
Sample characteristics
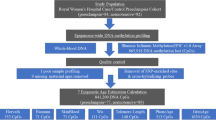
A sample of 77 women were recruited from Los Angeles, CA, into this pilot study as part of the Healthy Babies Before Birth (HB3) cohort, a longitudinal study designed to test the impact of antenatal maternal mood on pregnancy and postpartum outcomes. Demographics and previous pregnancy information were obtained at study entry and are presented in Table 1. The majority of participants were White (49%) or Latina (23%), married or cohabiting (87%), and primiparous (61%).
Epigenetic age indices
Prenatal whole blood samples were collected at 15.6 ± 2.15 weeks gestation. DNA methylation was assayed using standardized protocols (see “Methods” section). Average epigenetic age index values are presented in Table 1. Fifteen epigenetic age indices were considered: age acceleration residual, age-adjusted senescent T cells, intrinsic epigenetic age acceleration (IEAA), extrinsic epigenetic age acceleration (EEAA), phenotypic epigenetic age acceleration (PEAA), GrimAge acceleration (GrimAgeAccel), and age-adjusted DNAm PAI-1, DNAm ADM, DNAm B2M, DNAm cystatin C, DNAm GDF, DNAm Leptin, DNAm TIMP-1, DNAm smoking pack years, and DNAm telomere (TL).
Pregnancy outcomes
Birthweight and gestational length were obtained by medical chart review. Average gestational length was 39.4 ± 1.47 weeks (range 32.9–42 weeks), with 4% (N = 3) of the sample meeting clinical guidelines for preterm birth (< 37 weeks gestation). Average birthweight was 3371 ± 507 g (range 1086–4624 g), with 1% (N = 1) meeting clinical guidelines for low birthweight (< 2500 g). Bivariate correlations are presented in Suppl Table 1.
Pregnancy epigenetic age and gestational length
Results of linear regression models testing associations between gestational length and epigenetic age indices are presented in Table 2. Covariates were demographics, gestational age at blood sample collection, parity, and pre-pregnancy body mass index (BMI). Independent of covariates, four early second trimester epigenetic age indices were associated with gestational length. Higher GrimAgeAccel, an epigenetic age marker enriched for DNA methylation sites that are surrogate biomarkers for blood plasma proteins related to morbidity and mortality and cigarette smoking, estimated by packs per year [30], was associated with shorter gestational length, b = − .171, SE = .056, p = .004 (Fig. 1a). Higher DNAm PAI-1, b = − 1.95 × 10−4, SE = 8.5 × 10−5, p = .004 (Fig. 1b); DNAm ADM, b = − .033, SE = .011, p = .003 (Fig. 1c); and DNAm cystatin C, b = − 2.10 × 10−5, SE = 8.0 × 10−5, p = .012 (Fig. 1d), all DNA methylation indices validated by identifying the CpG sites most associated with blood plasma concentrations of plasminogen activator inhibitor (PAI)-1, adrenomedullin (ADM), and Cystatin C, respectively [30], were also associated with shorter gestational length. None of the other epigenetic age indices were associated with gestational length, p’s > .091.
Associations between a GrimAgeAccel, b age-adjusted DNAm PAI-1, c age-adjusted DNAm ADM, and d age-adjusted DNAm cystatin C and gestational length, adjusting for covariates. Higher early second trimester GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm Cystatin C were associated with shorter gestational length
Pregnancy epigenetic age and birthweight
Results of linear regression models testing associations between birthweight and epigenetic age indices are presented in Table 2. Covariates were demographics, gestational age at blood sample collection, and pre-pregnancy BMI. Independent of covariates, higher early second trimester GrimAgeAccel, b = − 75.2, SE = 19.7, p < .001 (Fig. 2a); DNAm PAI-1, b = − .079, SE = .031, p = .013 (Fig. 2b); DNAm ADM, b = − 13.8, SE = 3.87, p = .001 (Fig. 2c); and DNAm cystatin C, b = − .010, SE = .003, p = .001 (Fig. 2d), were associated with lower birthweight. None of the other epigenetic age indices were associated with birthweight, p’s > .075.
Exploratory analyses: birthweight and gestational length
Finally, linear regression models were run to determine if epigenetic age indices GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C were associated with birthweight independent of gestational length, and gestational length independent of birthweight. Independent of gestational length, higher early second trimester GrimAgeAccel, b = − 44.9, SE = 18.1, p = .016; DNAm ADM, b = − 8.37, SE = 3.72, p = .028; and DNAm cystatin C, b = − .006, SE = .003, p = .019, continued to be associated with lower birthweight. However, GrimAgeAccel, b = − .080, SE = .053, p = .137; DNAm ADM, b = − .015, SE = .011, p = .159; and DNAm cystatin C, b = − 7.54 × 10−6, SE = 8.0 × 10−5, p = .341, were not associated with gestational length independent of birthweight. DNAm PAI-1 was also not associated with either gestational length independent of birthweight, b = − 1.11 × 10−4, SE = 6.90 × 10−5, p = .113, nor birthweight independent of gestational length, b = − .044, SE = .025, p = .077.
Discussion
The purpose of this study was to determine if maternal epigenetic age acceleration indices during pregnancy were associated with gestational length and birthweight. Fifteen epigenetic age indices were considered: age acceleration residuals, IEAA, EEAA, PEAA, GrimAgeAccel, and age-adjusted DNAm PAI-1, DNAm ADM, DNAm B2M, DNAm cystatin C, DNAm GDF-15, DNAm leptin, DNAm TIMP-1, DNAm telomere, DNAm smoking pack years, and proportion of senescent T cells. Independent of covariates, higher early second trimester maternal GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C were significantly associated with shorter gestational length; no other epigenetic clock estimate variables were significant. In addition, higher early second trimester maternal GrimAgeAccel, DNAm ADM, and DNAm cystatin C were significantly associated with having a lower birthweight baby, while other epigenetic estimates of biological age of the mother were not. In exploratory analyses, higher early second trimester maternal GrimAgeAccel, DNAm ADM, and DNAm cystatin C continued to significantly be associated with lower birthweight of the child independent of gestational length of the pregnancy, but maternal GrimAgeAccel, DNAm ADM, and DNAm cystatin C were no longer associated with gestational length when controlling for birthweight. These findings suggest that maternal epigenetic age acceleration indices, specifically GrimAgeAccel, DNAm ADM, and DNAm cystatin, are associated with risk for adverse pregnancy outcomes, and highlight the potential role of maternal biological aging in birth outcomes.
Contrary to hypotheses, only a subset of epigenetic age acceleration indices was associated with gestational length and birthweight, specifically GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C. Several epigenetic aging indices have been developed over the last decade, with a trend toward moving from epigenetic indices that were predominately validated based on chronological age (e.g., Horvath epigenetic clock) toward indices that are validated or enriched for disease morbidity and biological risk factors. GrimAgeAccel is calculated by taking the residual of GrimAge regressed onto chronological age. GrimAge is constructed as a composite marker calculated from chronological age, sex, and epigenetic surrogate markers for 7 plasma proteins (adrenomedullin, β-2-microglobulin, cystatin-C, growth differentiation factor 15, leptin, plasminogen activator inhibitor 1, and tissue inhibitor metalloproteinase 1) and smoking pack years, based on self-reported smoking data [30], and is strongly associated with death. DNAm PAI-1, DNAm ADM, and DNAm cystatin C are the epigenetic surrogate marker for plasminogen activator inhibitor 1, adrenomedullin, and cystatin-C, respectively [30]. Plasminogen activator inhibitor-1 is a glycoprotein involved in suppressing fibrinolysis or the breakdown of blood clots, the production of which increases during pregnancy [31, 32]; adrenomedullin is a vasodilator peptide hormone, the production of which increases during pregnancy [33]; and cystatin-C is a protein that is typically used as an indicator of kidney function, the production of which decreases during pregnancy [34]. As only GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C were significant markers of pregnancy outcomes, this suggests that these epigenetic age acceleration indices might be most sensitive in capturing unique biological processes not represented in the other epigenetic age acceleration indices, and which could be salient to physiological processes related to pregnancy outcomes, particularly birthweight. However, it is not clear what biological activity is captured by these indices. For example, in the validation study, associations between DNAm PAI-1, DNAm ADM, and DNAm cystatin C and their respective plasma biomarkers were moderate (r’s = .36–.39) [30], suggesting that these indices could be capturing additional biological activity. As such, additional well-powered research is warranted before firm conclusions should be drawn. Future research should begin to explore the unique biological activity or processes captured by the epigenetic age acceleration indices, and the role this could play in placenta development and function, with implications for intrauterine growth, and the biology of parturition (e.g., cervical integrity and rupturing of membranes), with implications for gestational length. Regardless, our findings suggest that early second trimester GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C could be useful in markers of risk for shorter gestational length or low birthweight.
This is the first study to specifically consider associations between maternal epigenetic age acceleration indices during pregnancy and pregnancy outcomes. Of note, this study contributes to a burgeoning field of research that proposes biological aging of the mother and the placenta could contribute to timing of birth. Indeed, maternal chronological age is prognostic of birth outcomes [17,18,19,20,21], while less clear is the role of aging biology. Accelerated placenta epigenetic aging, as indexed by the Horvath clock, has been associated with lower birthweight [28]. In parallel, the placenta also can experience telomere length shortening that induces cellular senescence and the release of secretory factors (senescence-associated secretory phenotype) that promote parturition through increased inflammatory signaling cascades. In women with an older biological age, these processes may be accelerated [23, 35, 36]. As placental tissue was not evaluated here, additional research is needed to determine whether the placenta is responsible for associations between GrimAgeAccel and DNAm PAI-1 with risk for shorter gestation and lower birthweight.
Interestingly, findings from prior epigenome-wide association studies that reported associations between shorter gestation and differences in DNA methylation in immune and regulatory pathways is consistent with what is reported here, in that GrimAgeAccel and DNAm PAI-1 are epigenetic age acceleration indices developed based on immunological markers. A study of 154 women who presented with threatened preterm labor found differences in whole blood epigenetic profiles between women who did go on to have a preterm delivery and those women who did not, specifically in pathways related to signal transduction, transport, stress response, metabolic processes, immune system processes, mRNA processing, and cellular modification process [25]. Differences in DNA methylation patterns were also detected in a larger sample of 300 African American women [26]. Earlier birth was associated with hypomethylation at sites in the promoter regions of CYTIP and LINC00114, genes that are potentially involved in leukocyte trafficking, T-cell receptor signaling, transcriptional control, epigenetic modification, post-transcriptional control of mRNA, and cell differentiation [26]. In parallel, prior work has identified heightened circulating markers of inflammation, greater inflammatory gene expression, and related transcription factors [i.e., nuclear factor kappa B (NFкB), activator protein 1 (AP1)] among pregnant women who go on to have an early delivery and lower birthweight newborns [37, 38]. Collectively, this suggests that alterations in DNA methylation pathways that are shared with biological aging might also be related to immune and inflammatory activity that play a role in risk for shorter gestation. As biological aging is highly entwined with inflammatory processes [39], this potential pathway in the context of adverse birth outcomes warrants further research.
Associations between gestational length and birthweight and DNAm ADM and DNAm cystatin C are also consistent with previous literature. Both DNAm ADM and DNAm cystatin C were validated from markers involved in hemodynamics, including vasodilation and kidney glomerular filtration rate, respectively [31, 32, 34]. Hemodynamic adaptations are essential during pregnancy to ensure adequate circulation to the placenta and to meet the increased metabolic demands of the developing fetus, and include remodeling of cardiac tissue, increases in blood volume, increased cardiac output, and vasodilation of the vasculature and kidneys [40, 41]. Higher levels of cystatin C are associated with risk for preeclampsia diagnosis [42,43,44,45], a cardio-metabolic disease of pregnancy driven by distress signals released by an ischemic placenta. Preeclampsia is also associated with risk for shorter gestational length and low birthweight [46]. Similarly, higher levels of adrenomedullin could be associated with increased risk for low birthweight and shorter gestational length [47]. It is possible that DNAm ADM and DNAm cystatin C are indicators of these hemodynamic processes. However, these mechanisms must be interpreted with caution. Associations between DNAm ADM and DNAm cystatin C with protein serum levels of these markers are modest, r = .38–.39 [30], and these indices are calculated from blood samples. Nonetheless, these findings suggest that age-adjusted DNAm ADM and DNAm cystatin C could be capturing biological activity relevant to pregnancy hemodynamics and are potentially indicative of increased risk for shorter gestational length and lower birthweight.
Of interest, DNAm TL was not associated with gestational length or birthweight. This is contrary to previous research reporting associations between shorter telomere length in leukocytes and shorter gestational length [22, 23] but is consistent with a reported lack of association between leukocyte telomere length and birthweight [24]. It is possible that DNAm TL and leukocyte telomere length capture different physiological processes, accounting for differences in the pattern of findings. DNAm smoking pack years was also not associated with pregnancy outcomes, despite associations between maternal smoking or exposure to second-hand smoke and risk for shorter gestational length and lower birth weight [48,49,50]. DNAm smoking pack years was validated based on self-reported smoking habits. None of the women here reported any cigarette use during pregnancy, and few (> 10%) reported any tobacco use in the 3 months prior to conception. It is possible that no association was detected because there were too few smokers or previous smokers in the sample. Additional research is needed to better understand DNAm TL and DNAm smoking pack years and how they relate to leukocyte telomere length and self-reported smoking, respectively, in the context of pregnancy.
There are several study limitations to consider. Although our sample of N = 77 was larger and more diverse than other studies that have examined associations between pregnancy outcomes and DNA methylation patterns, it is still relatively small for DNA methylation research. Our sample has also generally higher income and educated, and generally low risk with few cases of clinically classified preterm birth or low birthweight. Future research should replicate these analyses in a larger, more diverse sample with high risk women. Finally, epigenetic age indices have not been validated in pregnant women. This is an important gap given that pregnancy is characterized by unique and dynamic physiological adaptations across physiological systems, including the immune, cardio-metabolic, and neuroendocrine [51]. How these adaptations affect blood cell DNA methylation patterns, or whether these patterns are stable or shift over the course of pregnancy, is not known. Future research should validate epigenetic clocks in pregnant samples.
Conclusions
In sum, to the best of our knowledge, this is the first study to assess whether maternal epigenetic age acceleration indices during pregnancy are associated with gestational length and birthweight. Higher maternal GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C during the early second trimester were associated with shorter gestation and lower birthweight, independent of maternal demographics, pre-pregnancy BMI, parity, and gestational age at blood sampling. Maternal biological aging, as indexed by GrimAgeAccel, DNAm PAI-1, DNAm ADM, and DNAm cystatin C, during pregnancy could affect risk for adverse pregnancy outcomes.
Methods
Participants
A sample of 77 women was recruited into this pilot study as part of the Healthy Babies Before Birth (HB3) cohort. HB3 study inclusion criteria were 18 years of age or older and singleton pregnancies up to 12 weeks gestation at time of recruitment. Exclusion criteria were medical intake involving current substance abuse, HIV-positive status, current smoking, or medications that could affect inflammatory processes, e.g., glucocorticoids. The current sample focused on women recruited at one of the two study sites (Los Angeles, CA), who had whole blood samples collected at study entry in early pregnancy. Study data were collected and managed using REDCap electronic data capture tool [52].
Protocol
Demographics and previous pregnancy information were obtained at study entry. Whole blood samples were collected at the first (8–16 weeks gestation) or second (20–26 weeks) pregnancy visit. Birthweight and gestational length were obtained by medical chart review.
DNA methylation
DNA was extracted from whole blood and assayed for DNA methylation by the UCLA Neurosciences Genomics Core using the Illumina Infinium HumanMethylation450 BeadChip (Illumina, Inc., San Diego, CA; 485,577 CpG sites). DNA methylation data were pre-processed as per standard protocols [9, 53], with detailed methods previously reported [54].
Epigenetic age indices
The epigenetic age of each blood sample was estimated using previously published methods [54] and using algorithms available through an online DNA methylation calculator [9] (https://labs.genetics.ucla.edu/horvath/dnamage/).
DNA methylation age (DNAm age; years), or biological age, was calculated using the Horvath method [9]. Differences between chronological and biological age are captured by the age acceleration residual, such that positive values indicate accelerated biological aging. Age-adjusted proportions of exhausted or senescent CD8+ T cells (CD8 + CD28-CD45FA- T cells) were calculated using estimation procedures validated by Horvath and described elsewhere [9, 55], adjusting for chronological age. Higher proportion of age-adjusted senescent CD8+ T cells is indicative of immunosenescence [56, 57]. Intrinsic epigenetic age acceleration (IEAA) captures the intrinsic biological age of immune cells, independent of age-related changes in immune cell populations in the blood. Extrinsic epigenetic age acceleration (EEAA) captures immune cell biological aging due to both intrinsic immune cell age and age-driven changes in immune cell populations. Phenotypic epigenetic age acceleration (PEAA) is calculated in accordance with the Levine method, using sites that were selected based on associations with both chronological age and phenotypic indicators of aging [58]. GrimAgeAccel is an epigenetic age marker enriched for DNA methylation sites that are surrogate biomarkers for blood plasma proteins related to morbidity and mortality and cigarette smoking, estimated by packs per year [30]. DNAm PAI-1, DNAm ADM, DNAm, B2M, DNAm cystatin C, DNAm GDF, DNAm leptin, DNAm TIMP1, and DNAm smoking pack years are surrogate DNA methylation index validated by identifying the CpG sites most associated with blood plasma protein concentrations or self-reported smoking [30].
Covariates
Demographics [race/ethnicity (White or not White), years of education, per capita household income, marital status (married and/or cohabiting or not)]; gestational age at blood sample collection; parity (nulliparous vs. parousFootnote 1); and pre-pregnancy BMI were included as covariates. Demographics were collected at study entry. Gestational age at blood sample collection was calculated by subtracting conception date (determined through ultrasound) from the assessment date. At study entry, participants reported their last pre-pregnancy weight. Height was measured at baseline by study personnel using a balance-beam scale. Pre-pregnancy BMI (kg/m2) was calculated by taking last pre-pregnancy weight (kg) and dividing by height squared (m2). Smoking prior to and during pregnancy was also considered as a covariate. Less than 10% (N = 6) reported nicotine use prior to pregnancy. Given that this variable was highly skewed, and preconception nicotine use was not correlated with epigenetic age indices, p’s > .129, or birth outcomes, p’s > .691, preconception nicotine use was not included as a covariate in analyses. No women reported any cigarette use during pregnancy.
Analytic strategy
All analyses were run using SPSS v. 24 [59]. Data was checked for outliers and normality prior to analysis. One birthweight outlier was Winsorized to − 3 SD (1850 g), and two gestational length outliers were Winsorized to − 3 SD (34.6 weeks).Footnote 2 Separate linear regression models were fit testing associations between gestational length and birthweight and each epigenetic age indices, controlling for covariates (demographics, gestational age at assessment, parity, and pre-pregnancy BMI).
Availability of data and materials
Data are available upon request from J. Carroll and C. Dunkel Schetter.
Notes
Pattern of results does not change whether parity is treated as a dichotomous or continuous variable.
Pattern of results is the same regardless of whether outliers are Winsorized or quartile linear regression is used.
Abbreviations
- ADM:
-
Adrenomedullin
- AP1:
-
Activator protein 1
- B2M:
-
β2 Microglobulin
- BMI:
-
Body mass index
- DNAm:
-
DNA methylation
- EEAA:
-
Extrinsic epigenetic age acceleration
- HB3:
-
Healthy Babies Before Birth
- IEAA:
-
Intrinsic epigenetic age acceleration
- GDF:
-
Growth and differentiation factor
- GrimAgeAccel:
-
GrimAge acceleration
- NFкB:
-
Nuclear factor kappa B
- PAI-1:
-
Plasminogen activator inhibitor-1
- PEAA:
-
Phenotypic epigenetic age acceleration
- TIMP-1:
-
Tissue inhibitor of metalloproteinases-1
- TL:
-
Telomere
References
Martin JA, Osterman MJ. Describing the increase in preterm births in the United States, 2014–2016. Hyattsville, MD: National Center for Health Statistics; 2018. Contract No.: 312.
Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Drake P. Births: final data for 2017. National Center for Health Statistics: Hyattsville, MD; 2018.
Ray JG, Park AL, Fell D. Mortality in infants affected by preterm birth and severe small for-gestational age birth weight. Pediatrics. 2017;140:e20171881.
Platt MJ. Outcomes in preterm infants. Public Health. 2014;128(5):399–403.
Escobar GJ, Littenberg B, Pettiti DB. Outcome among surviving very low birthweight infants: a meta-analysis. Arch Dis Child. 1991;66:204–11.
Hall ES, Greenberg JM. Estimating community-level costs of preterm birth. Public Health. 2016;141:222–8.
Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press (US); 2007.
Mitteldorf J. How does the body know how old it is? Introducing the epigenetic clock hypothesis. Interdiscip Top Gerontol. 2015;40:49–62.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115.
Ambatipudi S, Horvath S, Perrier F, Cuenin C, Hernandez-Vargas H, Le Calvez-Kelm F, et al. DNA methylome analysis identifies accelerated epigenetic ageing associated with postmenopausal breast cancer susceptibility. Eur J Cancer. 2017;75:299–307.
Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging. 2015;7:690–700.
Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (Atherosclerosis Risk in Communities). Circ Genom Precis Med. 2018;11(3):e001937.
Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25.
Horvath S, Pirazzini C, Giula Bacalini M, Gentelini D, di Blasio AM, Delledonne M, et al. Decreased epigenetic age of PBMCs from Italian semisupercentenarians and their offspring. Aging. 2015;7:1159–70.
Christiansen L, Lenart A, Tan QH, Vaupel JW, Aviv A, McGue M, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15(1):149–54.
Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, et al. Measuring biological aging in humans: a quest. Aging Cell. 2019:e13080.
Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: a large cohort study. PLoS One. 2018;13(1):e0191002.
Scime NV, Chaput KH, Faris PD, Quan H, Tough SC, Metcalfe A. Pregnancy complications and risk of preterm birth according to maternal age: a population-based study of delivery hospitalizations in Alberta. Acta Obstet Gynecol Scand. 2019.
Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–8.
Eichelberger KY. On odds, age, and preterm birth. BJOG. 2017;124(8):1245.
Kenny LC, Lavender T, McNamee R, O'Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. 2013;8(2):e56583.
Marrs C, Chesmore K, Menon R, Williams S. Maternal human telomerase reverse transcriptase variants are associated with preterm labor and preterm premature rupture of membranes. PLoS One. 2018;13(5):e0195963.
Hanna CW, Bretherick KL, Gair JL, Fluker MR, Stephenson MD, Robinson WP. Telomere length and reproductive aging. Hum Reprod. 2009;24(5):1206–11.
Akkad A, Hastings R, Konje JC, Bell SC, Thurston H, Williams B. Telomere length in small-for-gestational-age babies. BJOG. 2006;113(3):318–23.
Heng YJ, Pennell CE, Chua HN, Perkins JE, Lye SJ. Whole blood gene expression profile associated with spontaneous preterm birth in women with threatened preterm labor. PLoS One. 2014;9(5):e96901.
Hong X, Sherwood B, Ladd-Acosta C, Peng S, Ji H, Hao K, et al. Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: findings in maternal and cord blood samples. Epigenetics. 2018;13(2):163–72.
Parets SE, Conneely KN, Kilaru V, Menon R, Smith AK. DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics. 2015;10(9):784–92.
Tekola-Ayele F, Workalemahu T, Gorfu G, Shrestha D, Tycko B, Wapner R, et al. Sex differences in the associations of placental epigenetic aging with fetal growth. Aging (Albany NY). 2019;11(15):5412–32.
Knight AK, Smith AK. Epigenetic biomarkers of preterm birth and its risk factors. Genes (Basel). 2016;7(4):15.
Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303–27.
Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342(24):1792–801.
Ye Y, Vattai A, Zhang X, Zhu J, Thaler CJ, Mahner S, et al. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int J Mol Sci. 2017;18(8):1651.
Lorio RD, Marinoni E, Scavo D, Letizia C, Cosmi EV. Adrenomedullin in pregnancy. Lancet. 1997;349(9048):328.
Babay Z, Al-Wakeel J, Addar M, Mittwalli A, Tarif N, Hammad D, et al. Serum cystatin C in pregnant women: reference values, reliable and superior diagnostic accuracy. Clin Exp Obstet Gynecol. 2005;32(3):175–9.
Cheng SB, Davis S, Sharma S. Maternal-fetal cross talk through cell-free fetal DNA, telomere shortening, microchimerism, and inflammation. Am J Reprod Immunol. 2018;79(5):e12851.
Phillippe M. Cell-Free Fetal DNA, Telomeres, and the spontaneous onset of parturition. Reprod Sci. 2015;22(10):1186–201.
Ross KM, Carroll JE, Dunkel Schetter C, Hobel C, Cole SW. Pro-inflammatory immune cell gene expression during the third trimester of pregnancy is associated with shorter gestational length and lower birthweight. Am J Reprod Immunol. 2019;82(6):e13190.
Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007;109(1):121–7.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9.
Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24(1):11–4.
Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–8.
Niraula A, Lamsal M, Baral N, Majhi S, Khan SA, Basnet P, et al. Cystatin-C as a marker for renal impairment in preeclampsia. J Biomark. 2017;2017:7406959.
Wattanavaekin K, Kitporntheranunt M, Kreepala C. Cystatin C as a novel predictor of preterm labor in severe preeclampsia. Kidney Res Clin Pract. 2018;37(4):338–46.
Thilaganathan B, Ralph E, Papageorghiou AT, Melchiorre K, Sheldon J. Raised maternal serum cystatin C: an early pregnancy marker for preeclampsia. Reprod Sci. 2009;16(8):788–93.
Strevens H, Wide-Swensson D, Grubb A, Hansen A, Horn T, Ingemarsson I, et al. Serum cystatin C reflects glomerular endotheliosis in normal, hypertensive and pre-eclamptic pregnancies. BJOG. 2003;110(9):825–30.
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80.
Lenhart PM, Caron KM. Adrenomedullin and pregnancy: perspectives from animal models to humans. Trends Endocrinol Metab. 2012;23(10):524–32.
Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11(4):427–33.
Kupers LK, Xu X, Jankipersadsing SA, Vaez A, la Bastide-van Gemert S, Scholtens S, et al. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol. 2015;44(4):1224–37.
Ahern J, Pickett KE, Abrams B. Preterm birth among African American and white women: a multilevel analysis of socioeconomic characteristics and cigarette smoking. J Epidemiol Community Health. 2003;57:606–11.
Cunningham GF, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ. Song CY. McGraw Hill Professional: Williams Obstetrics; 2014.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Triche TJ Jr, Weisenberger DJ, Van Den Berg D, Laird PW, Siegmund KD. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 2013;41(7):e90.
Ross KM, Carroll J, Horvath S, Hobel CJ, Coussons-Read ME, Dunkel SC. Immune epigenetic age in pregnancy and 1 year after birth: associations with weight change. Am J Reprod Immunol. 2020;83(5):e13229.
Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):171.
Kananen L, Marttila S, Nevalainen T, Kummola L, Junttila I, Mononen N, et al. The trajectory of the blood DNA methylome ageing rate is largely set before adulthood: evidence from two longitudinal studies. Age (Dordr). 2016;38(3):65.
Marttila S, Kananen L, Hayrynen S, Jylhava J, Nevalainen T, Hervonen A, et al. Ageing-associated changes in the human DNA methylome: genomic locations and effects on gene expression. BMC Genomics. 2015;16:179.
Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573–91.
Corp IBM. IBM SPSS Statistics for Windows, Version 26.0. IBM Corp: Armonk, NY; 2018.
Acknowledgements
The authors thank the staff and the participants especially Dr. Roberta Mancuso, Project Coordinator in Denver, and Susan Jackman, RN, coordinator of the Cedars Sinai Health Center site, and the UCLA Neuroscience Genomics Core for completing methylation arrays.
Funding
Healthy Babies Before Birth (HB3) Study was supported by funding from NIH (R01 HD073491: MPI Coussons-Read & Dunkel Schetter). Additional epigenetic assays were funded by the NIH/National Center for Advancing Translational Science UCLA CTSI Grant (UL1TR001881) and the Cousins Center for Psychoneuroimmunology. REDCap is supported by the Colorado Clinical & Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support [NIH/NCRR Colorado CTSI Grant Number UL1 RR025780].
Author information
Authors and Affiliations
Contributions
K. Ross conducted analyses and led in writing the manuscript, J. Carroll advised on direction of analyses and manuscript, helped secure funding for the epigenetic work, completed DNA extractions and submitted biospecimens to the UCLA Neuroscience Genomics Core, S. Horvath developed the algorithms and advised on epigenetic clock and related analyses, and C. Dunkel Schetter advised on the manuscript and together with M. Coussons Read conducted the larger study as Joint PIs. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All protocols and methods were reviewed and approved by the appropriate university and medical center institutional review boards, and participants provided written informed consent.
Consent for publication
All authors have reviewed this manuscript and consented to its publication.
Competing interests
S. Horvath is the creator of the GrimAge clock. The other authors report no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Supplemental Table 1. Correlations
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ross, K.M., Carroll, J.E., Horvath, S. et al. Epigenetic age and pregnancy outcomes: GrimAge acceleration is associated with shorter gestational length and lower birthweight. Clin Epigenet 12, 120 (2020). https://doi.org/10.1186/s13148-020-00909-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-020-00909-2