Abstract
The onset and development of malignant tumors are closely related to epigenetic modifications, and this has become a research hotspot. In recent years, a variety of epigenetic regulators have been discovered, and corresponding small molecule inhibitors have been developed, but their efficacy in solid tumors is generally poor. With the introduction of the first synthetic lethal drug (the PARP inhibitor olaparib in ovarian cancer with BRCA1 mutation), research into synthetic lethality has also become a hotspot. High-throughput screening with CRISPR-Cas9 and shRNA technology has revealed a large number of synthetic lethal pairs involving epigenetic-related synthetic lethal genes, such as those encoding SWI/SNF complex subunits, PRC2 complex subunits, SETD2, KMT2C, and MLL fusion proteins. In this review, we focus on epigenetic-related synthetic lethal mechanisms, including synthetic lethality between epigenetic mutations and epigenetic inhibitors, epigenetic mutations and non-epigenetic inhibitors, and oncogene mutations and epigenetic inhibitors.
Similar content being viewed by others
Background
Epigenetics is a term which describes heritable changes of gene expression without alteration of the DNA sequence [1]. Epigenetic modifications include DNA methylation, histone modification (methylation, acetylation, phosphorylation and ubiquitination), chromatin remodeling, and RNA methylation [2]. Epigenetics has become a hot topic in the field of malignant tumor treatment. A variety of epigenetic-related genes have been found to be associated with tumor development, progression and drug resistance [3], and small molecule inhibitors targeting the protein products of these genes have also been synthesized. However, in clinical studies, these inhibitors are only efficacious against some hematologic malignancies and are not very effective for treating most solid tumors [2].
As early as 1922, there was a report about the synthetic lethality phenomenon, when Calvin Bridges observed that simultaneous mutation of a pair of genes in a fruit fly would lead to death, but mutation of either gene alone did not significantly affect survival [4]. The same phenomenon was observed at the cellular level thereafter. Functional changes in two genes resulted in cell death, whereas survival was maintained if either gene was changed.
Loss-of-function (LOF) mutations or low expression of tumor suppressor genes are very common in cancer. Compared to gain-of-function (GOF) mutations or overexpression, mutations that cause loss of function or low expression of tumor suppressor genes are considered as “undruggable” [5]. However, screening for synthetic lethal interactions can be performed by shRNA or CRISPR-Cas9 systems, with the aim of identifying synthetic lethal partner genes for the undruggable gene. Thus, cancer cells carrying the undruggable mutant gene can be selectively killed by specifically targeting the function of the synthetic lethal partner.
Epigenetic regulation is widespread in cancers, involving many targets, and epigenetic-related gene mutations are very common in a variety of cancers [6]. According to epigenetic synthetic lethality approaches, targeting synthetic lethal pair with epigenetic mutations or using epigenetic inhibitors against cancers with “undruggable” mutations may be possible to expand the range of drug treatments and to improve the efficacy of some epigenetic inhibitors in solid tumors.
In this review, we focus on epigenetic-related synthetic lethal interactions in cancers, involving mutations or deletions of epigenetic-related genes and inhibitors of epigenetic enzymes, which might have potential therapeutic value in the future.
Synthetic lethality between epigenetic alterations and epigenetic inhibitors
Synthetic lethality induced by ARID1A mutation and EZH2 inhibition
SWI/SNF (SWItch/Sucrose Non-Fermentable), a chromatin remodeling complex mainly including the components ARID1A, SMARCA4, SMARCB1, SMARCD1, and ACTL6A [7], is considered a tumor suppressor, and inactivation of SWI/SNF subunits is thought to drive tumorigenesis by altering gene expression [2, 8, 9]. PRC2 (Polycomb Repressive Complex 2) is another important complex, including EZH2, SUZ12, EED, and YY1, which catalyzes mono-, di-, and tri-methylation on lysine 27 of histone H3 and is involved into the development and progression of multiple cancers [10]. Recently, increasing evidence has shown that there is epigenetic antagonism between the SWI/SNF complex and the PRC2 complex. Therefore, PRC2 complex inhibitors may be potential synthetic lethal treatments for cancers with SWI/SNF mutations or deletions. The gene encoding ARID1A is frequently mutated in multiple cancers. In ovarian clear cell carcinoma, Bitler et al. found that there is a synthetic lethal interaction between ARID1A loss-of-function mutation and an inhibitor of EZH2 [11]. They found that GSK126, a specific small molecule inhibitor of EZH2, induced a significant decrease of cell proliferation in ARID1A knockdown cell lines. In contrast, the cell proliferation of wild-type ARID1A cell lines did not change significantly after treatment with GSK126. Moreover, in the mutant ARID1A cell lines, ectopic expression of wild-type ARID1A significantly reduced the sensitivity to GSK126. In a nude mouse xenograft model with tumors derived from ARID1A mutant cells, they also found that the size of tumors was significantly reduced by GSK126 treatment. The above results demonstrated that the synthetic lethal approach using an EZH2 inhibitor in ARID1A mutation patients might be a promising strategy for cancer therapy.
Synthetic lethality induced by loss of SMARCB1 and inhibition of EZH2/HDAC
SMARCB1 is widely described as a tumor suppressor gene. SMARCB1, also known as SNF5, is a subunit of the SWI/SNF complex. Complete loss of SMARCB1/SNF5 is very common in malignant rhabdoid tumors (MRT) and atypical teratoid/rhabdoid tumors (ATRT) [12,13,14,15]. MRT is a rare but highly malignant cancer with a poor prognosis, and so there is an urgent need for new improved therapies [16]. Experiments have shown that treatment with pharmacological concentrations of EZH2 inhibitors may significantly inhibit the proliferation of SMARCB1/SNF5-deficient MRT cells, while having no significant effect on the proliferation of wild-type SMARCB1/SNF5 MRT cells [17]. Further mechanistic research showed that the expression level of the tumor suppressor gene p16INK4a is downregulated in SMARCB1/SNF5-deficient tumor cells and increased in EZH2-deficient cells. In SMARCB1/SNF5-deficient tumor cells, EZH2 inhibitor treatment can increase p16INK4a expression by lowering the level of tri-methylated H3K27 in the p16INK4a gene region, which demonstrates that EZH2 epigenetically silences the expression of p16INK4a by catalyzing H3K27 tri-methylation [18, 19]. It was also found that SMARCB1/SNF5-deficient tumor cells are highly sensitive to EZH2 inhibitors, which significantly inhibit cell proliferation and increase apoptosis [20]. In addition, several reports have shown that some HDAC inhibitors can partially mimic the histone acetylation function of SWI/SNF in SMARCB1/SNF5-deficient MRTs, thus indicating that HDAC inhibitors may have anticancer effects in MRTs [21, 22]. Further experiments demonstrated that pan-HDAC inhibitors can significantly inhibit the proliferation and self-renewal ability of SMRCB1/SNF5-deficient MRTs [16]. This study may expand the applications of marketed HDAC inhibitors and uncover potential clinical applications of EZH2 inhibitors.
Synthetic lethality induced by CREBBP mutation and p300 inhibition
CREB binding protein (CREBBP), also known as CBP, has histone acetyltransferase (HAT) activity [23] and regulates chromatin structure and gene expression. Loss-of-function mutations of the CREBBP gene are very common in a variety of cancers. Approximately 10–15% of non-small cell lung cancers (NSCLC) and small cell lung cancers (SCLC) have CREBBP loss-of-function mutations [24, 25]. P300/EP300, also known as p300 HAT or E1A-associated protein p300, is a histone acetyltransferase, which regulates the transcription of genes via chromatin remodeling [26]. Screening with an siRNA library revealed that knockdown of the gene P300, which encodes the CREBBP paralog P300/EP300, is synthetically lethal with CREBBP deletions. The study found that G1-S phase cell-cycle arrest occurs after inhibition of P300 in CREBBP-deficient or CREBBP-knockdown lung cancer cells. The p300-specific inhibitor c646 significantly reduced the growth of CREBBP-deficient cells but not CREBBP wild-type cells. A similar phenomenon has also been found in human hematopoietic cancer [23]. Mechanistically, genome-wide gene expression analysis showed that MYC expression was downregulated in p300-inhibited CREBBP-deficient cells due to decreased levels of acetylation in the promoter region of the MYC gene. Expression of the oncogenic transcription factor MYC (c-myc) is upregulated in a variety of cancers. Therefore, it is possible that p300 inhibitors exhibit a synthetic lethal anticancer effect in CREBBP-deficient cancer cells by inhibiting the expression of MYC. This phenomenon provides a potential treatment for CREBBP-deficient cancers.
Synthetic lethality induced by MLL gene fusion and DOT1L inhibition
Mixed-lineage leukemia (MLL) is a very aggressive form of leukemia which is caused by chromosomal translocations affecting the MLL gene. Normally, the MLL gene encodes a SET domain histone methyltransferase that catalyzes the methylation of H3K4 [27]. As the rearrangement of the MLL gene, the catalytically active SET domain is lost, and MLL fuses with other genes such as AF4, AF9, AF10, and ENL to generate fusion proteins. The N-terminal portions of these fusion products are lost in the rearrangements, but C-terminal gene-specific recognition elements are retained. These fusion products are capable of interacting with another histone methyltransferase by these recognition elements, such as Disruptor of telomeric silencing 1-like (DOT1L), which is known the unique enzyme to catalyze the mono-, di-, and tri-methylation of histone H3 at lysine 79 (H3K79me1, H3K79me2, H3K79me3) [28,29,30,31]. As a result, fusion products gain the ability to recruit DOT1L by retained gene-specific recognition elements. [32,33,34]. Study shows that leukemia cells with MLL fusion genes are hypersensitive to DOT1L inhibitors such as EPZ5676. DOT1L inhibitors induce cell proliferation and apoptosis in MLL-rearranged leukemia cells but do not significantly inhibit cell proliferation or apoptosis in non-MLL-rearranged leukemia cells. Mechanistic studies have found that MLL fusion products recruit DOT1L which leads to an enhanced H3K79 methylation level in the promoter or enhancer region of MLL-fusion target genes, including HOXA9 and MEIS1, subsequently regulates their expression and mediates dysfunction of cell differentiation and proliferation [35,36,37]. Thus, treatment with DOT1L inhibitor in MLL fusion cells could reverse the dysfunction. Another mechanism research found that LAMP5 is positively correlated with the MLL fusion protein level and LAMP5 can be activated by H3K79me2 as a direct target which is generated by DOT1L. Therefore, inhibition of DOT1L can abolish the inhibition of autophagy by LAMP5 [38]. It was also found that MLL fusion protein levels decreased significantly after treatment with DOT1L inhibitors. This work may provide a potential therapy for MLL-fusion leukemia [34, 35].
Synthetic lethality between epigenetic alterations and non-epigenetic inhibitors
Synthetic lethality induced by SETD2 deficiency and inhibition of WEE1/PI3Kβ-AKT
SET domain containing 2 (SETD2), a histone methyltransferase that is specific for tri-methylation of Histone3 at lysine 36 (H3K36me3), has been shown to act as a tumor suppressor in human cancers [39]. H3K36me3 is frequently lost in multiple cancer types, and this may be caused by loss of the tumor suppressor SETD2 and overexpression of KDM4A (a H3K36me3 demethylase) [40]. WEE1 is a nuclear kinase of the Ser/Thr family and is an important factor in cell-cycle regulation checkpoint and DNA damage checkpoints. WEE1 inhibits CDK1 activity by phosphorylation on Tyr15 and Thr14, and decreased CDK1 activity prevents cells from entering mitosis [41,42,43]. A role for WEE1 in epigenetic regulation has also been reported. WEE1 can catalyze the phosphorylation of histone H2B tyrosine 37 and regulate histone expression [44, 45]. Pfister et al. and Martinelli et al. found that SETD2-deficient tumors cells such as mast cell leukemia and kidney cancers are very sensitive to WEE1 inhibitors. They found that compared to control cells, SETD2 knockout cells are hypersensitive to the WEE1 inhibitor Adavosertib (AZD1775) [40, 46]. They further found that H3K36me3 catalyzed by SETD2 promotes RRM2 expression, and WEE1 also promotes RRM2 expression through CDK1; thus, inhibition of WEE1 leads to inhibition of RRM2 [47, 48]. The expression of RRM2, which is a subunit of ribonuclease reductase, is associated with the intracellular level of dNTPs, and decreased expression of RRM2 can result in decreased levels of dNTPs. Inhibition of WEE1 in SETD2-deficient cells consequently results in extremely low dNTP levels, which leads to impaired DNA replication and increased cell death [49]. Therefore, there is a synthetic lethal interaction between SETD2 deficiency and WEE1 inhibition.
Terzo et al. also reported that there is a synthetic lethal interaction between SETD2-deficiency and PI3Kβ-AKT inhibition in kidney cancer [50]. The phosphoinositide 3-kinase (PI3K)-AKT axis is a very important signaling pathway in cell proliferation and it is frequently changed in cancers [51]. PI3Kβ-AKT and its downstream effectors are often overactivated and have high expression levels in kidney cancer [52, 53]. Research shows that kidney cancer cells with SETD2 knockout or mutation, when treated with PI3Kβ-specific inhibitors, displayed significantly decreased viability and migration compared to cells with wild-type SETD2. Treatment of SETD2-deficient or wild-type kidney cancer cells with an AKT-specific inhibitor resulted in similar effects. Therefore, these studies demonstrate that loss of SETD2 is synthetically lethal with PI3Kβ inhibition. They also show that AKT is a key part of the interaction between SETD2-deficiency and the PI3Kβ-AKT axis.
Synthetic lethality induced by KMT2C mutation and PARP inhibition
Poly (ADP-ribose) polymerase (PARP) is reported to be involved in DNA repair, genomic stability, and programmed cell death [54]. Studies have found that BRCA1/2 mutations and PARP inhibition are synthetically lethal. The PARP inhibitor olaparib, which was developed according to the principles of synthetic lethality, is the first drug for treating ovarian cancer carrying the BRCA1/2 mutation. Studies have shown that PARP inhibitors not only have synthetic lethal effects with BRCA1/2 mutations, but also have synthetic lethal interactions with low-activity KMT2C in bladder cancer. Lysine N-methyltransferase 2C (KMT2C), also known as myeloid/lymphoid or mixed-lineage leukemia protein 3 (MLL3), methylates histone3 at lysine 4 (H3K4), and the KMT2C gene has a high mutation rate in bladder cancer [55,56,57]. Studies have shown that the epigenetic state is changed in bladder cancer cells with low KMT2C activity, and there is decreased expression of genes involved in DNA repair. Therefore, PARP inhibitors have synthetic lethal effects in cancer cells with low KMT2C activity, especially in epithelial carcinoma, such as bladder cancer, colon cancer, NSCLC, and HNSCC [58]. This synthetic lethal interaction may provide a new clinical use of PARP inhibitors.
Synthetic lethality between non-epigenetic alterations and epigenetic inhibitors
Synthetic lethality induced by TP53 mutation and EZH2 inhibition
The TP53 gene, which encodes the widely studied tumor suppressor protein p53, is frequently mutated in various cancers [59, 60]. Previous studies have generally focused on loss-of-function mutations in TP53, and have suggested that the tumor suppressor capacity of p53 is lost, thereby promoting tumor development. However, recent investigations have shown that some TP53 mutations are gain-of-function changes that endow the p53 protein with new activities that can promote tumor development, including increased cell proliferation and cell migration, etc. [60,61,62,63]. Zhao et al. used RNA immunoprecipitation sequencing (RIP-seq) to demonstrate that EZH2 binds to the 5′ UTR of TP53 mRNA, which enhances transcription and translation of p53 protein. This mechanism is independent of the methyltransferase activity of EZH2. Furthermore, it was found that reduction of EZH2 expression by antisense oligonucleotides (ASO) instead of an EZH2 inhibitor induces synthetic lethality in a variety of cancer cells with gain-of-function mutations of p53, such as partial breast cancer and prostate cancer. And there is no significant growth inhibition in wild-type p53 cells [64]. TP53 mutation is very common in cancers, and therefore the synthetic lethal interaction of p53 and EZH2 may have clinical value for cancer treatments.
Conclusions
Epigenetic modifications are very common in malignant tumors, and multiple tumor types are dependent on specific epigenetic modifications. Mutations in numerous oncogenes and epigenetic-related genes are also common in a variety of cancers, and many loss-of-function mutations are considered to be undruggable. Therefore, the use of the principle of synthetic lethality is a breakthrough for treating tumors that carry such mutations.
Screens using CRISPR and shRNA technology have uncovered multiple sets of epigenetic-related synthetic lethal pairs, such as the SWI/SNF complex and PRC2 complex inhibitors, SETD2 and WEE1 inhibitors or PI3K-AKT inhibitors, KMT2C and PARP inhibitors, MLL fusions and DOT1L inhibitors, p53 and EZH2 inhibitors, and CREBBP and p300 inhibitors. These synthetic lethal pairs and their mechanisms are shown in Table 1 and Fig. 1. The inhibitors are all highly active in tumor cells carrying particular mutant genes but have no significant effect on wild-type cells. Synthetic lethal combinations have also been found that can alleviate the drug resistance of some tumors; for example, AKT inhibitors reverse the resistance to EGFR-TKIs in NSCLC with PBRM1 mutation, and EGFR-TKIs reverse the resistance to MET and ALK inhibitors in NSCLC with SMARCE1 deletion [65, 66]. Although research into using synthetic lethality to overcome drug resistance is limited, it will become an important new direction in the fight against drug resistance.
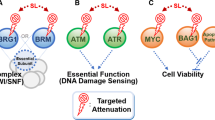
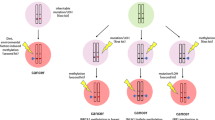
Epigenetic synthetic lethality approaches. a In normal cells, inhibiting one molecule in a synthetic lethal pair may lead to a compensatory response of another molecule, and therefore the cells survive. b Mutations in cancer cells are represented by red stars. In cancer cells with mutations, one molecule of synthetic lethal part has changed, and the specific inhibitor has inhibited another molecule, so it cannot produce complementarity or antagonism, leading to cell death. This review mainly introduces three epigenetic synthesis of lethal strategies. Strategy 1 uses epigenetic inhibitors in cells with epigenetic mutations cells; strategy 2 uses non-epigenetic inhibitors in cells with epigenetic mutations; and strategy 3 uses epigenetic inhibitors in cells with non-epigenetic mutations. Specific mutations and inhibitors are shown in the figure
Epigenetic modifications and gene mutations regulate gene expression at different levels. Therefore, the combination of the two ways may make it easier to find synthetic lethal pairs, breaking through the bottlenecks of cancer treatment, especially for some patients who have pathogenic mutations but lack targeted drugs, such as KRAS and TP53 mutations. In addition to screening for synthetic lethal pairs using the CRISPR-Cas9 system and siRNA libraries, screening synthetic lethal pairs for specific gene mutations also can be performed by chemical compound libraries or small molecule inhibitor libraries. And there is often complementarity or antagonism between synthetic lethal pairs, usually in related or same signaling pathway or cell development process. Therefore, it is possible to optimize and narrow the screening range and increase the screening efficiency.
In summary, this review has explored multiple epigenetic synthetic lethal relationships that may provide potential therapies for cancer.
Availability of data and materials
Not involved into this
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- ARID1A:
-
AT-Rich Interaction Domain 1A
- ASO:
-
Antisense oligonucleotides
- ATRT:
-
Atypical teratoid/rhabdoid tumors
- BRCA1/2:
-
Breast cancer type 1/2
- CDK1:
-
Cyclin-dependent kinase1
- CREB1:
-
CAMP Responsive Element Binding Protein 1
- CREBBP:
-
CREB Binding Protein
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- dNTP:
-
Deoxyribonucleoside triphosphate
- DOT1L:
-
Disruptor of telomeric silencing 1 like
- EGFR-TKIs:
-
Epidermal growth factor receptor-tyrosine kinase inhibitors
- EZH2:
-
Enhancer of Zeste Homolog 2
- HDAC:
-
Histone deacetylase
- HNSCC:
-
Head and neck squamous cell carcinoma
- KDM4A:
-
Lysine-specific demethylase 4A
- KMT2C:
-
Lysine Methyltransferase 2C
- LAMP5:
-
Lysosome-associated membrane protein 5
- MET:
-
MET proto-oncogene, receptor tyrosine kinase
- MLL:
-
Mixed-lineage leukemia
- MRTs:
-
Malignant rhabdoid tumors
- MYC:
-
MYC proto-oncogene, bHLH transcription factor
- NSCLC:
-
Non-small cell lung cancer
- PARP:
-
Poly (ADP-ribose) polymerase
- PBRM1:
-
Polybromo 1
- PI3Kβ-AKT:
-
The phosphoinositide 3-kinase-AKT
- PRC2:
-
Polycomb repressive 2
- RRM2:
-
Recombinant Human Ribonucleotide Reductase M2
- SETD2:
-
SET domain containing 2
- SMARCB1:
-
SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1
- SWI/SNF:
-
SWItch/Sucrose Non-Fermentable
- WEE1:
-
Wee1-like protein kinase
References
Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63.
Morel D, Almouzni G, Soria JC, Postel-Vinay S. Targeting chromatin defects in selected solid tumors based on oncogene addiction, synthetic lethality and epigenetic antagonism. Ann Oncol. 2017;28:254–69.
Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50.
Nijman SM, Friend SH. Cancer. Potential of the synthetic lethality principle. Science. 2013;342:809–11.
Epstein RJ. The unpluggable in pursuit of the undruggable: tackling the dark matter of the cancer therapeutics universe. Front Oncol. 2013;3:304.
Han M, Jia L, Lv W, Wang L, Cui W. Epigenetic enzyme mutations: role in tumorigenesis and molecular inhibitors. Front Oncol. 2019;9:194.
Collingwood TN, Urnov FD, Wolffe AP. Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J Mol Endocrinol. 1999;23:255–75.
Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–92.
Lu P, Roberts CW. The SWI/SNF tumor suppressor complex: regulation of promoter nucleosomes and beyond. Nucleus. 2013;4:374–8.
Chittock EC, Latwiel S, Miller TC, Muller CW. Molecular architecture of polycomb repressive complexes. Biochem Soc Trans. 2017;45:193–205.
Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, Schultz DC, Liu Q, Shih Ie M, Conejo-Garcia JR, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–8.
Biegel JA, Kalpana G, Knudsen ES, Packer RJ, Roberts CW, Thiele CJ, Weissman B, Smith M. The role of INI1 and the SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the workshop on childhood atypical teratoid/rhabdoid tumors. Cancer Res. 2002;62:323–8.
Sansam CG, Roberts CW. Epigenetics and cancer: altered chromatin remodeling via Snf5 loss leads to aberrant cell cycle regulation. Cell Cycle. 2006;5:621–4.
Roberts CW, Biegel JA. The role of SMARCB1/INI1 in development of rhabdoid tumor. Cancer Biol Ther. 2009;8:412–6.
Fruhwald MC, Biegel JA, Bourdeaut F, Roberts CW, Chi SN. Atypical teratoid/rhabdoid tumors-current concepts, advances in biology, and potential future therapies. Neuro-Oncology. 2016;18:764–78.
Muscat A, Popovski D, Jayasekara WS, Rossello FJ, Ferguson M, Marini KD, Alamgeer M, Algar EM, Downie P, Watkins DN, et al. Low-dose histone deacetylase inhibitor treatment leads to tumor growth arrest and multi-lineage differentiation of malignant rhabdoid tumors. Clin Cancer Res. 2016;22:3560–70.
Knutson SK, Warholic NM, Wigle TJ, Klaus CR, Allain CJ, Raimondi A, Porter Scott M, Chesworth R, Moyer MP, Copeland RA, et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A. 2013;110:7922–7.
Oruetxebarria I, Venturini F, Kekarainen T, Houweling A, Zuijderduijn LM, Mohd-Sarip A, Vries RG, Hoeben RC, Verrijzer CP. P16INK4a is required for hSNF5 chromatin remodeler-induced cellular senescence in malignant rhabdoid tumor cells. J Biol Chem. 2004;279:3807–16.
Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28:3457–64.
Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, Koellhoffer EC, Pomeroy SL, Orkin SH, Roberts CW. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18:316–28.
Yamamichi N, Yamamichi-Nishina M, Mizutani T, Watanabe H, Minoguchi S, Kobayashi N, Kimura S, Ito T, Yahagi N, Ichinose M, et al. The Brm gene suppressed at the post-transcriptional level in various human cell lines is inducible by transient HDAC inhibitor treatment, which exhibits antioncogenic potential. Oncogene. 2005;24:5471–81.
Algar EM, Muscat A, Dagar V, Rickert C, Chow CW, Biegel JA, Ekert PG, Saffery R, Craig J, Johnstone RW, et al. Imprinted CDKN1C is a tumor suppressor in rhabdoid tumor and activated by restoration of SMARCB1 and histone deacetylase inhibitors. PLoS One. 2009;4:e4482.
Ogiwara H, Sasaki M, Mitachi T, Oike T, Higuchi S, Tominaga Y, Kohno T. Targeting p300 addiction in CBP-deficient cancers causes synthetic lethality by apoptotic cell death due to abrogation of MYC expression. Cancer Discov. 2016;6:430–45.
Kishimoto M, Kohno T, Okudela K, Otsuka A, Sasaki H, Tanabe C, Sakiyama T, Hirama C, Kitabayashi I, Minna JD, et al. Mutations and deletions of the CBP gene in human lung cancer. Clin Cancer Res. 2005;11:512–9.
George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, Leenders F, Lu X, Fernandez-Cuesta L, Bosco G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53.
Eckner R, Ewen ME, Newsome D, Gerdes M, DeCaprio JA, Lawrence JB, Livingston DM. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–84.
Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–17.
Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem. 2006;281:18059–68.
Bitoun E, Oliver PL, Davies KE. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum Mol Genet. 2007;16:92–106.
Mueller D, Bach C, Zeisig D, Garcia-Cuellar MP, Monroe S, Sreekumar A, Zhou R, Nesvizhskii A, Chinnaiyan A, Hess JL, et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. 2007;110:4445–54.
Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17:198–212.
Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–7.
Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–78.
Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, Johnston LD, Scott MP, Smith JJ, Xiao Y, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53–65.
Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, Allain CJ, Klaus CR, Raimondi A, Scott MP, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122:1017–25.
Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012;7:283–301.
Kuntimaddi A, Achille NJ, Thorpe J, Lokken AA, Singh R, Hemenway CS, Adli M, Zeleznik-Le NJ, Bushweller JH. Degree of recruitment of DOT1L to MLL-AF9 defines level of H3K79 Di- and tri-methylation on target genes and transformation potential. Cell Rep. 2015;11:808–20.
Wang WT, Han C, Sun YM, Chen ZH, Fang K, Huang W, Sun LY, Zeng ZC, Luo XQ, Chen YQ. Activation of the lysosome-associated membrane protein LAMP5 by DOT1L serves as a bodyguard for MLL fusion oncoproteins to evade degradation in leukemia. Clin Cancer Res. 2019;25:2795–808.
Al Sarakbi W, Sasi W, Jiang WG, Roberts T, Newbold RF, Mokbel K. The mRNA expression of SETD2 in human breast cancer: correlation with clinico-pathological parameters. BMC Cancer. 2009;9:290.
Pfister SX, Markkanen E, Jiang Y, Sarkar S, Woodcock M, Orlando G, Mavrommati I, Pai CC, Zalmas LP, Drobnitzky N, et al. Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell. 2015;28:557–68.
Den Haese GJ, Walworth N, Carr AM, Gould KL. The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2. Mol Biol Cell. 1995;6:371–85.
Kim SY, Ferrell JE Jr. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell. 2007;128:1133–45.
Rowley R, Hudson J, Young PG. The wee1 protein kinase is required for radiation-induced mitotic delay. Nature. 1992;356:353–5.
Mahajan K, Fang B, Koomen JM, Mahajan NP. H2B Tyr37 phosphorylation suppresses expression of replication-dependent core histone genes. Nat Struct Mol Biol. 2012;19:930–7.
Mahajan K, Mahajan NP. WEE1 tyrosine kinase, a novel epigenetic modifier. Trends Genet. 2013;29:394–402.
Martinelli G, Mancini M, De Benedittis C, Rondoni M, Papayannidis C, Manfrini M, Meggendorfer M, Calogero R, Guadagnuolo V, Fontana MC, et al. SETD2 and histone H3 lysine 36 methylation deficiency in advanced systemic mastocytosis. Leukemia. 2018;32:139–48.
Beck H, Nahse-Kumpf V, Larsen MS, O'Hanlon KA, Patzke S, Holmberg C, Mejlvang J, Groth A, Nielsen O, Syljuasen RG, et al. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol. 2012;32:4226–36.
D'Angiolella V, Donato V, Forrester FM, Jeong YT, Pellacani C, Kudo Y, Saraf A, Florens L, Washburn MP, Pagano M. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell. 2012;149:1023–34.
Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, Hesley JA, Miller SC, Cromwell EF, Solow-Cordero DE, et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 2009;35:228–39.
Terzo EA, Lim AR, Chytil A, Chiang YC, Farmer L, Gessner KH, Walker CL, Jansen VM, Rathmell WK. SETD2 loss sensitizes cells to PI3Kbeta and AKT inhibition. Oncotarget. 2019;10:647–59.
Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–7.
Akbani R, Ng PK, Werner HM, Shahmoradgoli M, Zhang F, Ju Z, Liu W, Yang JY, Yoshihara K, Li J, et al. A pan-cancer proteomic perspective on The Cancer Genome Atlas. Nat Commun. 2014;5:3887.
Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB, et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42:343–53.
Herceg Z, Wang ZQ. Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001;477:97–110.
Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu Rev Biochem. 2012;81:65–95.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22.
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171:540–56 e25.
Rampias T, Karagiannis D, Avgeris M, Polyzos A, Kokkalis A, Kanaki Z, Kousidou E, Tzetis M, Kanavakis E, Stravodimos K, et al. The lysine-specific methyltransferase KMT2C/MLL3 regulates DNA repair components in cancer. EMBO Rep. 2019;20(3).
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31.
Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83.
Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–60.
Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–58.
Weissmueller S, Manchado E, Saborowski M, Morris JP IV, Wagenblast E, Davis CA, Moon SH, Pfister NT, Tschaharganeh DF, Kitzing T, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157:382–94.
Zhao Y, Ding L, Wang D, Ye Z, He Y, Ma L, Zhu R, Pan Y, Wu Q, Pang K, et al. EZH2 cooperates with gain-of-function p53 mutants to promote cancer growth and metastasis. EMBO J. 2019;38(5).
Vyse S, Howitt A, Huang PH. Exploiting synthetic lethality and network biology to overcome EGFR inhibitor resistance in lung cancer. J Mol Biol. 2017;429:1767–86.
Papadakis AI, Sun C, Knijnenburg TA, Xue Y, Grernrum W, Holzel M, Nijkamp W, Wessels LF, Beijersbergen RL, Bernards R, et al. SMARCE1 suppresses EGFR expression and controls responses to MET and ALK inhibitors in lung cancer. Cell Res. 2015;25:445–58.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81673652, 81572947, 81773216), the Natural Science Foundation of Liaoning Province (No. 20170540841, 20180550076), and Liaoning BaiQianWan Talents Program (To Lihui Wang).
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81673652, 81572947, 81773216), the Natural Science Foundation of Liaoning Province (No. 20170540841, 20180550076), and Liaoning BaiQianWan Talents Program (To Lihui Wang).
Author information
Authors and Affiliations
Contributions
HY contributed to drafting the manuscript and analyzing the data. LW and WC contributed to designing and drafting the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not involved into ethics
Consent for publication
-
1.
The contents of this manuscript have not been copyrighted or published previously.
-
2.
The contents of this manuscript are not now under consideration for publication elsewhere.
-
3.
The contents of this manuscript will not be copyrighted, submitted, or published elsewhere, while acceptance by Clinical Epigenetics is under consideration.
-
4.
There are no directly related manuscripts, published or unpublished, by any authors of this paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yang, H., Cui, W. & Wang, L. Epigenetic synthetic lethality approaches in cancer therapy. Clin Epigenet 11, 136 (2019). https://doi.org/10.1186/s13148-019-0734-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-019-0734-x