Abstract
Background
Exercise-induced improvements in cardiorespiratory fitness (CRF) often coincide with improvements in insulin sensitivity and reductions in liver fat content. However, there are limited data concerning the relationship between CRF and liver fat content in adults with varying degrees of metabolic dysfunction.
Methods
The aim of this study was to examine the association between CRF, liver fat content, and insulin resistance in inactive adults with obesity and with or without type 2 diabetes (T2D), via cross-sectional analysis. CRF was determined via a graded exercise test. Liver fat content was assessed via proton magnetic resonance spectroscopy and insulin resistance was assessed via homeostatic model of insulin resistance (HOMA-IR). A partial correlation analysis, controlling for age and gender, was performed to determine the association between CRF, demographic, cardiometabolic, and anthropometric variables. Independent t tests were performed to compare cardiometabolic outcomes between participants with T2D and participants without T2D.
Results
Seventy-two adults (46% male) with a mean age of 49.28 ± 10.8 years, BMI of 34.69 ± 4.87 kg/m2, liver fat content of 8.37 ± 6.90%, HOMA-IR of 3.07 ± 2.33 and CRF of 21.52 ± 3.77 mL/kg/min participated in this study. CRF was inversely associated with liver fat content (r = − 0.28, p = 0.019) and HOMA-IR (r = − 0.40, p < 0.001). Participants with T2D had significantly higher liver fat content (+ 3.66%, p = 0.024) and HOMA-IR (+ 2.44, p < 0.001) than participants without T2D. Participants with T2D tended to have lower CRF than participants without T2D (− 1.5 ml/kg/min, p = 0.094).
Conclusion
CRF was inversely associated with liver fat content and insulin resistance. Participants with T2D had lower CRF than those without T2D, however, the difference was not statistically significant. Further longitudinal studies are required to elucidate the relationship between CRF and the progression of obesity-related diseases such as T2D.
Registration: ACTRN12614001220651 (retrospectively registered on the 19th November 2014) and ACTRN12614000723684 (prospectively registered on the 8th July 2014).
Similar content being viewed by others
Background
Obesity plays a significant role in the development of many chronic diseases such as hypertension, coronary heart disease, numerous cancers, and type 2 diabetes (T2D) [48]. While the relationship between obesity and increased cardiometabolic risk is well established [1], the location of adipose tissue, particularly in and/or around the liver, heart, muscles, and pancreas, also known as ectopic fat, is significantly more predictive of adverse health outcomes such as hypertension and hyperglycaemia [36]. Metabolic dysfunction-associated fatty liver disease (MAFLD), characterised by excessive liver fat (LF) content, is strongly associated with insulin resistance and is highly prevalent in individuals with T2D [41]. As T2D and MAFLD share a similar underlying pathological process, they often present alongside other disorders such as obesity, dyslipidaemia, and hypertension [49]. A known strategy for managing these risk factors is increased physical activity [23, 43], which importantly leads to improved cardiorespiratory fitness (CRF) [8]. However, individuals with T2D and/or MAFLD reportedly experience disease-related aerobic impairments, which contribute to, or are further affected by, mitochondrial dysfunction, cardiac dysfunction, insulin resistance, and diastolic dysfunction - which may manifest as low CRF [6, 44].
Low CRF is a well-established risk factor for all-cause morbidity and mortality [18] and increases an individual’s risk for developing T2D [40]. Multiple studies have shown that individuals who engage in structured exercise can improve CRF, insulin sensitivity, and reduce ectopic fat [13, 29,30,31], however, it is unclear whether these improvements are mediated through changes in CRF or through simply undertaking regular exercise. Recent observational data have shown that while physical activity levels were not associated with LF [16], low CRF was strongly and independently associated with MAFLD prevalence [9]. However, these findings are yet to be confirmed in participants with varying T2D-status using gold-standard LF quantification techniques and maximal exercise tests to determine CRF.
Decoupling CRF from physical activity-related improvements has proven difficult due to the interrelated nature of the two measures [24]. It could therefore be assumed that individuals who have higher physical activity and CRF levels also partake in other healthy behaviours, which provide further protection from a variety of obesity-related complications [34]. Furthermore, it is becoming increasingly accepted that both modifiable ,such as physical activity levels, and non-modifiable factors, such as gene-specific variations, contribute to variations in CRF [5, 33], with the relative genetic contribution to CRF reported to be ~ 50% [3, 33]. Consequently, it is important to explore the association between CRF and cardiometabolic risk in adults with similar levels of self-reported physical activity in order to decouple physical activity from CRF and better assess CRF-related benefits independently. There are limited studies which have investigated the relationship between CRF and insulin resistance in adults with and without T2D, and even fewer studies assessing the relationship between CRF and LF, as quantified via gold-standard proton magnetic spectroscopy (1H-MRS). Therefore, the aim of this cross-sectional study, was to determine the association between CRF, LF, insulin resistance, and other cardiometabolic outcomes in inactive adults with obesity, and with or without T2D. A secondary aim was to compare cardiometabolic and CRF differences between adults with T2D to those without T2D. It was hypothesised that CRF would be inversely associated with LF content and insulin resistance. It was also hypothesised that inactive adults with obesity and T2D would have significantly lower CRF than inactive adults with obesity but without T2D.
Methods
Participants
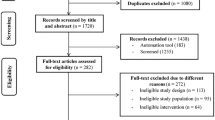
The participants of this study were recruited via electronic bulletins, clinical databases, and media advertisements between June 2011 and February 2019. Eligible volunteers were between the ages of 18–65 years, had a BMI ≥ 30.0 kg/m2, self-reported being physically inactive (exercising < 3 days/week) and/or not currently meeting physical activity guidelines [4]. All participants were screened by a medical practitioner prior to enrolment and were excluded if there was evidence of an unstable cardiac condition, uncontrolled hypertension, or uncontrolled blood glucose. The analysis included 72 volunteers from two larger intervention trials (ACTRN12614001220651 and ACTRN12614000723684) for which the results have been published elsewhere [14, 31, 46]. Eligible participants were screened via telephone interview and those who met the inclusion criteria and provided written informed consent, were enrolled in the study and were assessed at the University of Sydney (NSW, Australia). The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the procedures were approved by the University of Sydney Human Research Ethics Committee.
Anthropometry and blood pressure assessment
Relevant anthropometric data were collected in accordance with international standards [38]. The height of the participants was measured via stadiometer (SECA model 220 Telescopic Height Rod, Hamburg, Germany). Participants body weight was measured using Tanita BC-418 Body Composition Analyzer (Tanita Corporation, Tokyo, Japan) to the nearest 0.1 kg and BMI (kg/m2) was calculated. Waist circumference (WC) was measured (SECA Model 201, Hamburg, Germany) thrice horizontally, between the inferior margin of the ribs and the superior border of the iliac crest after expiration but before inspiration. After 10 to 15 mins of quiet sitting, systolic (SBP) and diastolic (DBP) blood pressures were measured manually on each arm with a sphygmomanometer (Welch Allyn® 767 Series Aneroid; New York, USA) and the average of three measures was recorded from the arm which produced the highest SBP and DBP readings.
Biochemical parameters
Venous blood was collected after an overnight fast (> 10 h) for the purpose of determining fasting blood glucose (FBG), insulin, high-sensitivity C-reactive protein (CRP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Concentration of plasma free fatty acids (FFA) was measured using stored plasma. All biochemical and lipid assessments were completed by a private accredited laboratory. Insulin resistance was assessed via the homeostatic model of insulin resistance (HOMA-IR) [45].
Proton magnetic resonance spectroscopy (1H-MRS)
LF% was measured via 1H-MRS using a Phillips Intera 1.5 Telsa Achieva MRI system (Philips Medical Systems, Best, Netherlands). Spectral data were post-processed by an assessor (NAJ), who was blinded to participant details, using a magnetic resonance user interface software (jMRUI, version 5.2; www.jmrui.eu) [25, 37]. LF% ≥ 5.5% was considered consistent with the presence of metabolic dysfunction-associated fatty liver disease (MAFLD) [39]. The LF% quantification methodology employed in this study is comprehensively detailed elsewhere [31].
Cardiorespiratory fitness
CRF was assessed via a graded maximal exercise test on an electronically-braked cycle ergometer (Lode Corival, Netherlands) under the supervision of an Accredited Exercise Physiologist [35]. All tests incorporated a three-min warm up at 35 W and 65 W for women and men, respectively, and workloads were incrementally adjusted by 25 W every 150 s until volitional fatigue as described elsewhere [15]. Heart rate, blood pressure, and ratings of perceived exertion (RPE) were obtained at each stage of exercise, with RPE measured using the Borg scale [2]. The test was terminated when the pedalling rate fell below 50 revolutions per min despite encouragement, or volitional fatigue. Peak work capacity (Wpeak) was measured [20] and peak oxygen consumption (VO2Peak) estimated as described previously [10]. CRF was assessed within one-week of LF assessment.
Statistical analysis
Data were analysed using Statistical Package for the Social Sciences (SPSS version 24.0; IBM Corp., Armonk, NY, USA). All data are reported as the mean values ± standard deviation (SD) unless otherwise stated. Independent t tests were performed to compare differences in CRF and other biochemical and anthropometric measures between individuals with and without T2D. Homogeneity of variances was assessed via Levene’s test for equality of variance and χ2 for categorical data. Abnormally distributed primary outcome measures were transformed via natural logarithm prior to between-group comparisons. Partial correlation coefficients (r), controlling for age and sex, were used for correlations between continuous variables, and where one of the variables was dichotomous categorical data and the other was continuous. Associations between two categorical variables were assessed by χ2 test for independence, whereby the φ coefficient determined the magnitude of the correlation. The magnitude of correlations were qualitatively assessed as: trivial (r < 0.1), small (r > 0.1 to 0.3), moderate (r > 0.3 to 0.4), strong (r > 0.5 to 0.7), very strong (r > 0.7 to 0.9), nearly perfect (r > 0.9), and perfect (r = 1.0) [11]. Male and female participants were divided into CRF quartiles, respectively, and analysis of variance was employed to determine significant differences in cardiometabolic outcomes between CRF quartiles (lowest fitness, IQR, highest fitness). Least significant difference post-hoc comparisons were used to identify and compare significant differences between CRF quartiles. Effect sizes were calculated as standardised difference in the means and expressed as Cohen’s d. Statistical significance was set at p < 0.05. A two-tailed sensitivity analysis using the effect size of the difference in LF content between participants with T2D versus participants without T2D revealed that the study achieved 78% power (G-Power software; University of Trier, Trier, Germany).
Results
Participants
Participant characteristics are summarised in Table 1. A total of 72 (33 male and 39 female) volunteers participated in this study. The mean age was 49.28 ± 10.18 years, BMI 34.69 ± 4.87 kg/m2, WC 108.55 ± 14.43 cm, LF was 8.37 ± 6.90%, and CRF was 21.52 ± 3.77 ml/kg/min. The prevalence of MAFLD was 43% in participants without T2D , 65% in participants with T2D, and 54% amongst all participants. Participants with T2D had significantly higher WC, LF%, HOMA-IR, FBG, ALT, TC, HDL, LDL, and FFA than those without T2D (p < 0.05 for all). There were no differences in other variables between participants with or without T2D. Male participants had significantly higher WC, TC, HDL, LDL, CRP, HOMA-IR and SBP than female participants (Supplementary Table 1).
Partial correlations
The associations between CRF, demographic, anthropometric, and cardiometabolic variables are summarised in Table 2. CRF was inversely associated with LF% (r = − 0.271), HOMA-IR (r = − 0.411), BMI (r = − 0.527), WC (r = − 0.490), FFA (r = − 0.283), CRP (r = − 0.344) FBG (r = − 0.259), insulin (r = − 0.403), and SBP (r = − 0.292) (p < 0.05 for all).
CRF and cardiometabolic outcomes
Participant characteristics stratified by CRF are summarised in Table 3. Participants with the lowest CRF had significantly higher LF% and HOMA-IR than those with the highest CRF (+ 5.31%, p = 0.021, + 2.50, p = 0.001, respectively) (Fig. 1). When compared to participants with the highest CRF, those with the lowest CRF also had significantly higher BMI (+ 7.54 kg/m2, p < 0.001), WC (+ 18.65 cm, p < 0.001), CRP (+ 3.80 mg/L, p = 0.012), FBG (+ 2.06 mmol/L, p = 0.034), insulin (+ 5.57 mU/L, p = 0.008), FFA (+ 153.34 umol/L, p = 0.015), and SBP (+ 16.57 mmHg, p = 0.001). When compared to participants with CRF scores within the interquartile range, those with the lowest CRF had significantly higher BMI (+ 7.54 kg/m2, p < 0.001), WC (+ 18.65 cm, p < 0.001), CRP (+ 3.93 mg/L, p = 0.003) and SBP (+ 10.89 mmHg, p = 0.014).
Discussion
This study is one of the first to show significant associations between key cardiometabolic risk factors, such as LF content, quantified via gold-standard 1H-MRS, and CRF in individuals with and without T2D. The analyses showed that the prevalence of MAFLD in participants with T2D was 65%, which is consistent with previous reports [50]. When compared to participants without T2D, those with T2D had significantly higher cardiometabolic abnormalities. Furthermore, adults with T2D also had lower CRF than those without T2D, however, the difference was not statistically significant. Further analyses showed that even relatively small variations in CRF were associated with increased LF content and insulin resistance, which were significantly higher in individuals with relatively low CRF than those with relatively high CRF. Similarly, individuals with the highest CRF had lower cardiometabolic and inflammatory abnormalities than those with the lowest CRF.
The association between cardiorespiratory fitness and liver fat
In accordance with the original hypothesis, CRF was inversely associated with LF content. This finding supports previously reported data, where Kantartzis and colleagues showed that LF content was inversely associated with CRF (r = − 0.22, p = 0.005) in a mixed sample of adults with MAFLD or adults at risk of metabolic disease [12]. Similarly, the findings from The Young Finns study showed that for every 1 ml/kg/min increase in CRF, the risk of MAFLD significantly declined (risk ratio = 0.90, 95% confidence interval 0.88 to 0.93; p < 0.001), however, LF content was measured up to three years after initial assessment of CRF and done so using ultrasound, which is less accurate than 1H-MRS [27]. Another study reported low CRF was inversely associated with increasing MAFLD activity and steatohepatitis severity measured via liver biopsy and sampled up to four months after CRF assessment [19]. The results of the study reported herein add to previous findings and suggest that even relatively small variations in CRF are associated with increased LF in inactive adults with or at risk of T2D. Importantly, the assessments of CRF and LF content were undertaken within a narrow timeframe (< 1 week) and LF% was quantified using gold-standard 1H-MRS, thus highlighting the novelty and methodological rigour of the present study.
The association between cardiorespiratory fitness and insulin resistance and other cardiometabolic outcomes
In accordance with the original hypothesis, CRF was inversely associated with insulin resistance. Further analyses revealed that, on average, individuals with relatively poor fitness had abnormally high levels of insulin resistance, blood pressure, inflammation and LF content, whereas individuals with relatively high fitness, only had slightly abnormal LF content (11.40% vs. 6.08% for low vs. high CRF, respectively). These data, which are supported by previous findings [12, 13, 22, 29,30,31, 47], suggest that CRF may play an important role in the context of metabolic disease.
A recent meta-analysis reported that CRF was inversely associated with T2D prevalence in a dose-dependent manner [28]. The results of the current study showed that while participants with T2D had lower CRF than those without T2D, the difference was not statistically significant. Furthermore, as physical activity has been shown to be inversely associated with LF content independent of BMI [17], only inactive participants were included in this study in an attempt to control for higher levels of physical activity - which incur cardiometabolic benefits. Because of this, the mean level of CRF of participants was quite low at 21.5 mL/kg/min. Reports show that CRF < 29.1 mL/kg/min increases the likelihood of developing metabolic syndrome six-fold [21], consequently a greater number of participants with higher levels of CRF are required to provide more robust results.
Mechanisms
While the mechanistic interplay between low CRF, MAFLD, and T2D remains unclear, it is purported that low CRF and ensuing mitochondrial defects contribute to the incomplete oxidation of fatty acids, which contribute to the accumulation of fatty acid by-products, such as ceramides and diacylglycerol in skeletal muscle and liver cells. The intracellular accumulation of these by-products impair insulin signalling pathways and contribute to insulin resistance [7, 26]. Importantly, T2D-related exercise intolerance appears to be reversed by regular exercise, which is made evident by the amelioration of skeletal muscle mitochondrial impairments, as well as improved insulin sensitivity, and CRF [29, 32, 42].
Strengths
This study adds to existing literature by highlighting the importance of CRF for metabolic health in adults with or at risk of metabolic disease. While previous studies have shown similar findings [12, 27], this is the first study to do so involving a mixed sample of inactive adults with obesity and with or without T2D. Furthermore, CRF and LF were measured within a week of each other, whereas previous studies had measured CRF up to three years after LF assessment [27]. Finally, this study quantified LF% using 1H-MRS, which is currently considered the gold-standard non-invasive technique for LF quantification.
Limitations
This study has limitations that should be considered when interpreting the results. Firstly, the results of this study, by nature, incorporated measures of CRF and LF content at a time-specific point and did not track the progression of any outcome to determine their relative importance in the development of MAFLD or T2D disease progression. Secondly, this study was completed using baseline data from previous interventional studies which prevented an a priori power analysis. However, the two-tailed sensitivity analysis revealed that the study achieved 78% power, which is just shy of the commonly accepted 80% value. Thirdly, this study assessed the amount of LF% per se and the methodology employed cannot determine the amount of fibrosis or classification of more severe liver diseases such as non-alcoholic steatohepatitis and/or their association with CRF. Additionally, while 1H-MRS is currently considered the gold-standard non-invasive measurement technique for LF%, HOMA-IR is comparatively more limited and cannot provide inference into tissue-specific impairments in insulin sensitivity. Fourthly, although CRF was assessed using a validated graded exercise test model [10], the gold-standard of aerobic capacity testing involves direct measures of gas analysis, and where possible, this method should be implemented. Finally, while an attempt was made to control for high levels of physical activity by only recruiting individuals who reported to be inactive (exercising < 3 days/week), inter-participant variations in physical activity levels likely contributed to the associations between CRF and LF.
Conclusions
The results of this study showed that CRF was inversely associated with 1H-MRS-quantified LF content in inactive adults with obesity. CRF was also inversely associated with insulin resistance and other key cardiometabolic risk factors. Adults with T2D had lower CRF than adults without T2D, however, the difference did not achieve statistical significance. These findings suggest that CRF may play a key role in metabolic dysfunction, however, further longitudinal studies are required to elucidate the relationship between CRF and the progression of obesity-related diseases such as MAFLD and T2D.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRF:
-
Cardiorespiratory fitness
- LF:
-
Liver fat
- 1H-MRS:
-
Proton magnetic resonance spectroscopy
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- T2D:
-
Type 2 diabetes
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- WC:
-
Waist circumference
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- FBG:
-
Fasting blood glucose
- CRP:
-
C-reactive protein
- TC:
-
Total cholesterol
- TG:
-
Total triglyceride
- HDL:
-
High density lipoprotein cholesterol
- LDL:
-
Low-density lipoprotein cholesterol
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- RPE:
-
Rate of perceived exertion
- Wpeak :
-
Peak work capacity
- VO2Peak :
-
Peak oxygen capacity
References
Aronne LJ, Isoldi KK. Overweight and obesity: key components of cardiometabolic risk. Clin Cornerstone. 2007;8(3):29–37. https://doi.org/10.1016/S1098-3597(07)80026-3.
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
Bouchard C, Daw EW, Rice T, Perusse L, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30(2):252–8. https://doi.org/10.1097/00005768-199802000-00013.
Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. https://doi.org/10.1136/bjsports-2020-102955.
Bye A, Klevjer M, Ryeng E, et al. Identification of novel genetic variants associated with cardiorespiratory fitness. Prog Cardiovasc Dis. 2020;63(3):341–9. https://doi.org/10.1016/j.pcad.2020.02.001.
Canada JM, Abbate A, Collen R, et al. Relation of hepatic fibrosis in nonalcoholic fatty liver disease to left ventricular diastolic function and exercise tolerance. Am J Cardiol. 2019;123(3):466–73. https://doi.org/10.1016/j.amjcard.2018.10.027.
Chan Z, Ding C, Chooi YC, et al. Ectopic fat and aerobic fitness are key determinants of glucose homeostasis in nonobese Asians. Eur J Clin Investig. 2019;49(5):e13079. https://doi.org/10.1111/eci.13079.
Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297(19):2081–91. https://doi.org/10.1001/jama.297.19.2081.
Croci I, Coombes JS, Bucher Sandbakk S, et al. Non-alcoholic fatty liver disease: prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT study. Prog Cardiovasc Dis. 2019;62(2):127–34. https://doi.org/10.1016/j.pcad.2019.01.005.
Hawley JA, Noakes TD. Peak power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur J Appl Physiol Occup Physiol. 1992;65(1):79–83. https://doi.org/10.1007/BF01466278.
Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. https://doi.org/10.1249/MSS.0b013e31818cb278.
Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58(9):1281–8. https://doi.org/10.1136/gut.2008.151977.
Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157–66. https://doi.org/10.1016/j.jhep.2012.02.023.
Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–82. https://doi.org/10.1016/j.jhep.2015.02.022.
Keating SE, Machan EA, O'Connor HT, et al. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J Obes. 2014;2014:834865.
Keating SE, Parker HM, Pavey TG, et al. Objectively quantified physical activity and sedentary behavior in predicting visceral adiposity and liver fat. J Obes. 2016;2016:2719014.
Kistler KD, Brunt EM, Clark JM, et al. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106:460–8 quiz 469.
Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. https://doi.org/10.1001/jama.2009.681.
Krasnoff JB, Painter PL, Wallace JP, et al. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47(4):1158–66. https://doi.org/10.1002/hep.22137.
Kuipers H, Verstappen FT, Keizer HA, et al. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6(04):197–201. https://doi.org/10.1055/s-2008-1025839.
Lakka TA, Laaksonen DE, Lakka HM, et al. Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc. 2003;35(8):1279–86. https://doi.org/10.1249/01.MSS.0000079076.74931.9A.
Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32(2):257–62. https://doi.org/10.2337/dc08-1377.
Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29.
Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7). https://doi.org/10.3390/nu11071652.
Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12(2-3):141–52. https://doi.org/10.1007/BF02668096.
Nassir F, Ibdah JA. Role of mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(5):8713–42. https://doi.org/10.3390/ijms15058713.
Palve KS, Pahkala K, Suomela E, et al. Cardiorespiratory fitness and risk of fatty liver: the young Finns study. Med Sci Sports Exerc. 2017;49(9):1834–41. https://doi.org/10.1249/MSS.0000000000001288.
Qiu S, Cai X, Yang B, et al. Association between cardiorespiratory fitness and risk of type 2 diabetes: a meta-analysis. Obesity. 2019;27(2):315–24. https://doi.org/10.1002/oby.22368.
Sabag A, Little JP, Johnson NA. Low-volume high-intensity interval training for cardiometabolic health. J Physiol. 2021. https://doi.org/10.1113/JP281210.
Sabag A, Way KL, Keating SE, et al. Exercise and ectopic fat in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2017;43(3):195–210. https://doi.org/10.1016/j.diabet.2016.12.006.
Sabag A, Way KL, Sultana RN, et al. The effect of a novel low-volume aerobic exercise intervention on liver fat in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2020;43(10):2371–8. https://doi.org/10.2337/dc19-2523.
Sargeant JA, Gray LJ, Bodicoat DH, et al. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obesity Rev. 2018;19(10):1446–59. https://doi.org/10.1111/obr.12719.
Schutte NM, Nederend I, Hudziak JJ, Bartels M, de Geus EJ. Twin-sibling study and meta-analysis on the heritability of maximal oxygen consumption. Physiol Genomics. 2016;48(3):210–9. https://doi.org/10.1152/physiolgenomics.00117.2015.
Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546–50. https://doi.org/10.1007/s11606-010-1609-1.
Smart NA, Williams A, Lyndon K. The role and scope of accredited exercise physiologists in the Australian healthcare system. J Clin Exerc Physiol. 2016;5(2):16–20. https://doi.org/10.31189/2165-6193-5.2.16.
Snel M, Jonker JT, Schoones J, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012;2012:983814. https://doi.org/10.1155/2012/983814.
Stefan D, Cesare FD, Andrasescu A, et al. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol. 2009;20(10):104035. https://doi.org/10.1088/0957-0233/20/10/104035.
Stewart A, Marfell-Jones M, Olds T, De Ridder J. International Standards for Anthropometric Assessment, vol. 137; 2011.
Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8. https://doi.org/10.1152/ajpendo.00064.2004.
Tarp J, Støle AP, Blond K, Grøntved A. Cardiorespiratory fitness, muscular strength and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetologia. 2019;62(7):1129–42. https://doi.org/10.1007/s00125-019-4867-4.
Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. https://doi.org/10.1038/nrgastro.2016.147.
van Tienen FH, Praet SF, de Feyter HM, et al. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab. 2012;97(9):3261–9. https://doi.org/10.1210/jc.2011-3454.
Wahid A, Manek N, Nichols M, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(9). https://doi.org/10.1161/JAHA.115.002495.
Wahl MP, Scalzo RL, Regensteiner JG, Reusch JEB. Mechanisms of aerobic exercise impairment in diabetes: a narrative review. Front Endocrinol. 2018;9:181. https://doi.org/10.3389/fendo.2018.00181.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. https://doi.org/10.2337/diacare.27.6.1487.
Way KL, Sabag A, Sultana RN, et al. The effect of low-volume high-intensity interval training on cardiovascular health outcomes in type 2 diabetes: a randomised controlled trial. Int J Cardiol. 2020;320:148–54. https://doi.org/10.1016/j.ijcard.2020.06.019.
Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. https://doi.org/10.7326/0003-4819-130-2-199901190-00002.
Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6(2):97–106. https://doi.org/10.1002/j.1550-8528.1998.tb00322.x.
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. https://doi.org/10.1038/nrgastro.2017.109.
Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. https://doi.org/10.1016/j.jhep.2019.06.021.
Acknowledgements
Not applicable.
Funding
This research was supported by funding from the Collaborative Research Network for Advancing Exercise & Sports Science (CRN-AESS)/Research Capacity Building Seeding Grant Scheme and by funding from Diabetes Australia Research Trust. (Establishment Grant: N.A. Johnson). The funding bodies were not involved in the study design, data collection, data analysis and interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
AS, SEK, and NAJ contributed to the design of the study. AS, SEK, KLW, and RNS collected the data. AS analysed and interpreted the data. AS drafted the manuscript. AS, SEK, KLW, RNS, SML, SMT, NAJ critically appraised, read, and approved the final manuscript.
Authors’ information
Affiliations.
1. NICM Health Research Institute, Western Sydney University, NSW, Australia
Angelo Sabag.
2. Faculty of Medicine and Health, discipline of exercise and sport science, the University of Sydney, NSW, Australia
Angelo Sabag, Kimberley L Way, Rachelle N Sultana, Nathan A Johnson.
3. The Boden collaboration for obesity, nutrition, exercise and eating disorders, the University of Sydney, NSW, Australia
Angelo Sabag, Kimberley L Way, Rachelle N Sultana, Nathan A Johnson.
4. Centre for Research on Exercise, Physical Activity and Health, School of Human Movement and Nutrition Sciences, The University of Queensland, QLD, AustraliaShelley E Keating.
5. Exercise Physiology and Cardiovascular Health Lab, Division of Cardiac Prevention and Rehabilitation, University of Ottawa Heart Institute, CanadaKimberley L Way.
6. Faculty of Medicine, University of Ottawa, Canada.
Kimberley L Way.
7. School of Health Sciences, Faculty of Health and Medicine, University of Newcastle, NSW, AustraliaSean M Lanting.
8. Central Clinical School, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, NSW, Australia.
Stephen M Twigg
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the procedures were approved by The University of Sydney Human Research Ethics Committee. Eligible participants were screened via telephone interview and those whom met the inclusion criteria and provided written informed consent, were enrolled in the study and underwent assessments at The University of Sydney (NSW, Australia).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest relevant to this article
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1
: Participant characteristics stratified by gender.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sabag, A., Keating, S.E., Way, K.L. et al. The association between cardiorespiratory fitness, liver fat and insulin resistance in adults with or without type 2 diabetes: a cross-sectional analysis. BMC Sports Sci Med Rehabil 13, 40 (2021). https://doi.org/10.1186/s13102-021-00261-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-021-00261-9