Abstract
Background
Human immunodeficiency virus (HIV) and tuberculosis (TB) are the leading independent global causes of death among patients with infectious diseases. Additionally, due to the shared immune defense mechanisms, they are the leading cause of co-morbidities globally. However, little information was found regarding the proportion of TB/HIV co-infection in the study area. Thus, this study determined the proportion and associated factors of TB/HIV co-infection.
Methods
All TB patients treated from January/2011 to December/2014 were included in this study. Data were collected from three health centers namely; Kobo, Robit and Gobiye. Data were entered, cleared, and analyzed using SPSS version 20. Frequency, percentage, median and range were used to present the data. To assess the associated factors, logistic regression was employed.
Results
Of the total 990 TB patients enrolled in the study, 98.2 % were screened for HIV; of these, 24.3 % were co-infected with TB and HIV. The odds of having TB/HIV co-infection were 3.4 times higher among in the age group of 25–45 years compared to older (≥45 years) age TB patients (OR = 3.4; 95 % CI 2–5). Moreover, the odds of having TB/HIV co-infection were 2.8 and 1.7 times higher among smear positive and smear negative patients with pulmonary TB respectively than patients with extra pulmonary TB. Of 236 co-infected patients, 71.2 % took co-trimoxazole preventive therapy and 76.3 % took antiretroviral treatment.
Conclusion
TB/HIV co-infection is one of the serious public health problems in the study area. Thus, Collaborative TB/HIV activities that reduce the co-morbidities and mortalities should be addressed.
Similar content being viewed by others
Background
Human immunodeficiency virus (HIV) and tuberculosis (TB) are the first and second leading causes, respectively of death globally due to a single infectious agent [1]. Due to the shared immune defense mechanisms between the two diseases, TB is a leading preventable cause of death among people living with HIV and vice versa [2–4]. Most of these deaths occur in resource-limited settings [5] like Ethiopia.
According to 2011 Ethiopian demographic health survey, the prevalence of HIV was 1.5 % [6]. Similarly, in urban and rural Amhara region where this study was conducted, the prevalence of HIV was 10.7 and 1.5 %, respectively [7].
The prevalence of TB in Ethiopia was 211/100,000 [8]. Tuberculosis threatens the poorest and most marginalized populations [9, 10]. The resurgence of TB has been fueled by multi factors like HIV and drug resistant TB. And also, the current increasing HIV associated tuberculosis shifted the clinical pattern TB towards smears negative pulmonary (PTB−) and extra pulmonary TB (EPTB) [11, 12].
The prevalence of TB among HIV positive clients in Ethiopia was 7.8 % and in Amhara region, it it was 4.9 % [13]. Conversely, the prevalence of HIV among TB patients in Ethiopia and Amhara region was 20 and 26.5 %, respectively [13].
Information is limited on the proportion of TB, HIV and TB/HIV co-infection in the study area. Also, studies done nationally and in Amhara region could not reflect the real TB/HIV situation in the study area due to methodological variation [13–15]. Unlike others, this study used all TB registers data across 2011–2014 among TB clinics who were treating TB patients from urban and rural area of Kobo and Raya kobo woredas, East of Amhara and/or north eastern Ethiopia to assess the proportion of TB/HIV co-infection and associated factors.
Methods
Study setting and period
All patients diagnosed and treated from January/2011 to December/2014 were included in this study. Health centers in Ethiopia in general are a primary health care unit that serves a population of 15,000–25,000 and is comprised of clinics like TB, antiretroviral treatment (ART), maternal and child health, and outpatient. Public health officers and clinical nurses with BSc degree work as practitioner. Moreover, most of laboratory and pharmacy professionals were diploma holder.
Diagnoses of pulmonary TB in the health centers were done using clinical and Ziehl-Neelsen (ZN) microscopy information. Clinical specimen for diagnosis of pulmonary TB was collected using spot-morning-spot sample collection strategy. EPTB cases were diagnosed and referred from nearby Kobo, Woldiya, Dessie, Alamata hospitals and privates clinics. Diagnosis of EPTB was mainly using clinical history with X-ray, ultrasound and/or pathologic techniques. For those diagnosed with active TB, the standard TB treatment regimen, 2(RHZE)/4 (RH) and 2S (HRZE)/1(HRZE)/5(HR)E was started for new and previously treated TB patients, respectively [16]. For HIV screening, nationally approved rapid serological testing algorisms (KHB → StatPak → Uni-gold) were used. As soon as HIV is identified in a TB patient, the patient is enrolled to HIV chronic care and co-trimoxazole preventive therapy (CPT) started [17].
Operational definitions
Cured
A patient with bacteriologically confirmed pulmonary TB at the beginning of treatment who was smear or culture-negative in the last month of treatment.
Treatment completed
A patient with TB who completed treatment without evidence of failure, but with no record of sputum smear or culture results, in the last month of treatment.
Treatment success (good treatment outcome)
The sum of cured and treatment completed.
Died
A TB patient who died from any cause during treatment.
Failed
A TB patient whose sputum smear or culture is positive at month 5 or later during treatment.
Lost to follow-up
A TB patient who did not start treatment or whose treatment was interrupted for two consecutive months or more.
Poor treatment outcome
The sum of treatment failed, died and loss to follow up.
Inclusion and exclusion criteria
Patients with TB whose record data were missing age, sex and type of TB were excluded from the study. Patients with complete demographic and clinical data (regardless of age groups and sex, type of TB and HIV status) were included in the study.
Sample size
A total of 990 patients with TB were treated between January/2011 and December/2014. All these patients were included in the study.
Data collection and tools
Data were collected from three health centers namely Kobo, Robit and Gobiye. Demographic and clinical data such as age, sex, type of TB, HIV, CPT and ART status were retrieved from TB registry.
Data analysis
All data were entered, cleared, and analyzed using SPSS statistical software package (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Frequency, percentage, median and range were used to present the data. To assess the association between dependent and independent variables, logistic regression was used. Associations between variables were determined using odds ratio and 95 % confidence interval (CI). P value of <0.05 was considered as statistical significance.
Ethical considerations
Ethical clearance was obtained from Amhara Regional Ethical Review Committee (RERC) with reference number of HRTT/11/71/2014. In addition, a support letter was written to Zonal Health Department and Kobo and Raya Kobo Woreda Health Offices. Data did not include the patient’s name and kept confidential. The data were used for the purpose of the study only.
Results
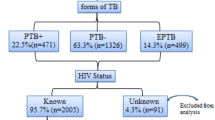
Of the total 990 TB patients enrolled to this study, 551 (55.7 %) were males. The median age of participants were 29 years (range 1–84). The majority of TB patients were in the age groups of 25–45 years. The proportion of EPTB, PTB− and PTB+ were 462 (46.7 %), 332 (33.5 %) and 196 (19.8 %), respectively (Table 1).
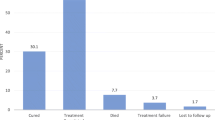
Among the total TB patients, 972 (98.2 %) were screened for HIV. The remaining 18 (1.8 %) patients had no HIV status data. Of the total HIV screened TB patients, the proportion of TB/HIV co-infection was 236 (24.3 %). The odds of having TB/HIV co-infection was 3.4 times higher in the age group of 25–45 year old as compared to patients with ≥46 years of age (AOR = 3.4; 95 % CI 2–5; P = 0.00). Moreover, the odds of having TB/HIV co-infection were 2.8 and 1.7 times higher among PTB+ and PTB− patients than EPTB, respectively (Table 2). Of that TB/HIV co-infected 71.2 and 76.3 % took CPT and ART, respectively. The proportion of treatment success was 86.2 % (Table 1).
Discussion
In the present study, 98.1 % of TB patients were screened for HIV which was higher than the latest national data, 71 % [10, 18]. In 2013, 48 % of TB patients globally had a documented HIV test result. In the African Region, 76 % of TB patients knew their HIV status [8]. Among Ethiopian regional states, the highest proportion of people living with HIV/AIDS (PLHIV) was seen in Amhara [7]. This showed that HIV screening practice is better in Amhara region and in the study area in particular. Moreover, it seems that there was better commitment from health professionals and local administrative bodies in terms of screening, communication and social mobilization.
Among those HIV screened TB patients; the proportion of TB/HIV co-infection was 24.3 % which was much higher than reports from Ethiopia, 6.3–20 % [10, 13, 16, 17, 19]. It was also higher when compared to a similar study in Nigeria, 20.5 % [20]. Overlapping co-morbid diseases are growing in resource-limited countries like Ethiopia [11]. This might be due multi-TB and HIV related risk factors. First, it is well known that, people in this area have the practice of dating sisters-in-law or brothers-in-law [observation]. This practice would have a significant impact on HIV transmission. Second, the community especially farmers in the study area are well known drinkers of local beer (Teji in local Amharic Language), this action might push people to have unprotected sexual practice. Third, the area is one of draughts prone and food unsecured area [21]. Thus, there are many migrants to Middle East for home maid and daily labor after having divorced. Moreover, so many beautiful ladies enforced to sex work due to financial constraints. Furthermore, the associations between poverty and TB have been known [9, 10, 22–24]. This might increase TB/HIV co-infection cases in the area. Fourth, the presence of many illegal private TB drug sellers in the area might contributed for high TB and drug resistant TB. Lastly, high number of HIV means, there would be many HIV associated tuberculosis. All these factors might have direct or indirect contribution to the high TB/HIV co-infection rate. To identify which factors are more important than other, population based study must be done.
In contrast, our TB/HIV proportion data was lower than studies done in Debre markos 44 % [14], Dabat-Gondar 34 % [15] and Kenya 41.8 % [25]. This might be due to differences in the study site. These studies were conducted at referral and teaching hospitals where many chronic, end stage diseases and referral cases were seen. Our study was conducted in health centers. Globally, 13–14.8 % of people who developed TB are HIV positive [7, 9, 26, 27]. Prevalence of TB/HIV co-infection was 31.25 % in African countries [7, 28]. In 2012, the prevalence of TB/HIV co-infection was 43 % in Africa [9] and as high as 50–80 % in parts of sub-Saharan Africa [27].
The ART coverage in the present study was higher, 76.3 %, as compared to a WHO report from Ethiopia, 68 %, but lowers than WHO target of 100 % [10, 18]. Other reports showed that ART coverage was 50.5 and 40.2 % in Ethiopia and Amhara region [13], respectively. This might be due to proximity of health centers to the community. Moreover, communication and social mobilization might be done by local stake holders to diagnose and linked more patients to ART. Furthermore, according to unpublished reports from the community, the practice of safe sex and use of condoms is low but people’s awareness of the value of ART is high.
The proportion of TB/HIV co-infection among women was relatively higher in the present study (Table 2). This corroborates WHO reports. More young women notified with TB/HIV than young men in Africa [29–31]. Out of the 346 TB cases in Kenya, 41.8 % of were co-infected and female to male TB/HIV co-infection prevalence ratio was 1.35:1 [25]. The possible reason might be due to socio economic factors and health seeking behavior of women. As compared to men, women have no control of financial resources at household levels. Moreover, there is higher rate of transient and permanent immune suppression with pregnancy, lactation and HIV in women than men [29].
In the present study, TB/HIV co-infection was significantly associated with age group of 25–45 years and PTB+, P < 0.05 (Table 2). It was well known that TB and HIV affect reproductive age groups of the population [8]. In contrast to the literature evidence, PTB+ was significantly associated with HIV than PTB− and EPTB in the present study. This might be due to multi factors. First, the relatively higher ART and CPT coverage was found in the study area. If PTB+/HIV co-infected people adhered well on ART and CPT, they would have high level of innate and cell mediated immunity. Thus, these people would have high probability for being smear positive. Second, it is known that EPTB and PTB− are HIV associated than PTB+ [12]. However, this study could not differentiated HIV associated TB from non HIV associated TB. Thus, if TB infection and diagnosis occurred before HIV infection, the proportion of PTB+/HIV would be higher than EPTB/HIV and PTB−/HIV co-infection. The third, possible reason might be due to, over-diagnosis of EPTB and PTB− cases. Because, higher proportion of EPTB and PTB− cases were diagnosed and transferred in from private clinics which did not have any imaging and pathologic techniques. This justification was supported by, health professionals in the study area (expert opinion). Additionally, Ehlers et al. reported that only 2 of the 76 PTB− patients had been diagnosed correctly [32]. Moreover, a study by Iwnetu et al. concluding that up to 15 % of all EPTB cases could be wrongly diagnosed [33]. The last possible reason might be due to zoonotic transmission of TB and genetic features of the pathogen and/or the host population. This was in line with the latest report by Berg et al. According to this report, having regular and direct contact with live animals, was a significant risk factor for lymph node (TBLN) when compared to pulmonary TB. In addition, no association was found with HIV infection [34]. The study area is known with large live stock populations, sharing of shelter between human and livestock, use of raw meat and milk.
TB/HIV co-infection was significantly associated with poor TB treatment outcome in the present study (AOR: 2; 95 % CI 1.2–3.1; P = 0.003) (Table 2). This might be due to the presence of TB and HIV drug interaction that make low adherence to anti-TB drugs. Tuberculosis case-fatality rates in Africa were 16–35 % among HIV positives and 4–9 % in HIV-negative patients [35, 36]. This indicates that full DOTS implementation alone is insufficient to control TB. Thus, collaborative TB/HIV activities aimed to reduce the burden of diseases are very important [37].
Conclusion and recommendations
The proportion of TB/HIV co-infections were higher in the study area which indicates that people’s awareness on transmission and control mechanism of TB and HIV is low. On the other hand, 98.1 % of TB patients were screened for HIV but ART coverage was 76.3 % which was lower than WHO target of 100 %. Moreover, proportion of TB/HIV co-infection was associated with women, age group of 25–45 years, PTB+ and poor TB treatment outcome. Hence, sex and age targeted interventions like awareness creation training should be given to female and sexually active age (25–45 years) groups of the population. Moreover, communication and social mobilization must further strengthen to achieve 100 % ART coverage. TB/HIV co-infected people were found to be more infectious. Thus, the laboratory professionals’ capacity must be strengthen to detect those infectious cases. Additionally, diagnostic quality of EPTB and PTB− must strengthen by implementing rapid and sensitive diagnostic technologies like, Fluorescent Microscopy, Xpert MTB/Rif. Furthermore, to have good TB treatment outcome, prompt and community involved DOTS based anti-TB treatment should be in place.
Abbreviations
- AIDS:
-
acquired immunodeficiency syndrome
- AOR:
-
adjusted odds ratio
- ART:
-
antiretroviral therapy
- CI:
-
confidence interval
- COR:
-
crude odds ratio
- CPT:
-
co-trimoxazole preventive therapy
- DOTS:
-
directly observed treatment for short course
- E:
-
ethambutol
- EPTB:
-
extra pulmonary TB
- H:
-
isoniazid
- HIV:
-
human immunodeficiency virus
- ICF:
-
intensified case-finding of TB
- IPT:
-
isoniazid preventive therapy
- PLHIV:
-
people living with HIV
- PTB−:
-
smears negative pulmonary TB
- PTB+:
-
smear-positive pulmonary TB
- R:
-
rifampicin
- RERC:
-
Regional Ethical Review Committee
- S:
-
streptomycin
- SPSS:
-
statistical package social sciences
- TB:
-
tuberculosis
- TBLN:
-
lymph node TB
- WHO:
-
World Health Organization
- Z:
-
pyrazinamide
- ZN:
-
Ziehl-Neelsen
References
WHO media center. Tuberculosis fact sheet. Geneva: WH; 2015. http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed 30 Sep 2015.
WHO. Priority research questions for TB/HIV in HIV prevalent and resource limited settings. Geneva: WHO; 2010. http://whqlibdoc.who.int/publications/2010/9789241500302_eng.pdf. Accessed 10 April 2015.
WHO. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva: WHO; 2012. http://www.who.int/tb/publications/2012/tb_hiv_policy_9789241503006/en/. Accessed 10 April 2015.
WHO. WHO three i’s meeting: Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for people living with HIV. Report of a joint World Health Organization HIV/AIDS and TB department meeting, Geneva: WHO; 2008. http://www.who.int/hiv/pub/tb/3is_mreport/en/. Accessed 10 April 2015.
WHO. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Department of HIV/AIDS stop TB department. Geneva: WHO; 2011. http://www.who.int/tb/challenges/hiv/ICF_IPTguidelines/en/. Accessed 20 April 2015.
WHO Country office for Ethiopia. HIV/AIDS progress in 2014. Addis Ababa: 2015. http://www.afro.who.int/en/ethiopia/who-country-office-ethiopia.html/. Accessed 30 Sep 2015.
Federal Democratic Republic of Ethiopia Ministry of Health. Health sector development programme IV. 2010/11–2014/15. Draft version 2011. https://www.google.com.et/?gws_rd=cr,ssl&ei=VYjtVciXMcKPsgH_uKPgCw#q=Federal+Democratic+Republic+of+Ethiopia+Ministry+of+Health.+Health+Sector+Development+Programme+IV. Accessed 10 August 2015.
WHO. Global tuberculosis report. Geneva: WHO; 2014. http://www.who.int/tb/publications/global_report/en/. Accessed 2nd May 2015.
United States Government agency for international development (USAID). The Twin epidemics: HIV and TB co-infection. http://www.usaid.gov/news-information/fact-sheets/twin-epidemics-hiv-and-tb-co-infection. Accessed 20 Sep 2015.
WHO. The stop Tb strategy. building on and enhancing DOTS to meet the TB-related millennium development goals. Geneva: WHO; 2006. http://www.who.int/tb/strategy/en/. Accessed 10 May 2015.
Lawn D, Zumla I. Tuberculosis. Lancet. 2011;378:57–72.
American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167: 603–2. Reprinted in MMWR Recomm Rep 2003;52 suppl RR-11:1–77.
Ethiopian Health and Nutrition Research Institute. TB/HIV sentinel surveillance. One year report (July 2011–June 2012). Addis Ababa: 2013. http://www.ephi.gov.et/images/pdf/National_tbhiv_Surveillance_report.pdf. Accessed 1st Sep 2015.
Esmael A, Tsegaye G, Wubie M, Endris M. Tuberculosis and human immune deficiency virus co-infection in debre Markos referral hospital in Northwest Ethiopia: a 5 Years retrospective study. J AIDS Clin Res. 2013;4:263.
Tadesse S, Tadesse T. HIV co-infection among tuberculosis patients in Dabat, northwest Ethiopia. J Infect Dis and Immun. 2013;5(Suppl 3):29–32.
Federal Democratic Republic of Ethiopia. Ministry of Health. Guideline for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. Addis Ababa: 2012. http://www.etharc.org/resources/download/finish/33/709. Accessed 13 May 2015.
The Federal Democratic Republic of Ethiopia, Ministry of Health. National training package, provider-initiated HIV testing and counseling. Trainer’s manual. Addis Ababa: 2010. https://www.google.com.et/?gws_rd=cr,ssl&ei=uZbtVaS0JIOrswHBhIDADA#q=The+Federal+Democratic+Republic+of+Ethiopia+Ministry+of+Health+National+Training+Package%2C+Provider−Initiated+HIV+Testing+and+Counseling.+Trainer%E2%80%99s+Manual+2010. Accessed 23 Aug 2015.
WHO country office for Ethiopia. Tuberculosis update in Ethiopia. Progress in 2014. Addis Ababa: 2015. http://www.afro.who.int/en/ethiopia/who-country-office-ethiopia.html. Accessed 30 Sep 2015.
Kaufmann E and Parida K. Tuberculosis in Africa: learning from pathogenesis for biomarker identification. Cell Host Microbe. 2008;4(Suppl 3):219–28.
Oshi Ch, Oshi N, Alobu I, Ukwaja N. Profile, outcomes, and determinants of unsuccessful tuberculosis treatment outcomes among HIV-infected tuberculosis patients in a Nigerian state. Cairo: Hindawi Publishing Corporation, Tuberculosis Research and Treatment 2014; 2014.
Seid Yassin. Small-scale irrigation and household food security: a case study of three irrigation schemes in Gubalafto Woreda of North Wollo zone, Amhara region, Master’s Thesis, Addis Ababa: Graduate School of the University of Addis Ababa; 2002. p. 35.
Barter DM, Agboola SO, Murray MB, Bärnighausen T. Tuberculosis and poverty: the contribution of patient costs in sub-Saharan Africa. a systematic review. BMC Public Health. 2012;12:980.
Spence DPS, Hotchkiss J, Williams CSD, Davies PD. Tuberculosis and poverty. BMJ. 1993;307:759–61.
Oxlade O, Murray M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS One. 2012;7(suppl 11):e47533.
Nyamogoba DN, Mbuthia G, Mining S, Kikuvi G, Biegon R, Mpoke S, et al. HIV co-infection with tuberculosis and non-tuberculous mycobacteria in western Kenya: challenges in the diagnosis and management. Afr Health Sci. 2012;12(suppl 3):305–11.
WHO. Global tuberculosis report. WHO/HTM/TB/2012.6. Geneva: WHO; 2012. http://www.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf. Accessed 3rd April 2015.
Luetkemeyer A, and Daley L. Tuberculosis and HIV. Comprehensive, up-to-date information on HIV/AIDS treatment, prevention, and policy from the University of California San Francisco. HIV in site knowledge base chapter. San Francisco: University of California; 2013. http://hivinsite.ucsf.edu/InSite?page=kb-05-01-06. Accessed 27 Aug 2015.
Gao J, Zheng P, Fu H. Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PLoS One. 2013;8(Suppl 5):e64915.
WHO fact sheet. Gender and tuberculosis. Department of gender and women’s health. Geneva: WHO; 2003. http://whqlibdoc.who.int/gender/2002/a85584.pdf. Accessed 11 April 2015.
WHO fact sheet. Tuberculosis in women. Geneva: WHO; 2014. http://www.who.int/tb/publications/factsheets/en/. Accessed 14 May 2015.
World Health Organization. WHO fact sheet. HIV-associated tuberculosis. Geneva: WHO; 2014. http://www.who.int/entity/tb/publications/tbhivfactsheet_24oct2013.pdf. Accessed 14 May 2015.
Ehlers VJ, Aragaw GS. An audit of diagnosis and treatment of tuberculosis in Ethiopia. Afr J Prm Health Care Fam Med. 2014;6(suppl 1):6.
Iwnetu R, van den Hombergh J, Woldeamanuel Y, Asfaw M, Gebrekirstos C, Negussie Y, Ashenafi S, et al. Is tuberculous lymphadenitis over-diagnosed in Ethiopia? Comparative performance of diagnostic tests for mycobacterial lymphadenitis in a high-burden country. Scand J Infect Dis. 2009;41(suppl 6–7):462–8.
Berg S, Schelling E, Hailu E, Firdessa R, Gumi B, Erenso G, Gadisa E, et al. Investigation of the high rates of extrapulmonary tuberculosis in Ethiopia reveals no single driving factor and minimal evidence for zoonotic transmission of Mycobacterium bovis infection. BMC Infect Dis. 2015;15:112.
Ya M, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–52.
Corbett L, Marston B, Churchyard J, Cock De. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–37.
WHO. A guide to monitoring and evaluation for collaborative TB/HIV activities. Geneva: WHO; 2009. https://www.who.int/hiv/pub/tb/hiv_tb_monitoring_guide.pdf. Accessed 30 Sep 2015.
Authors’ contributions
DM was involved in the design, implementation of the study, performed statistical analysis, and drafted the manuscript. AD was involved in data collection and entry, performed statistical analysis and critically revised the manuscript. ED contributes to the scientific content of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We express our deep appreciation to Amhara National Regional Health Bureau Research and Technology department for approving the proposal, Mr Hailu Mekonnen for his unreserved support in data collection. Moreover, we also thank North Wollo zonal health department, Kobo and Raya Kobo woreda health offices and health centers staffs. Our deepest appreciation goes to Dr. Elizabeth Thomas American Society of Microbiology Lab Cap consultant for editing the language. We also thank the study participants.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mekonnen, D., Derbie, A. & Desalegn, E. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in Northeastern Ethiopia: a 4 years retrospective study. BMC Res Notes 8, 666 (2015). https://doi.org/10.1186/s13104-015-1664-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1664-0