Abstract
Methyl CpG binding protein 2 (MeCP2) is a highly abundant chromosomal protein within the brain. It is hence not surprising that perturbations in its genome-wide distribution, and at particular loci within this tissue, can result in widespread neurological disorders that transcend the early implications of this protein in Rett syndrome (RTT). Yet, the details of its role and involvement in chromatin organization are still poorly understood. This paper focuses on what is known to date about all of this with special emphasis on the relation to different epigenetic modifications (DNA methylation, histone acetylation/ubiquitination, MeCP2 phosphorylation and miRNA). We showcase all of the above in two particular important neurological functional alterations in the brain: depression (major depressive disorder [MDD]) and cocaine addiction, both of which affect the MeCP2 homeostasis and result in significant changes in the overall levels of these epigenetic marks.
Similar content being viewed by others
“Au temps déjà lointain où, étudiant de la sublime Science, nous nous penchions sur le mystère tout rempli de lourdes énigmes…”
Fulcanelli (1925), Le mystère des cathédrales [1]
Background
It was not until 1999, when it was realized that mutations in MeCP2 may result in RTT (an autistic type of neurodevelopmental disorder) [2], that the scientific community began to pay a great deal of attention to this protein. A significantly large amount of research has been carried out since that time, and what was initially thought to be a relatively simple, unique repressor protein [3], developed into a fascinating transcriptional regulator [4, 5]. Part of its functional multiplicity is due to the intrinsically disordered conformation of MeCP2 [4, 6], which makes it amenable to interaction with multiple interacting protein partners, as well as to its labelling with various post-translational modifications (PTMs), such as phosphorylation, acetylation and ubiquitination [4]. Such PTMs further modulate the interaction of MeCP2 with chromatin [7].
In the brain, where MeCP2 is highly abundant [8, 9], it can bind to both DNA methylated and non-methylated regions of chromatin [10]. Yet, the chromatin organization resulting from these interactions is still unclear, and the molecular details involved are not completely understood. The large occurrence of MeCP2 in neurons has led to the realization in recent years that alterations in the homeostatic levels of the protein can have important functional consequences for several neurodevelopmental and neurodegenerative diseases that highly transcend RTT [4]. In this regard, a significantly large amount of research and information has been recently gathered about two types of brain alterations, which have important societal implications: depression [11] and cocaine addiction [12]. Both have a strong epigenetic component that involves changes in MeCP2 levels and alterations of the histone PTMs (such as acetylation and phosphorylation). The molecular details regarding the involvement of MeCP2 in these brain disorders has brought about a significant amount of exciting functional information, relevant not only for the particular mechanisms involved in each of them, but more importantly, for the overall molecular biology of MeCP2 in the brain.
A brief history of MeCP2: Cancer, Rett syndrome, MDD and cocaine addiction
In 1989, the search for a protein “reader” that specifically binds to regions of methylated CpG in the mammalian genome [13] led to the identification of a protein known as MeCP-1 [14], a protein that binds in vitro to DNA sequences containing at least 12 symmetrically methylated CpGs [15]. Three years later, an additional methylated CpG binding protein was identified called MeCP2 [16, 17]. Due to its 5-methyl cytosine (5mC) binding activity, the protein was initially assigned a repressive role, supposedly acting at the methylated regions of transcriptionally repressed genes [3]. However, more recently, the protein has been shown to additionally bind to 5-hydroxy-methylated cytosines (5hmC) at the elongation regions of transcriptionally active genes [18]. It has also been shown to bind to both repressive and activating co-factors [19, 20]. Therefore, MeCP2 should be viewed as a transcriptional regulator [4]. By virtue of its ability to bind to methylated DNA and to chromatin modifying complexes, such as HDACs and CREB, MeCP2 can be considered both an epigenetic ‘reader’ and a ‘writer’.
The initially identified form of MeCP2 turned out to be what is today known as the MeCP2-E2 isoform. It was not until 2004 that the MeCP2-E1 isoform (initially called MeCP2 B) was discovered [21]. MeCP2-E1 is the major form of MeCP2 [22] and the one that is most abundant in the brain, corresponding to approximately 90 % of the total MeCP2 in this tissue [23]. Of note, the predicted half-life of the two isoforms is very different, with that of the E1 isoform (4 hours) being significantly different from that of E2 (100 hours) [4].
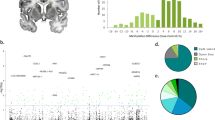
The gene organization and schematic representation of the protein structure of these two MeCP2 isoforms are shown in Fig. 1a. The MeCP2 gene is negatively regulated by the high mobility group nucleosome binding (HMGN) of non-histone chromosomal proteins [24], and positively regulated by the transcription factor myocyte enhancer factor 2C (MEF2C) [25]. MicroRNAs, such as miR-132 [26], miR-7b development [27], miR-483-5p [28], miR-155/miR-802 [29] and miR-181 [30] also play a very important role in the regulation of the expression of the gene.
a Gene organization of MeCP2. Two isoforms E1 and E2 result from alternative splicing. The red arrows indicate the starting sites of transcription. The domain organization of the resulting protein isoforms is also shown. CTD; C-terminal domain; ID: intervening domain; MBD: methyl-binding domain; NTD; N-terminal domain; TRD: transcriptional repression domain. b Number of publications since MeCP2 was first described
At the protein level, the common methyl binding domain (MBD) (Fig. 1a) is the only region of these proteins that shows a well-characterized tertiary structure [31] within what it is one of the best examples of an intrinsically disordered protein [4]. As such, the protein is subject to numerous PTMs [4], of which the phosphorylation of serine residues at position 80 (S80 of human E2 isoform) [32] and 421 (S421of mouse E1 isoform) [33], commonly referred to as S80 and S421 [34], have been the most extensively characterized. Importantly, the levels of MeCP2 and its homeostasis in the brain (where the protein is more abundant) appear to be critical to its proper developmental function [35], although normal physiologically-relevant fluctuations can occur in a circadian-regulated manner [36].
It took seven years since the discovery of MeCP2 for the number of publications on this protein (Fig. 1b) and the general interest of the scientific community to pick up on Huda Zoghbi’s team demonstration at the Baylor College of medicine, which demonstrated that RTT, for the most part, is due to mutations in MeCP2 [2]. Rett syndrome is an X-chromosome-linked neurodevelopmental disease predominantly affecting girls. It is related to autism and is characterized by intellectual disability and developmental regression [37]. Studies published previous to the knowledge of the connection of MeCP2 with RTT included the characterization of the interaction of MeCP2 with histone de-acetylases (HDACs) [38]. Together with the interaction of MeCP2 with CpG methylated DNA, this finding reinforced the notion of a transcriptionally repressive role initially associated with this protein [39], while establishing the first link between MeCP2 and histone acetylation. This concept fits well with that of DNA CpG hypermethylation, which is usually found at the promoters of tumor-suppressor genes in many cancers [40–43] where MBD proteins, including MeCP2, play an important role [44]. A paper appeared during the interim period of time that preceded the RTT-MeCP2 association, which involved MeCP2 in the repression of the retinoblastoma gene [45], and it paved the way in terms of MeCP2’s involvement in cancer. Indeed, MeCP2 has been recently recognized as playing the role of a bona fide oncogene [46].
Although the presence of MeCP2 in the brain is exceedingly larger than in any other tissue [9], the last sentence of the prior paragraph should serve as a reminder that the protein plays additional important roles in many other tissues and physiological aspects of the body. An underscoring example of this can be drawn from a recent observation describing a decrease in both the levels of mRNA and of MeCP2 itself during chronic heart failure [47]. The same holds equally true for the brain and, in this case, it is the massive presence of MeCP2 in this organ that is responsible for many other neurodevelopmental and neurodegenerative processes (Fig. 2) [4] far beyond RTT. As will be discussed later, alterations in the levels of MeCP2 have been described, for both major depressive disorder (MDD) and cocaine addiction [48], two brain affections which pervade our modern society and are an important public health concern [49–51].
Chromatin, the variance of the histones and chromatin epigenetics
Chromatin is the name given to the nucleoprotein complex that results from the association of DNA and histones. Histones are basic proteins, rich in both Lysine and Arginine residues. They have been broadly classified into two structurally different major groups [52]: core and linker histones. Core histones (H2A, H2B, H3 and H4) form an octameric protein complex consisting of 2 H2A-H2B dimers and an H3-H4 tetramer that serves as a protein core, around which 145-147 bp DNA is wrapped in approximately one and three quarters of left-handed super-helical turns to form the nucleosome core particle (NCP). In the chromatin fiber, NCPs are connected to each other by shorter linker DNA regions of variable nucleotide length. The average distance in nucleotides between the centers of two adjacent nucleosomes is known as the nucleosome repeat length (NRL), which may vary between different tissues, with neurons exhibiting an unusually short one (160 bp) in metazoan tissues [53]. Linker histones (of the histone H1 family) bind to the NCP close to its dyad axis, at the entry and exit sites of the linker DNA connecting adjacent nucleosomes [52, 54]. The former have been further classified into canonical replication-dependent histones and replication-independent replacement histone variants (i.e. H2A.Z, H2A.X, H2A.B, macro H2A, H3.3) [55].
However, what we have over the years referred to as histone variants [56] due to their lower abundance in metazoans may have been the primordial canonical histones, while what we are currently calling replication-dependent canonical histones may actually represent the real variants. As a matter of fact, most protein-encoding eukaryotic genes, in contrast to the replication-dependent histone genes, contain introns and are poly-adenylated, as compared to the replication histone genes, which are intron-less and are poly A- [57]. Of note, yeast H2A is H2A.X and H3 is H3.3 [58]. Moreover, H2A.X in metazoans is encoded by both poly A+ and poly A- mRNAs [59]. It appears that as genomes became increasingly larger, there was a need for specialization in the histone genes to adapt to the synthesis demands that ensure the fast and efficient coverage of the genomic DNA during DNA replication. This was achieved by a completely different regulation of the expression of these genes, as well as the loss of their introns and poly-adenylation [57]. Indeed, some of these replication-dependent histones are actively expressed in differentiating and aging retinal neurons at a time when replication has already ceased [60].
Regardless of their ancestry or origin, replication-independent histone variants play an important role in brain development [61, 62]. A few examples have been recently brought to the forefront. Histone H2A.Z has been shown to play a critical role in recent and remote memory consolidation [63], and histone H3.3 has been shown to play an essential role in neuronal plasticity and cognition [64].
In addition to the histone variation, histones in general, regardless of their replication dependent or independent nature, can be post-translationally modified (PTM) at specific amino acid sites in their sequence. These chemical modifications (i.e. acetylation, methylation, phosphorylation and ribosylation, to name just a few [65]) set the molecular basis for the proposal of the histone code fifteen years ago [66]. From then on, chromatin went from being considered a passive structural template that sustained the DNA metabolic functions to acquiring an epigenetic role of its own, in which the histones play a crucial role. As a matter of fact, histone variants and histone PTMs provide the molecular basis for their epigenetic involvement [55, 67, 68].
The histone code involves a complex network of protein ‘writers’, ‘erasers’ and ‘readers’ responsible for the downstream functional implications of the histone PTMs. However, not all histone variants and PTMs have an exclusive epigenetic function. For instance, in addition to its epigenetic involvement, global histone acetylation of pan-acetylated H3/H4/H2A.Z can alter the structural organization of chromatin on their own [69], as is also the case in H4 K16 acetylation [70].
In addition to histone variants and their PTMs [71], other chromatin trans-acting factors, such as MeCP2, by virtue of binding to methylated [72] and hydroxymethylated [18] DNA could also have an important involvement, thus connecting epigenetics and neuronal function [73]. How MeCP2 and histone epigenetic baggage affect neuronal chromatin organization is still one of the many remaining enigmas that will be analyzed in the next two sections.
MeCP2 and the chromatin organization of neurons
As we and others have experimentally shown, MeCP2 is a very abundant chromosomal protein within the brain, with approximately one MeCP2 mole for every nucleosome in the neurons of an adult brain [8, 9]. The question then arises as to how this high MeCP2 prevalence is accommodated within the histone crowded chromatin ensemble. Moreover, the levels of MeCP2 in neurons increase during brain development, and are accompanied by a decrease in the NRL from approximately 200 bp at the early embryonic stages to 160 bp in the adult brain [74]. In addition, it has long been known, and recently corroborated, that the levels of linker histones (histones of the H1 family) in the brain are approximately 50 % of what would be present in other somatic tissues [8, 75]. It was initially shown that MeCP2 is able to displace histone H1 in vitro in a DNA methylation-dependent manner [76]. When all this information is considered together, the question arises as to whether MeCP2 is present in a quasi-even alternating nucleosome arrangement, or clustered in regions that are highly enriched, others in which H1 prevails, or a mixture of the two.
With regards to the potential role of MeCP2 in the organization of chromatin, and neuronal chromatin in particular, one of the most puzzling and yet unexplained observations is that of its early release during micrococcal nuclease digestion [9]. Using a relatively simple chromatin fractionation method (Fig. 3a), it was demonstrated that a large amount of MeCP2—more than 50 percent—is released into a supernatant S1, which is deficient in histones (particularly H1) (Fig. 3b) and which consists of mono-nucleosomes (Fig 3c) along with small oligonucleotides that are released during nuclease digestion and contain a higher level of methylated cytosine (Fig. 3d) [9]. Thus, a significant amount of MeCP2 appears to be bound to chromatin regions that are highly accessible to micrococcal nuclease. Such an observation is highly enigmatic and appears to be in contrast with the initial repressive role associated with the protein [3], as well as with the highly compacted nucleoprotein complexes it forms in vitro upon interaction with nuclesosome arrays [77]. Interestingly, as shown in Fig. 3c, during the early stages of digestion, most of the MeCP2 present in S1 appears to arise from the highly compact “insoluble” structures present in fraction P.
a After micrococcal nuclease (MNase) digestion of cellular nuclei, small-sized nucleosomes chromatin (nI) (see Fig. 4) that leak through the nuclear membrane pores can be recovered in the supernatant (SI) after centrifugation. The nuclear pellet can next be hypotonically lysed in the presence of 0.25 mM EDTA and centrifuged once more to yield a supernatant (SE) fraction and an insoluble pellet (P). b Protein composition of the SI, SE and P fractions as analysed by polyacrylamide gel electrophoresis (PAGE) in the presence of SDS detergent. Histones H1, H2A, H2B, H3 and H4 are indicated, as well as myelin (M). c Analysis of the SI, SE and P fractions during a time-course Mnase digestion of rat whole brain nuclei. The upper part of the Figure shows a Western blot analysis using MeCP2 and H4 antibodies. The lower part shows a native PAGE analysis of the DNA composition of the fractions obtained at different time of digestion. CE: chicken erythrocyte histones used as a control; M: pBR322-Cfo I–digested DNA used as a marker. The numbers on the right had side of the native PAGE indicate the DNA fragment sizes in base pairs (bp). The red lines highlight the shift in the center of the mononucleosome DNA (nI) distribution in SE and P. d Relative meC/C percentile composition of the SI, Se and P fractions at limit Mnase digestion. (Section c was reproduced from Fig. 2A from [9], with permission)
Equally intriguing is the difference in NRL observed between chromatin in the P fraction (approx. 200 bp) and that of the SE fraction (centered at approx. 174 bp). This difference is reminiscent of the transition in NRL observed during neuronal development (from 200 bp at the onset of development to 160-170 bp in mature neurons [74]) in a way that is independent of the chromatin H1 content [53]) and for which there is yet no explanation. Interestingly, the NRL change is dependent on developmental factors of the brain, such as thyroid hormone [78], and follows the transition of the neuronal nucleus from a small heterochromatic to a larger euchromatic nucleus [79, 80]. Whether all these transitions are dependent on MeCP2 remains to be determined.
Based on these results, we would like to put forward a hypothetical model (Fig. 4) that still requires further experimental testing but which is different than that we had proposed earlier [9]. In this model, an important amount of MeCP2 is concentrated at the chromocenters and the nucleolus periphery, and therefore, while attached to these ‘insoluble’ chromatin domains, it should be highly accessible to digestion by nucleases. Indeed, immuno-gold labelling using electron microscopy initially demonstrated a preferential localization of MeCP2 at the periphery of highly dense chromatin structures [9]. This is in agreement with earlier observations of its co-localisation with DAPI-positive, heterochromatic regions that surround the nucleolus [81]. Of note, most of the transcriptional dynamics in neurons occur in the nucleolus and at different euchromatin sites [82]. Moreover, in neurons, MeCP2 has been shown to bind to the methylated CA sites of exceptionally long genes and, more importantly, disruption of MeCP2 alters the levels of rRNA [72].
Hypothetical model for the MeCP2 distribution within the neuronal chromatin organization. MeCP2 is sparsely distributed in the chromatin fibers in the nucleoplasm binding to DNA methylated sites that are depleted of H1. An important fraction of MeCP2 binds preferentially to the periphery of chromocenters/nucleoli. Upon micrococcal nuclease (MNase) digestion, MeCP2 is very quickly released from the insoluble pellet-able (P) chromocenter periphery and leaks through the nuclear membrane pores into the SI fraction (see Fig. 2). MeCP2 associated to nucleosomes is also released from nuclear chromatin fibers (SE fraction), albeit at a much lower level as indicated by the thickness of the arrows. nl: mononucleosome
The presence of two MeCP2 isoforms with putative, disparately different half-time lives may impinge in the dynamics of neuronal chromatin, giving it a very fluid organization. Although highly speculative, one could envision an organization in which MeCP2 that already binds highly dynamically to chromatin as determined by FRAP [83, 84] could utilize its E1 and E2 isoforms to differentially alter its association with different chromatin domains in a quick response to rapid changes in the environment (such as in response to the circadian cycle [36], or in the short-term response to drugs, such as cocaine [85]). It is likely that the PEST sequences common to both isoforms play an additional important role in such turnover [86]. PEST sequences consist of at least 12 amino acids residues in length and typically signal the protein containing them for rapid proteolytic degradation by the 26S ubiquitin proteasome system (UPS) through phosphorylated serine-mediated ubiquitination at a contiguous lysine residue within the PEST domain [87, 88]. In this regard, although experimental evidence involving these domains is still missing, a ubiquitin ligase (RNF4) [89] has recently been identified that may be involved in the process, and serine 80 and lysine 82 (nomenclature referred to the E2 isoform) within the PEST 1 sequence in mice have been shown to be phosphorylated [32] and ubiquitinated [90] respectively.
The regulation of MeCP2 homeostasis and additional brain epigenetic markers, to be summarized in the following section, provide an extra layer of complexity to this chromatin organization and ultimately are responsible for the chromatin alterations that affect its function in the brain.
Critical brain epigenetic markers
Four main epigenetic contributors deserve special attention when it comes to the physiologically relevant aspects of MeCP2 and neuronal chromatin in normal and altered functional states of the brain: DNA methylation, MeCP2 phosphorylation, histone acetylation and microRNAs.
DNA methylation
MeCP2 is a methylated cytosine ‘reader’ and any changes in DNA methylation are going to have immediate downstream effects on the MeCP2-dependent organization of chromatin. Contrary to what was earlier believed, DNA methylation in neurons is highly dynamic and may change quite rapidly to alter the MeCP2 distribution and their connections or synapses. The process involves a group of enzymes called Tet (ten eleven translocation), which initially oxidize 5mC to 5hmC [91] and subsequently lead to active DNA de-methylation [92] in conjunction with the base-excision repair (BER) pathway. Tet 3 has recently been shown to regulate synaptic transmission and homeostatic plasticity through this mechanism [93]. DNA methylation is then restored by Dnmt1 and Dnmt3a DNA methyl-transferases [94] that close the cycle, and thus contribute to the process of synapsis function. Interestingly, as was mentioned previously, MeCP2 binds very tightly to 5hmC [72], and in doing so it plays a very important role in active transcription [18] within mature neurons. Furthermore, the histone variant H2A.X, which increases during neuronal development [61], has been shown to be associated with BER [95, 96] and was the only histone variant found to co-immuno precipitate with MeP2-containing nucleosomes [9].
MeCP2 phosphorylation
MeCP2 phosphorylation is by far the most studied PTM of this protein. MeCP2 has been reported to be phosphorylated at various serine residues, such as S13, S80, S149, S164, S229, S274, S401 and S421 [32, 90].
Among these MeCP2 serine residues, phosphorylation at residues S80 and S421 have been proposed to have opposite effects on neuronal activity [32, 34, 97]. In neurons at rest, MeCP2 is tightly associated with Bdnf promoter III, acting as a repressor. Upon neuron depolarization, and following calcium influx, MeCP2 S421 becomes phosphorylated by a Camk2/4-dependent mechanism [33] and is released from the Bdnf promoter III, allowing for its activity-dependent transcription. Phosphorylation at S421 has also been shown to be associated with accurate synapse development and behaviour [33]. In an opposite mode, MeCP2 S80 becomes dephosphorylated upon neuronal depolarization, allowing for the dissociation of MeCP2 from chromatin. Conversely, MeCP2 is phosphorylated at S80 by homeodomain-interacting protein kinase 2 (HIPK2) and contributes to induction of apoptosis [98]. This opposite regulation of MeCP2 by neuronal activity suggests that S421 phosphorylation plays an important role in active neurons, while S80p is more important in resting neurons.
More recently, neuronal depolarization has been shown to result in the phosphorylation of MeCP2 at threonine 308 in the transcriptional repressor domain (TRD) of the protein. Phosphorylation of this residue blocks the interaction of MeCP2 with the nuclear receptor co-repressor (NCoR) complex. Among other effects, this elicits Npas4 transcription (a transcription factor that promotes the development of inhibitory synapses on excitatory neurons) and the ensuing activation of Bdnf transcription [99].
Histone acetylation
The direct or indirect molecular mechanisms behind the relation between MeCP2 and histone acetylation are obscure and require further analysis. Knockout mice in which MeCP2 expression has been completely ablated exhibit an almost 3-fold increase in the histone acetylation in neurons when compared to the wild type [8]. Conversely, treatment of HeLa cells with sodium butyrate (an inhibitor of histone de-acetylases) decreases the levels of MeCP2 by almost 3-fold (Thambirajah and Ng, unpublished results). While it can be argued that the former is the result of the well-documented interaction of MeCP2 with repressive chromatin remodelling complexes containing HDACs, the latter has no straightforward explanation.
Global histone pan-acetylation results in a highly labile nucleosome [100] and affects the folding of the chromatin fiber in the absence of linker histones [101]. It also decreases the inter-fiber chromatin interactions [102]. MeCP2 has been shown to interact with chromatin in a way reminiscent of the interaction between linker histones and chromatin [4]. Indeed, MeCP2 was shown early on to compete with histone H1 in a DNA-methylation-dependent way [76]. Although treatment of cells with sodium butyrate does not significantly affect the levels of histone H1 [102], histone acetylation has been known to impair the binding of histone H1 to the nucleosome [103]. However, a role of histone acetylation in facilitating MeCP2 binding could not be demonstrated [104].
MicroRNAs
MicroRNAs represent one of the most recently identified epigenetic constituents. MicroRNAs (miRNAs) are non-coding RNA transcripts that control gene expression by binding to complementary sequences (miRNA response elements; MRE) in the 3'-UTR of target mRNAs, thus controlling their degradation and translation [105]. Furthermore, DNA methylation has been found to be crucial in miRNA biogenesis [106], and MeCP2 long 3'UTR contains conserved MREs for several miRNAs that modulate its expression [107].
As was mentioned earlier, MeCP2 homeostasis plays an important role in the proper functional outcome of this protein. MicroRNA miR-132 has been found to play a crucial role in this regard. Blocking miR-132 in cultured rat neurons results in an increase of MeCP2 expression. This, in turn, increases the expression of Bdnf that induces miR-132 and represses MeCP2 translation. Taken together, these findings suggest a feedback loop involved in MeCP2 homeostasis [26].
Another regulatory microRNA, miR-7b, is expressed in various regions of the adult mouse brain, and also targets MeCP2 at the 3'-UTR. Two CpG islands have been identified at the 5'-flanking region of the gene encoding for miR-7b. An increase in the methylation of these CpG islands during postnatal neuron maturation increases the recruitment of MeCP2 to these regions. This inhibits miR-7b gene expression and its repressive effect on MeCP2. Overall, miR-7b acts as a negative regulator of MeCP2 gene expression and is at the same time a down-stream target of MeCP2, forming a bi-directional feedback that may also be important for MeCP2 homeostasis during brain development [27].
In humans, the intragenic miR-483-5p, derived from the gene Igf2 (insulin-like growth factor – 2), has been found to regulate MeCP2 levels. This miRNA binds to the MREs in MeCP2 3'-UTR and inhibits MeCP2 gene expression. miR-483-5p is enriched in the fetal brain and is down-regulated after birth, thus controlling MeCP2 expression during human brain development [28].
The miR-155 and miR-802 also target MeCP2 and have recently shown to play an important role in Down syndrome pathology [29].
The miR-181 family is expressed in astrocytes and has been shown to be involved in their neuro-inflammatory response. Despite the current lack of information, MeCP2 has been found to be a target for miR-181 [30].
MeCP2 in depression and cocaine abuse
One of the more fascinating aspects of chromatin’s epigenetic involvement is, in our opinion, the connection between chromatin alterations in response to environmental cues, such as early life stress (ELS), and the resulting behavioral output. It has now been fully demonstrated in rodents that maternal/parental care can affect gene expression [26, 28] and epigenetically affect the offspring in a trans-generational way [108]. More importantly, preclinical studies suggest that early life stressors, such as inconsistent and harsh parental discipline on their children [109], can result in increased stress responses leading to depressive disorders later on in adulthood [110]. At the molecular level, as will be discussed in the next paragraphs, an important part of the connection regards alterations in the function of the brain-derived neurothrophic factor (Bdnf) gene [111] that encodes for a member of the neurtrophin family of growth factors and its involvement in many important brain functions. It is a long gene with 4 promoters, which transcribe 4 mRNAs containing one of the four 5′ noncoding exons (I, II, III, or IV) spliced to the common 3′ coding exon [112], and is regulated by MeCP2. In addition to Bdnf, MeCP2 regulates the expression of many other similarly long genes that encode for proteins such as the calcium/calmodulin-dependent Camk2d kinase and those involved in axon guidance and synapsis formation [72]. Hence, as we have previously mentioned, is not surprising that MeCP2 in conjunction with epigenetic neuronal chromatin modifications are involved in many alterations of brain function that result in a plethora of neurological and psychiatric disorders [4, 113–120] (Fig. 2). The integration of environmental effects, such as stress, and the genetic and epigenetic modifications underlie what is currently known as synaptic and behavioral megaplasticity [121, 122]. In this section, I focus on two of them that have broad important social connotations in our current era: major depressive disorder (MDD), which has been quite extensively studied in recent years, and addiction (focusing on cocaine), for which a large number of studies are also available.
Whilst the genetic risk factors of both depression [123, 124] and addiction [125] are being established, the biochemical details and molecular mechanisms involved in the epigenetic counterpart are further ahead in their elucidation. At the chromatin level in a general mechanism, neuronal stimulation results in a Ca2+ influx that triggers the action of neuronal kinases (such as Camk2d) that phosphorylate different substrates, including MeCP2, CREB (cAMP response element-binding protein) and histone H3S10, amongst others [126]. Phosphorylation of MeCP2 weakens its interaction with chromatin, and phosphorylation of CREB allows it to bind to CBP (CREB-binding protein a histone acetyl transferase) [127], which acetylates histones and leads to a further chromatin relaxation, as was described in the previous section. All these modifications are conducive to a more open chromatin conformation, which enhances the accessibility of transcriptional co-activators to cis acting regulatory elements, like MEF2C, which results in gene activation (such as that of Bdnf) [126, 128].
One of the first molecular connections between ELS and depression was established through the arginine vasopressin gene (Avp). It was discovered that ELS was able to control the DNA methylation dynamics in post-meiotic neurons (see previous section), to result in stable persistent hypo-methylation of Avp expression that triggers the neuroendocrine and behavioral changes often observed in depression [129]. Early Avp derepression is driven by neuronal activity that results in the Ca2 + - Camk2d dependent MeCP2 phosphorylation and chromatin dissociation described in the previous paragraph, followed by DNA hypo-metylation. A vicious cycle is thus established in which MeCP2 occupancy uncouples from the original stimulus, leading to the ELS hard-coding at the level of DNA methylation [130]. The situation is by far more complex, and in addition to Avp, MeCP2 also regulates the ELS-dependent programming of other genes, such as Crh (corticotropin releasing hormone) and Pomc (Proopiomelanocortin) [131], all of which enhance the hypothalamic-pituitary-adrenal (HPA) axis which drives the ELS response, and are driven initially by MeCP2 S421 phosohorylation [48].
Whilst Avp, Crh, and Pomc, explain the connection between ELS and depression, Bdnf, as expected for any neuronal disturbance, also plays a very important role in depression [111], and its expression is decreased in stress and depression [132]. With the use of antidepressants, it has been shown that H3K27 methylation and histone deacetylation increase at the Bdnf III and IV promoters, a process that can be reverted with the use of histone methylation and HDAC5 inhibitors in mice [133]. It has been shown that the antidepressant citalopram decreases the levels of H3K27me3 at promoter IV of Bdnf in humans [134]. The histone PTM involvement in the stress-mediated neuronal response goes far beyond those briefly described here, and the reader is referred to [135] for a more comprehensive description.
MicroRNAs are also involved in depression. An increase was observed in the levels of miR-132 in the hippocampus of a rat model of stress-induced depression, and also in peripheral blood samples of patients with MDD. As expected from its role in MeCP2 homeostasis, a negative correlation between the expression levels of miR-132 and those of MeCP2 and BDNF was observed in these studies [11]. Also, high levels of miR-144-5p were detected in the plasma of MDD patients when compared to healthy controls, suggesting that miR-144-5p can be used as a biomarker for the disease [136].
Cocaine abuse and MDD share some similarities, let alone the fact that ELS is often a common risk factor for addiction [137, 138], which is considered a brain disorder of experience-dependent neuroplasticity [139]. Like ELS, cocaine affects the DNA methylation dynamics [140, 141] and in particular, within the nucleus acumbens (NAc) [142], a reward-related central region of the brain, both for 5mC and 5hmC [143]. Hence, it also affects MeCP2 [12, 144] and bdnf expression [145, 146].
The role of MeCP2 in cocaine addiction involves different aspects of the protein metabolism and its gene regulation and, in particular, the calcium-dependent MeCP2 S421 phosphorylation, one of its important PTMs that plays a crucial role [97, 147, 148]. The neuronal-activity mediated phosphorylation of MeCP2 S421 has been shown to have important transient and permanent effects in drug abuse and ELS respectively [129].
Of particular interest to this review is the interplay at the chromatin level between the induced levels of MeCP2 expression observed during cocaine intake [149] and the changes in histone acetylation [150]. After repeated (chronic) exposure to cocaine, the global levels of histone acetylation in the cocaine-targeted GABAergic neurons were observed to decrease in general agreement with the HDAC-mediated MeCP2 repressive activity [144]. However, the situation appears to be not that simple. In what could be considered a seminal paper on this topic, it was shown that an increase in the levels of histone H4 hyperacetylation in NAc occurs at the promoters of certain genes (such as the immediate early gene c-Fos), and is observed within 30 minutes of a single cocaine injection (acute exposure). However, the effect fades away during chronic exposure. Conversely, the levels of H3 acetylation increased at promoters of genes such as Bdnf and Cdkl5 [151]. A genome-wide ChIP-chip analysis using antibodies against pan-acetylated H3/H4 has, interestingly, revealed that the increases in acetylation after chronic exposure do not affect the genome randomly [152], but rather increase the magnitude of their promoter distribution [150]. They have also confirmed the lack of overlap between H3 and H4 acetylated promoters [152]. All of this indicates that the cocaine-induced histone acetylation chromatin remodelling may be different for histones H3 and H4, in agreement with their different structural roles [153, 154].
Along with the shared molecular mechanisms with MDD, the cocaine-induced increase in MeCP2 represses the transcription of miR-132/miR-212 micro RNAs, which reduce the miRNA repression of bdnf through the feedback loop described in the previous section [139, 155]. Furthermore, it has been shown that in the dorsal striatum of rats, the expression of miR-212 is increased in those that show compulsive-like cocaine-taking behavior [156].
We would like to close this chapter with another intriguing aspect, which is that involving gender [157]. As was mentioned earlier, both depression and drug addiction share genetic and epigenetic contributions. It has now been well documented that women have a higher genetic predisposition to depression [158], which in turn may lead to a potential higher risk of drug abuse, which also exhibits important differences between both sexes [159]. Epigenetic studies of the brain have only recently started grasping this issue, but they have been postulated to also have a role in risk and resilience to mental health between the sexes [160]. For instance, 248 genes and loci associated with synaptic function were identified in mouse brains, with increased H3K4me3 in females [161]. Perinatal testosterone exposure resulted in important alterations of the DNA methylome [162], underscoring the contribution of the hormonal component [163]. Therefore, it is very important that when conducting future research in any of the areas described in this work, including those directly related to MeCP2, attention be paid to the sex differences and the differential involvement of gonadal hormones [164], the epigenetics new frontier [165].
Conclusions
It has already been twenty-four years since the first description of MeCP2 [16, 17]. For a long time now we have been trying to decipher its mysteries. Significant progress has been made, yet many ‘heavy enigmas’ still remain. In this review, we have analysed the relevance and potential implications of this chromosomal protein, an abundant transcription regulator in neurons, for its architectural and functional role within the chromatin context. It is clear that in neuronal nuclei, MeCP2 is very weakly bound to chromatin, as a highly significant part of it is easily detached under very low ionic strength conditions, and it is bound to highly accessible nuclease domains [9]. We propose a model (Fig. 4) to account for these observations and their apparent disparity with the originally repressive role assigned to the protein. Whether the model is correct will require further experimental evidence. It will be critical at this point to be able to distinguish between the two MeCP2-E1 and MeCP2-E2 isoforms.
Finally, and although the broad attention by the scientific community to MeCP2 was triggered by the discovery of its massive involvement in RTT [2], a neurodevelopmental disease of autistic characteristics, its massive presence in the brain, and in neurons in particular, indicate its potential for a broader group of brain pathologies [4]. To underscore this, we have focused here on a brain disease (MDD) and on the neuronal disturbances resulting from cocaine abuse, both of which are highly pervasive issues within our society. A substantially large amount of information about some of the molecular details involved has been gathered for both of them. They provide an excellent example of how some of the critical epigenetic components that operate in the brain (DNA methylation, MeCP2 phosphorylation, histone acetylation and microRNAs) are intertwined. Similar mechanisms can be envisioned to participate in many other neurological disorders, and are currently being deciphered [4]. It will be important to take into consideration the relevant gender epigenetic differences [165].
Abbreviations
- Avp :
-
arginine vasopressin gene
- Bdnf:
-
brain-derived neurotrophic factor
- BER:
-
base-excision repair
- Camk2:
-
Calcium/Calmodulin-Dependent Protein Kinase II
- CBP:
-
CREB-binding protein a histone acetyl transferase
- Cdkl5:
-
cyclin-dependent kinase-like 5
- ChIP:
-
chromatin immuno-precipitation
- CREB:
-
cAMP response element-binding protein
- Crh :
-
corticotropin releasing hormone gene
- Dnmt:
-
DNA methyl-transferases
- ELS:
-
early life stress
- Fos:
-
FBJ murine osteosarcoma
- FRAP:
-
fluorescence recovery after photobleaching
- HDAC:
-
histone de-acetylase
- Hipk2:
-
homeodomain-interacting protein kinase 2
- Hipk2:
-
homeodomain-interacting protein kinase 2
- HMGN:
-
high mobility group nucleosomal
- HPA:
-
hypothalamic-pituitary-adrenal
- Igf2:
-
insulin-like growth factor 2
- MBD:
-
methyl binding domain
- MDD:
-
major depressive disorder
- MeCP2:
-
methyl CpG binding protein 2
- MEF2C:
-
factor myocyte enhancer factor 2C
- miRNA:
-
microRNA
- MRE:
-
miRNA response elements
- NAc:
-
nucleus acumbens
- NCoR:
-
the nuclear receptor co-repressor
- NCP:
-
nucleosome core particle
- Npas4:
-
neuronal PAS domain pritein 4
- NRL:
-
nucleosome repeat length
- PEST:
-
enriched in proline, glutamic, serine and threonine
- Pomc :
-
Proopiomelanocortin gene
- PTMs:
-
post-translational modifications
- RNF4:
-
RING finger protein 4
- RTT:
-
Rett syndrome
- Tet:
-
ten eleven translocation
- UPS:
-
ubiquitin proteasome system
References
Fulcanelli. Le mystère des cathédrales et l’interprétation ésotérique des symboles hermétiques du grand œuvre. Paris: chez Jean-Jacques Pauvert; 1925.
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature genetics. 1999;23:185–8.
Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–9.
Ausió J, de Paz A, Esteller M. MeCP2: the long trip from a chromatin protein to neurological disorders. Trends Mol Med. 2014;20:487–98.
Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annual review of cell and developmental biology. 2011;27:631–52.
Adams VH, McBryant SJ, Wade PA, Woodcock CL, Hansen JC. Intrinsic disorder and autonomous domain function in the multifunctional nuclear protein, MeCP2. The Journal of biological chemistry. 2007;282:15057–64.
Bellini E, Pavesi G, Barbiero I, Bergo A, Chandola C, Nawaz MS, et al. MeCP2 post-translational modifications: a mechanism to control its involvement in synaptic plasticity and homeostasis? Front Cell Neurosci. 2014;8:236.
Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Molecular cell. 2010;37:457–68.
Thambirajah AA, Ng MK, Frehlick LJ, Li A, Serpa JJ, Petrotchenko EV, et al. MeCP2 binds to nucleosome free (linker DNA) regions and to H3K9/H3K27 methylated nucleosomes in the brain. Nucleic acids research. 2011;40:2884–97.
Chen L, Chen K, Lavery LA, Baker SA, Shaw CA, Li W, et al. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:5509–14.
Su M, Hong J, Zhao Y, Liu S, Xue X. MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA132 in rats with depression. Mol Med Rep. 2015.
Feng J, Nestler EJ. MeCP2 and drug addiction. Nature neuroscience. 2010;13:1039–41.
Antequera F, Macleod D, Bird AP. Specific protection of methylated CpGs in mammalian nuclei. Cell. 1989;58:509–17.
Boyes J, Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991;64:1123–34.
Meehan RR, Lewis JD, McKay S, Kleiner EL, Bird AP. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989;58:499–507.
Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–14.
Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic acids research. 1992;20:5085–92.
Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 Binds to 5hmC Enriched within Active Genes and Accessible Chromatin in the Nervous System. Cell. 2012;151:1417–30.
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science (New York NY). 2008;320:1224–9.
Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19416–21.
Mnatzakanian GN, Lohi H, Munteanu I, Alfred SE, Yamada T, MacLeod PJ, et al. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nature genetics. 2004;36:339–41.
Kriaucionis S, Bird A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic acids research. 2004;32:1818–23.
Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Human molecular genetics. 2002;11:115–24.
Abuhatzira L, Shamir A, Schones D, Schäffer A, Bustin M. The chromatin-binding protein HMGN1 regulates the expression of methyl CpG-binding protein 2 (MECP2) and affects the behavior of mice. J Biol Chem. 2011;286:42051–62.
Zweier M, Gregor A, Zweier C, Engels H, Sticht H, Wohlleber E, et al. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Human mutation. 2010;31:722–33.
Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nature neuroscience. 2007;10:1513–4.
Chen Y, Shin B, Thamotharan S, Devaskar S. Differential methylation of the micro-RNA 7b gene targets postnatal maturation of murine neuronal Mecp2 gene expression. Dev Neurobiol. 2014;74:407–25.
Han K, Gennarino VA, Lee Y, Pang K, Hashimoto-Torii K, Choufani S, et al. Human-specific regulation of MeCP2 levels in fetal brains by microRNA miR-483-5p. Genes & development. 2013;27:485–90.
Bofill-De Ros X, Santos M, Vila-Casadesus M, Villanueva E, Andreu N, Dierssen M, et al. Genome-wide miR-155 and miR-802 target gene identification in the hippocampus of Ts65Dn Down syndrome mouse model by miRNA sponges. BMC genomics. 2015;16:907.
Hutchison E, Kawamoto E, Taub D, Lal A, Abdelmohsen K, Zhang Y, et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61:1018–28.
Ho KL, McNae IW, Schmiedeberg L, Klose RJ, Bird AP, Walkinshaw MD. MeCP2 binding to DNA depends upon hydration at methyl-CpG. Molecular cell. 2008;29:525–31.
Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, et al. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4882–7.
Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–69.
Chao HT, Zoghbi HY. The yin and yang of MeCP2 phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4577–8.
Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Human molecular genetics. 2004;13:2679–89.
Martinez de Paz A, Vicente Sanchez-Mut J, Samitier-Marti M, Petazzi P, Saez M, Szczesna K, et al. Circadian cycle-dependent MeCP2 and brain chromatin changes. PloS one. 2015;10:e0123693.
Lombardi LM, Baker SA, Zoghbi HY. MECP2 disorders: from the clinic to mice and back. The Journal of clinical investigation. 2015;125:2914–23.
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature genetics. 1998;19:187–91.
Ashraf SI, Ip YT. Transcriptional control: repression by local chromatin modification. Curr Biol. 1998;8:R683–686.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. The New England journal of medicine. 2003;349:2042–54.
Jones PA, Laird PW. Cancer epigenetics comes of age. Nature genetics. 1999;21:163–7.
Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature reviews Genetics. 2007;8:286–98.
Lopez-Serra L, Ballestar E, Ropero S, Setien F, Billard LM, Fraga MF, et al. Unmasking of epigenetically silenced candidate tumor suppressor genes by removal of methyl-CpG-binding domain proteins. Oncogene. 2008;27:3556–66.
Di Fiore B, Palena A, Felsani A, Palitti F, Caruso M, Lavia P. Cytosine methylation transforms an E2F site in the retinoblastoma gene promoter into a binding site for the general repressor methylcytosine-binding protein 2 (MeCP2). Nucleic acids research. 1999;27:2852–9.
Neupane M, Clark AP, Landini S, Birkbak NJ, Eklund AC, Lim E, et al. MECP2 Is a Frequently Amplified Oncogene with a Novel Epigenetic Mechanism that Mimics the Role of Activated RAS in Malignancy. Cancer Discov. 2016;6:45–58.
Mayer S, Gilsbach R, Preissl S, Monroy Ordonez EB, Schnick T, Beetz N, et al. Adrenergic Repression of the Epigenetic Reader MeCP2 Facilitates Cardiac Adaptation in Chronic Heart Failure. Circ Res. 2015;117:622–33.
Zimmermann CA, Hoffmann A, Raabe F, Spengler D. Role of mecp2 in experience-dependent epigenetic programming. Genes (Basel). 2015;6:60–86.
WHO. 2001. Mental health. A call for action by World Health Ministers. Ministerial Round Tables 54th World Health Assembly 2001.
Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53:849–57.
Cornish JW, O'Brien CP. Crack cocaine abuse: an epidemic with many public health consequences. Annu Rev Public Health. 1996;17:259–73.
Gonzalez-Romero R, Ausio J. dBigH1, a second histone H1 in, and the consequences for histone fold nomenclature. Epigenetics. 2014;9.
Woodcock CL, Skoultchi AI, Fan Y. Role of linker histone in chromatin structure and function: H1 stoichiometry and nucleosome repeat length. Chromosome Res. 2006;14:17–25.
Zhou BR, Jiang J, Feng H, Ghirlando R, Xiao TS, Bai Y. Structural Mechanisms of Nucleosome Recognition by Linker Histones. Molecular cell. 2015;59:628–38.
Cheema SM, Ausió J. The Structural Determinants behind the Epigenetic Role of Histone Variants. Genes (Basel). 2015;6:688–713.
van Holde KE. Chromatin. NY: Springer-Verlag; 1988.
Marzluff WF, Wagner EJ, Duronio RJ. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nature reviews Genetics. 2008;9:843–54.
Waterborg JH. Evolution of histone H3: emergence of variants and conservation of post-translational modification sites. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2012;90:79–95.
Mannironi C, Bonner WM, Hatch CL. H2A.X. a histone isoprotein with a conserved C-terminal sequence, is encoded by a novel mRNA with both DNA replication type and polyA 3' processing signals. Nucleic acids research. 1989;17:9113–26.
Banday AR, Baumgartner M, Al Seesi S, Karunakaran DK, Venkatesh A, Congdon S, et al. Replication-dependent histone genes are actively transcribed in differentiating and aging retinal neurons. Cell cycle (Georgetown, Tex). 2014;13:2526–41.
Pina B, Suau P. Changes in histones H2A and H3 variant composition in differentiating and mature rat brain cortical neurons. Developmental biology. 1987;123:51–8.
Bosch A, Suau P. Changes in core histone variant composition in differentiating neurons: the roles of differential turnover and synthesis rates. European journal of cell biology. 1995;68:220–5.
Zovkic IB, Paulukaitis BS, Day JJ, Etikala DM, Sweatt JD. Histone H2A.Z subunit exchange controls consolidation of recent and remote memory. Nature. 2014;515:582–6.
Maze I, Wenderski W, Noh KM, Bagot RC, Tzavaras N, Purushothaman I, et al. Critical Role of Histone Turnover in Neuronal Transcription and Plasticity. Neuron. 2015;87:77–94.
Huang H, Sabari BR, Garcia BA, Allis CD, Zhao Y. SnapShot: Histone Modifications. Cell. 2014;159:458–8. e451.
Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5.
Henikoff S, Smith MM. Histone variants and epigenetics. Cold Spring Harbor perspectives in biology. 2015;7:a019364.
Maze I, Noh KM, Soshnev AA, Allis CD. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nature reviews Genetics. 2014;15:259–71.
Calestagne-Morelli A, Ausio J. Long-range histone acetylation: biological significance, structural implications, and mechanisms. Biochem Cell Biol. 2006;84:518–27.
Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science (New York NY). 2006;311:844–7.
Maze I, Noh KM, Allis CD. Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacology. 2013;38:3–22.
Gabel HW, Kinde B, Stroud H, Gilbert CS, Harmin DA, Kastan NR, et al. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature. 2015;522:89–93.
Shahbazian MD, Zoghbi HY. Rett syndrome and MeCP2: linking epigenetics and neuronal function. American journal of human genetics. 2002;71:1259–72.
Jaeger AW, Kuenzle CC. The chromatin repeat length of brain cortex and cerebellar neurons changes concomitant with terminal differentiation. The EMBO journal. 1982;1:811–6.
Pearson EC, Bates DL, Prospero TD, Thomas JO. Neuronal nuclei and glial nuclei from mammalian cerebral cortex. Nucleosome repeat lengths, DNA contents and H1 contents. European journal of biochemistry/FEBS. 1984;144:353–60.
Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–81.
Georgel PT, Horowitz-Scherer RA, Adkins N, Woodcock CL, Wade PA, Hansen JC. Chromatin compaction by human MeCP2. Assembly of novel secondary chromatin structures in the absence of DNA methylation. The Journal of biological chemistry. 2003;278:32181–8.
Cestelli A, Di Liegro I, Castiglia D, Gristina R, Ferraro D, Salemi G, et al. Triiodothyronine-induced shortening of chromatin repeat length in neurons cultured in a chemically defined medium. Journal of neurochemistry. 1987;48:1053–9.
Singleton MK, Gonzales ML, Leung KN, Yasui DH, Schroeder DI, Dunaway K, et al. MeCP2 is required for global heterochromatic and nucleolar changes during activity-dependent neuronal maturation. Neurobiology of disease. 2011;43:190–200.
Manuelidis L. Different central nervous system cell types display distinct and nonrandom arrangements of satellite DNA sequences. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3123–7.
Payen E, Verkerk T, Michalovich D, Dreyer SD, Winterpacht A, Lee B, et al. The centromeric/nucleolar chromatin protein ZFP-37 may function to specify neuronal nuclear domains. The Journal of biological chemistry. 1998;273:9099–109.
Casafont I, Navascues J, Pena E, Lafarga M, Berciano MT. Nuclear organization and dynamics of transcription sites in rat sensory ganglia neurons detected by incorporation of 5'-fluorouridine into nascent RNA. Neuroscience. 2006;140:453–62.
Stuss DP, Cheema M, Ng MK, Martinez De Paz A, Williamson B, Missiaen K, et al. Impaired in vivo binding of MeCP2 to chromatin in the absence of its DNA methyl-binding domain. Nucleic acids research. 2013;41:4888–900.
Kumar A, Kamboj S, Malone BM, Kudo S, Twiss JL, Czymmek KJ, et al. Analysis of protein domains and Rett syndrome mutations indicate that multiple regions influence chromatin-binding dynamics of the chromatin-associated protein MECP2 in vivo. Journal of cell science. 2008;121:1128–37.
Bodetto SP, Romieu P, Sartori M, Tesone-Coelho C, Majchrzak M, Barbelivien A, et al. Differential regulation of MeCP2 and PP1 in passive or voluntary administration of cocaine or food. Int J Neuropsychopharmacol. 2014;17:2031–44.
Thambirajah AA, Eubanks JH, Ausio J. MeCP2 post-translational regulation through PEST domains: two novel hypotheses: potential relevance and implications for Rett syndrome. Bioessays. 2009;31:561–9.
Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends in Biochemical Sciences. 1996;21:267–71.
Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science (New York, NY. 1986;234:364–8.
Wang Y. RING finger protein 4 (RNF4) derepresses gene expression from DNA methylation. The Journal of biological chemistry. 2014;289:33808–13.
Gonzales ML, Adams S, Dunaway KW, LaSalle JM. Phosphorylation of distinct sites in MeCP2 modifies cofactor associations and the dynamics of transcriptional regulation. Molecular and cellular biology. 2012;32:2894–903.
Guo JU, Su Y, Zhong C, Ming GL, Song H. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell cycle (Georgetown Tex). 2011;10:2662–8.
Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–34.
Yu H, Su Y, Shin J, Zhong C, Guo JU, Weng YL, et al. Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Nature neuroscience. 2015;18:836–43.
Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nature neuroscience. 2010;13:423–30.
Taricani L, Shanahan F, Pierce RH, Guzi TJ, Parry D. Phenotypic enhancement of thymidylate synthetase pathway inhibitors following ablation of Neil1 DNA glycosylase/lyase. Cell cycle (Georgetown Tex). 2010;9:4876–83.
Dong H, Shi Q, Song X, Fu J, Hu L, Xu D, et al. Polychlorinated biphenyl quinone induces oxidative DNA damage and repair responses: The activations of NHEJ, BER and NER via ATM-p53 signaling axis. Toxicol Appl Pharmacol. 2015;286:10–6.
Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science (New York, NY). 2003;302:885–9.
Bracaglia G, Conca B, Bergo A, Rusconi L, Zhou Z, Greenberg M, et al. Methyl-CpG-binding protein 2 is phosphorylated by homeodomain-interacting protein kinase 2 and contributes to apoptosis. EMBO Rep. 2009;10:1327–33.
Ebert D, Gabel H, Robinson N, Kastan N, Hu L, Cohen S, et al. Activity-dependent phosphorylation of MeCP2 threonine 308 regulates interaction with NCoR. Nature. 2013;499:341–5.
Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, et al. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59.
Garcia Ramirez M, Rocchini C, Ausió J. Modulation of chromatin folding by histone acetylation. J Biol Chem. 1995;270:17923–8.
Wang X, He C, Moore SC, Ausió J. Effects of histone acetylation on the solubility and folding of the chromatin fiber. J Biol Chem. 2001;276:12764–8.
Ridsdale JA, Hendzel MJ, Delcuve GP, Davie JR. Histone acetylation alters the capacity of the H1 histones to condense transcriptionally active/competent chromatin. The Journal of biological chemistry. 1990;265:5150–6.
Ishibashi T, Thambirajah AA, Ausio J. MeCP2 preferentially binds to methylated linker DNA in the absence of the terminal tail of histone H3 and independently of histone acetylation. FEBS letters. 2008;582:1157–62.
Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett R, et al. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–5.
Wu H, Tao J, Chen PJ, Shahab A, Ge W, Hart RP, et al. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18161–6.
Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79.
Holmes SJ, Robins LN. The influence of childhood disciplinary experience on the development of alcoholism and depression. J Child Psychol Psychiatry. 1987;28:399–415.
Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological psychiatry. 2001;49:1023–39.
Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature neuroscience. 2007;10:1089–93.
Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–35.
Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nature reviews Neuroscience. 2007;8:355–67.
Telese F, Gamliel A, Skowronska-Krawczyk D, Garcia-Bassets I, Rosenfeld MG. "Seq-ing" insights into the epigenetics of neuronal gene regulation. Neuron. 2013;77:606–23.
Day JJ, Kennedy AJ, Sweatt JD. DNA methylation and its implications and accessibility for neuropsychiatric therapeutics. Annual review of pharmacology and toxicology. 2015;55:591–611.
Qureshi IA, Mehler MF. Understanding neurological disease mechanisms in the era of epigenetics. JAMA neurology. 2013;70:703–10.
Mehler MF. Epigenetics and the nervous system. Annals of neurology. 2008;64:602–17.
Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, et al. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–9.
Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet neurology. 2009;8:1056–72.
Landgrave-Gomez J, Mercado-Gomez O, Guevara-Guzman R. Epigenetic mechanisms in neurological and neurodegenerative diseases. Front Cell Neurosci. 2015;9:58.
Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends in neurosciences. 2013;36:3–13.
Schmidt MV, Abraham WC, Maroun M, Stork O, Richter-Levin G. Stress-induced metaplasticity: from synapses to behavior. Neuroscience. 2013;250:112–20.
consortium C. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–91.
Ledford H. First robust genetic links to depression emerge. Nature. 2015;523:268–9.
Szalavitz M. Genetics: No more addictive personality. Nature. 2015;522:S48–49.
Cortes-Mendoza J, Diaz de Leon-Guerrero S, Pedraza-Alva G, Perez-Martinez L. Shaping synaptic plasticity: the role of activity-mediated epigenetic regulation on gene transcription. Int J Dev Neurosci. 2013;31:359–69.
Parker D, Ferreri K, Nakajima T, LaMorte VJ, Evans R, Koerber SC, et al. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Molecular and cellular biology. 1996;16:694–703.
Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annual review of neuroscience. 2008;31:563–90.
Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature neuroscience. 2009;12:1559–66.
Murgatroyd C, Wu Y, Bockmuhl Y, Spengler D. Genes learn from stress: how infantile trauma programs us for depression. Epigenetics. 2010;5:194–9.
Wu Y, Patchev AV, Daniel G, Almeida OF, Spengler D. Early-life stress reduces DNA methylation of the Pomc gene in male mice. Endocrinology. 2014;155:1751–62.
Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Molecular psychiatry. 2011;16:1088–95.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9:519–25.
Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, Berlim MT, et al. Epigenetic regulation of BDNF expression according to antidepressant response. Molecular psychiatry. 2013;18:398–9.
Bagot RC, Labonte B, Pena CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues in clinical neuroscience. 2014;16:281–95.
Wang X, Sundquist K, Hedelius A, Palmer K, Memon AA, Sundquist J. Circulating microRNA-144-5p is associated with depressive disorders. Clinical epigenetics. 2015;7:69.
Brents LK, Tripathi SP, Young J, James GA, Kilts CD. The role of childhood maltreatment in the altered trait and global expression of personality in cocaine addiction. Journal of psychiatric research. 2015;64:23–31.
Lewis CR, Staudinger K, Scheck L, Olive MF. The Effects of Maternal Separation on Adult Methamphetamine Self-Administration, Extinction, Reinstatement, and MeCP2 Immunoreactivity in the Nucleus Accumbens. Front Psychiatry. 2013;4:55.
Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues in clinical neuroscience. 2014;16:335–44.
Zwiller J. [Epigenetics and drug addiction: a focus on MeCP2 and on histone acetylation]. Med Sci (Paris). 2015;31:439–46.
Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2010;35:2450–61.
Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, et al. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 2015;35:8042–58.
Feng J, Shao N, Szulwach KE, Vialou V, Huynh J, Zhong C, et al. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nature neuroscience. 2015;18:536–44.
Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich JB, et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–92.
McCarthy DM, Brown AN, Bhide PG. Regulation of BDNF expression by cocaine. Yale J Biol Med. 2012;85:437–46.
Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–44.
Deng JV, Wan Y, Wang X, Cohen S, Wetsel WC, Greenberg ME, et al. MeCP2 phosphorylation limits psychostimulant-induced behavioral and neuronal plasticity. J Neurosci. 2014;34:4519–27.
Mao LM, Horton E, Guo ML, Xue B, Jin DZ, Fibuch EE, et al. Cocaine increases phosphorylation of MeCP2 in the rat striatum in vivo: a differential role of NMDA receptors. Neurochemistry international. 2011;59:610–7.
Carouge D, Host L, Aunis D, Zwiller J, Anglard P. CDKL5 is a brain MeCP2 target gene regulated by DNA methylation. Neurobiology of disease. 2010;38:414–24.
Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 2013;38:94–110.
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14.
Renthal W, Kumar A, Xiao G, Wilkinson M, Covington 3rd HE, Maze I, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–48.
Kan PY, Caterino TL, Hayes JJ. The H4 tail domain participates in intra- and internucleosome interactions with protein and DNA during folding and oligomerization of nucleosome arrays. Molecular and cellular biology. 2009;29:538–46.
Pepenella S, Murphy KJ, Hayes JJ. A distinct switch in interactions of the histone H4 tail domain upon salt-dependent folding of nucleosome arrays. The Journal of biological chemistry. 2014;289:27342–51.
Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature neuroscience. 2010;13:1120–7.
Bali P, Kenny PJ. MicroRNAs and Drug Addiction. Frontiers in genetics. 2013;4:43.
Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–37.
Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med. 2001;31:605–16.
Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47.
Kigar SL, Auger AP. Epigenetic mechanisms may underlie the aetiology of sex differences in mental health risk and resilience. J Neuroendocrinol. 2013;25:1141–50.
Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, Houston I, et al. Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Experimental neurology. 2014;261:21–9.
Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biology of sex differences. 2014;5:8.
Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–20.
Chung WC, Auger AP. Gender differences in neurodevelopment and epigenetics. Pflugers Archiv : European journal of physiology. 2013;465:573–84.
McCarthy MM, Nugent BM. At the frontier of epigenetics of brain sex differences. Front Behav Neurosci. 2015;9:221.
Acknowledgements
I wish to thank Marlee K. Ng for the experimental elaboration of the data shown in Fig. 3 and Abby Truman for editing the manuscript. This grant was supported by a Canadian Institute of Health Research (CIHR) grant (MOP -130417]) to JA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that he has no competing interests.
Author information
JA is a Professor at the Department of Biochemistry and Microbiology at the University of Victoria. BC. Canada.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ausió, J. MeCP2 and the enigmatic organization of brain chromatin. Implications for depression and cocaine addiction. Clin Epigenet 8, 58 (2016). https://doi.org/10.1186/s13148-016-0214-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-016-0214-5