Abstract
Background
Hypertension is a multifactorial disease caused by the interaction between genetic and environmental factors. Mutations in the methylenetetrahydrofolate reductase gene (MTHFR) have been known to be associated with the risk of cardiovascular disease as well as hypertension. This case–control study was conducted out to measure the association of the polymorphism C677T of MTHFR with the risk of hypertension.
Methods
Polymerase chain reaction followed by restriction fragment analysis length was used to identify MTHFR C677T genotypes in patients 101 patients and 102 age and sex matched healthy controls. Odds ratio with 95 % confidence interval was used to assess the risk of association.
Results
The distribution of demographic and clinical features of patients showed no particular trend (p > 0.05). However, the frequency of homozygous 677T allele was higher in patients with a family history of heart disease (30.4 vs. 9 %, p = 0.031). Interestingly, the mutant 677TT genotype was significantly associated with the susceptibility of hypertension when compared to the wild type 677CC genotype (OR 5.4, CI 1.4–19.8, p = 0.008). In addition, the recessive model 677TT vs. 677CC/CT was found to be associated with the risk of hypertension (OR 5.3, CI 1.5–19.1, p = 0.005). However, the dominant model was not associated with the risk of hypertension in our cohort (OR 1.3, CI 0.7–2.2, p = 0.4).
Conclusions
Based on our findings, the homozygous mutant for 677TT of MTHFR gene is associated with the risk of hypertension in our population. Further studies with larger sample sizes are needed to confirm the results of this study.
Similar content being viewed by others
Background
Hypertension is a major public health concern in the world. It is found to be the main factor in the occurrence of myocardial infarction, stroke, cardiac and renal failure and later lesions of the retina of the eyes [1, 2]. In Morocco, the prevalence of hypertension in men and women is 30.2 and 37 %, respectively, with an overall prevalence of 33.6 % [3, 4]. The etiology of hypertension, like most of multifactorial diseases is not known. However, the pathogenesis of hypertension can be explained by the interaction between many factors such as sodium intake, overweight, alcohol, smoke and the genetic background of subjects [5, 6]. Many authors have shown the fundamental role of genetic factors not only in the development of hypertension, but also in the individualization of treatment [7, 8]. Methylenetetrahydrofolate reductase gene (MTHFR) is particularly involved in the metabolism of homocysteine and folate by catalyzing the 5, 10-methylenetetrahydrofolate into 5-methylenetetrahydrofolate. The MTHFR gene has been mapped to chromosome 1p36.3 and encodes an enzyme composed of 656 amino acids [9]. It was reported that, conditions such as hyperhomocysteinemia and homocystinuria are associated with mutations in the MTHFR gene. The single nucleotide polymorphism 677C > T (Alanine222Valine) of MTHFR reduces the enzymatic activity [10]. As a result, individuals this mutation are exposed to a high level of homocysteine in plasma and are more likely to develop cardiovascular disease, due to the absence of conversion of homocysteine to methionine by 5-methylenetetrahydrofolate [11, 12]. It has been shown that the MTHFR enzyme activity decrease is attributable to the loss of cofactor of Riboflavin. Thus, the supplementation of this latter, contributes to the reduction of hyperhomocysteinemia in hypertensive patients with the variant of MTHFR [13–15]. A recent study showed that supplementation with Riboflavin in hypertensive patients carrying the homozygous genotype mutant 677TT of MTHFR, without other cardiovascular diseases, has considerably improved the value of systolic blood pressure, paving the way for personalized medicine [16]. It is worth noting, that numerous studies investigated the relationship between the C677T polymorphism of MTHFR and many diseases, including breast cancer [17, 18], colorectal cancer [19], ischemic stroke [20, 21], inflammatory bowel disease [22] and hypertension [7, 23, 24]. In the light of the limited data on the variants of the MTHFR and hypertension in our population, and the role of vitamin B2 in the improvement of blood pressure in hypertensive patients with a mutant variant of the MTHFR gene, this case–control study was carried out to investigate the association of 677C > T polymorphism of MTHFR with the risk of hypertension in a sample of the Moroccan population.
Methods
Study population
The study included 101 outpatients (77 females and 24 males) with a mean age of 61.6 ± 9 (range 40–87 years) and 102 age and sex matched unrelated healthy control subjects with a mean age of 59.24 ± 10.7 (range 40–87 years). Patients have been confirmed with hypertension by cardiologists according to the criteria of International Society of Hypertension [systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg] [25]. Patients were recruited from the department of cardiology of the Ibn Rochd University Hospital in Casablanca, Morocco from 2011 to 2013. The control group was normotensive after medical examination (SBP < 140 mmHg or DBP < 90 mmHg) without any family history of cardiovascular, diabetes and thyroid diseases and was recruited at the laboratory of genetic and molecular diseases, Faculty of Medicine, the Hassan II University in Casablanca. The control subjects were composed of employees working at the faculty of medicine. The study received the approval from the local Ethics Committee and all participants accepted and signed the informed consent. Five milliliters of venous blood were collected from each participant in an EDTA tube for the genotyping of the MTHFR gene.
Identification of MTHFR C677T polymorphism
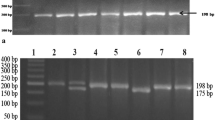
Genomic DNA was extracted from white blood cells by using the salting-out method described previously by Miller et al. [26]. Polymerase chain reaction followed by the restriction fragment length polymorphism was used to genotype the C677T polymorphism of MTHFR. The conditions of amplification and digestion have been well documented previously in our laboratory [19]. The digested PCR products after separation on a 3 % agarose gel, stained with ethidium bromide, showed one band of 198 bp corresponding to the wild type homozygous (CC), three bands of 198, 175 and 23 bp for the heterozygous (CT), two bands of 175 and 23 bp for the mutated homozygous (TT).
Statistical analysis
The statistical package for the social sciences SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. The difference in the distribution of demographic data, clinical characteristics of patients as well as their family history and MTHFR C677T genotypes were conducted by Fisher’s exact test. Hardy–Weinberg equilibrium in the cases and controls was evaluated by Chi square test. The estimation of the association between MTHFR C677T profiles and the risk of hypertension was evaluated by Fisher’s exact test followed by the calculation of an odds ratio (OR) with 95 % confidence interval. Statistical tests were considered significant when the p value was less than 0.05.
Results
The present case–control study assessed the distribution of the C677T polymorphism of MTHFR in 101 patients with hypertension and 102 controls without any history of hypertension. The frequencies of alleles and genotypes of C677T in patients and controls did not deviate from Hardy–Weinberg equilibrium (p > 0.05). As shown in Table 1, the distribution of CC, CT and TT genotypes in patients with respect to demographic data and clinical features (gender, age, body mass index, diabetes, dyslipidemia, risk stage, smoking habit) did not show a particular trend (p > 0.05). The stratification of patients according to their family history showed that carriers of mutated homozygous TT with a family history of heart disease were significantly associated with the risk of hypertension (30.4 vs. 9 %, p = 0.031). The distribution of other family histories such as hypertension, stroke, heart disease, diabetes, kidney disease and dyslipidemia were comparable between the different genotypes of MTHFR C677T with p > 0.05 (Table 2). In Table 3, the frequency of the homozygous wild type CC was higher in the control group than in patients (52.9 vs. 46.5 %). However, the frequency of the mutant TT was higher in cases than controls (13.9 vs. 2.9 %) and the TT genotype was found to be statistically associated with the risk of hypertension when compared with carriers of homozygous wild type CC (OR 5.4, CI 1.4–19.8, p = 0.008). Furthermore, the comparison of TT to CC/CT carriers in the recessive model showed the same trends with a risk of 5.3 (p = 0.005). However, the dominant model did not show any particular effect concerning the risk of hypertension. The frequency of mutant 677T allele was higher in cases compared to controls (34 vs. 25 %), but not significantly.
Discussion
Hypertension is a multifactorial disease that involves both genetic and environmental factors and it is known to be a common risk factor for kidney and heart complications. Hereditary genetic differences in MTHFR gene and the risk of hypertension have been discussed by many authors with conflicting results. In the current study, the genotype correlation of patients with regard to parameters such as gender, age, body mass index, diabetes, dyslipidemia, the stage of risk and smoking showed no significant trend. Similar results were reported by Gay et al. in the Chinese population [27]. The frequency of the homozygous mutant 677TT was statistically higher in patients with a history of heart disease in the family. This finding might help in the genetic counseling. However, other family histories showed a comparable distribution among the different genotypes of MTHFR C677T. However, Alghasham et al. in Saudi Arabia showed no association of the patient’s family history and MTHFR C677T genotypes [28]. We found that, the frequency of the wild type homozygous CC was higher in controls than in patients and subjects carriers of 677TT were associated with an increased risk hypertension (OR 5.4, p = 0.008). In addition, in the recessive model, we noticed that patients with the 677TT genotype were significantly associated with the risk of hypertension (OR 5.3, p = 0.005). Markan et al. reported similar observations in the Indian population [29]. Yan et al. also reported in a meta-analysis a strong association of MTHFR C677T with the risk of hypertension among Asians, Caucasians and Chinese subjects [30]. Similar findings were obtained in the population of Turkey [7]. Many authors have reported that the MTHFR C677T polymorphism has been found to be an independent factor of hypertension in different ethnic groups [31–33] as well as of severe diastolic hypertension in pregnant women [34]. These above studies showed that the MTHFR C677T polymorphism is associated with a high level of homocysteine. This study did not assess the level of homocysteine. However, a previous study conducted by Bennouar et al. in 2007 showed that the 677TT genotype was statistically higher in subjects with coronary artery disease and was found to be associated with hyperhomocysteinemia in Morocco [35]. In this study, it should be noted some limitations. First, our sample size was small, which reduces the statistical power. Secondly, the absence of the determination of homocysteine in patients and controls failed to establish the correlation between the variants of MTHFR and the values of homocysteine. Thirdly, hypertension is a complex disease; other candidate genes may contribute to the susceptibility of hypertension. With respect to these limitations, the results of this study should be interpreted with caution. Other studies of large sample, overcoming the current limitations might be interesting in the understanding of the role of candidate genes in the susceptibility of hypertension in our population.
Conclusion
We have looked the influence of MTHFR C677T and the risk of hypertension in a sample of the Moroccan population. We have shown that, the homozygous mutant 677TT was significantly associated with the risk of hypertension. Additionally, the recessive model showed the same trend. Considering the size of our sample, further studies will be needed to confirm our findings.
References
Messerli F, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603.
Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010;55:1210–6.
Tazi MA, Abir-Khalil S, Chaouki N, Cherqaoui S, Lahmouz F, Sraïri JE, et al. Prevalence of the main cardiovascular risk factors in Morocco: results of a National Survey, 2000. J Hypertens. 2003;21:897–903.
Ziyyat A, Ramdani N, El N, Bouanani H, Vanderpas J, Hassani B, et al. Epidemiology of hypertension and its relationship with type 2 diabetes and obesity in eastern Morocco. Springerplus. 2014;30:644. doi:10.1186/2193-1801-3-644.
Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–201.
Mein CA, Caulfield MJ, Dobson RJ, Munroe PB. Genetics of essential hypertension. Hum Mol Genet. 2004;13(Spec No 1):R169–75 (Epub 2004 Feb 5).
Bayramoglu A, Urhan Kucuk M, Guler HI, Abaci O, Kucukkaya Y, Colak E. Is there any genetic predisposition of MMP-9 gene C1562T and MTHFR gene C677T polymorphisms with essential hypertension? Cytotechnology. 2015;67:115–22.
Fontana V, Luizon MR, Sandrim VC. An update on the pharmacogenetics of treating hypertension. J Hum Hypertens. 2015;29:283–91.
Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, et al. Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet. 1994;7:195–200.
Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43:414–21.
Moat SJ, Doshi SN, Mcdowell IFW. Folate, homocysteine, endothelial function and cardiovascular disease. What is the link? Biomed Pharmacother. 2001;55:425–33.
Guilland J-C, Favier A, Potier de Courcy G, Galan P, Hercberg S. Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease? 2. Epidemiological data. Pathol Biol (Paris). 2003;51:111–21.
Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, Ludwig ML. The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat Struct Biol. 1999;6:359–65.
McNulty H, Dowey LRC, Strain JJ, Dunne A, Ward M, Molloy AM, et al. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C→T polymorphism. Circulation. 2006;113:74–80.
Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA. 2001;98:14853–8.
Wilson CP, McNulty H, Ward M, Strain JJ, Trouton TG, Hoeft BA, et al. Blood pressure in treated hypertensive individuals with the MTHFR 677TT genotype is responsive to intervention with riboflavin: findings of a targeted randomized trial. Hypertension. 2013;61:1302–8.
Diakite B, Tazzite A, Hamzi K, Jouhadi H, Nadifi S. Methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Moroccan women. Afr Health Sci. 2012;12:204–9.
Ergul E, Sazci A, Utkan Z, Canturk NZ. Polymorphisms in the MTHFR gene are associated with breast cancer. Tumour Biol. 2003;24:286–90.
Diakite B, Benmoussa A, Hamzi K, Jouhadi H, Nadifi S. Cancer colorectal et polymorphisme du méthylènetétrahydrofolate réductase (C677T) au Maroc. Journal Africain du Cancer. 2012;4:238–44.
Hamzi K, Tazzite A, Nadifi S. Large-scale meta-analysis of genetic studies in ischemic stroke: Five genes involving 152,797 individuals. Indian J Hum Genet. 2011;17:212–7.
They-They TP, Battas O, Nadifi S. Synergistic effect of MTHFR C677T and F2 G20210A polymorphisms on ischemic stroke. Neurosci Bull. 2013;29:725–30.
Senhaji N, Serbati N, Diakité B, Arazzakou S, Hamzi K, Badre W, et al. Methylenetetrahydrofolate reductase C677T variant in Moroccan patients with inflammatory bowel disease. Gene. 2013;521:45–9.
Wu Y, Hu C, Lu S, Gong F, Feng F, Qian Z, et al. Association between methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphisms and essential hypertension: a systematic review and meta-analysis. Metabolism. 2014;63:1503–11.
Cai W, Yin L, Yang F, Zhang L, Cheng J. Association between Hcy levels and the CBS844ins68 and MTHFR C677T polymorphisms with essential hypertension. Biomed Rep. 2014;2:861–8.
Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92.
Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Gai X, Lan X, Luo Z, Wang F, Liang Y, Zhang H, et al. Association of MMP-9 gene polymorphisms with atrial fibrillation in hypertensive heart disease patients. Clin Chim Acta. 2009;408:105–9.
Alghasham A, Settin AA, Ali A, Dowaidar M, Ismail H. Association of MTHFR C677T and A1298C gene polymorphisms with hypertension. Int J Health Sci. 2012;6:3–11.
Markan S, Sachdeva M, Sehrawat BS, Kumari S, Jain S, Khullar M. MTHFR 677CT/MTHFR 1298CC genotypes are associated with increased risk of hypertension in Indians. Mol Cell Biochem. 2007;302:125–1231.
Yang K, Jia J, Mao L, Men C, Tang K, Li Y, et al. Methylenetetrahydrofolate reductase C677T gene polymorphism and essential hypertension: a meta-analysis of 10,415 subjects. Biomed Rep. 2014;2:699–708.
Ilhan N, Kucuksu M, Kaman D, Ilhan N, Ozbay Y. The 677C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch Med Res. 2008;39:125–30.
Heux S, Morin F, Lea RA, Ovcaric M, Tajouri L, Griffiths LR. The methylentetrahydrofolate reductase gene variant (C677T) as a risk factor for essential hypertension in Caucasians. Hypertens Res. 2004;27:663–7.
Qian X, Lu Z, Tan M, Liu H, Lu D. A meta-analysis of association between C677T polymorphism in the methylenetetrahydrofolate reductase gene and hypertension. Eur J Hum Genet. 2007;15:1239–45.
Kosmas IP, Tatsioni A, Ioannidis JP. Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene with hypertension in pregnancy and pre-eclampsia: a meta-analysis. J Hypertens. 2004;22:1655–62.
Bennouar N, Allami A, Azeddoug H, Bendris A, Laraqui A, El Jaffali A, et al. Thermolabile methylenetetrahydrofolate reductase C677T polymorphism and homocysteine are risk factors for coronary artery disease in Moroccan population. J Biomed Biotechnology. 2007;2007(1):80687.
Authors’ contributions
SN and YK designed and conducted the research, analyzed and interpreted the data, created the tables, and wrote the paper; FK and RH designed the research, participated in the collection of the sample, SN designed the research and critically revised the article. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully thank the Hassan II Academy of Science and Technology for its financial support.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nassereddine, S., Kassogue, Y., Korchi, F. et al. Association of methylenetetrahydrofolate reductase gene (C677T) with the risk of hypertension in Morocco. BMC Res Notes 8, 775 (2015). https://doi.org/10.1186/s13104-015-1772-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1772-x