Abstract
Background
Following the ban on antimicrobial usage for growth promotion in animal husbandry in the EU, non-antimicrobial agents including heavy metal ions (e.g. zinc and copper), prebiotics or probiotics have been suggested as alternatives. Zinc has extensively been used in pig farming, particularly during weaning of piglets to improve animal health and growth rates. Recent studies, however, have suggested that high dietary zinc feeding during weaning of piglets increases the proportion of multi-drug resistant E. coli in the gut, contraindicating the appropriateness of zinc as an alternative. The underlying mechanisms of zinc effects on resistant bacteria remains unclear, but co-selection processes could be involved. In this study, we determined whether E. coli isolates from intestinal contents of piglets that had been supplemented with high concentrations of zinc acquired a higher tolerance towards zinc, and whether multi-drug resistant isolates tolerated higher zinc concentrations. In addition, we compared phenotypic zinc and copper resistance of E. coli isolates for possible correlation between phenotypic resistance/tolerance to different bivalent ionic metals.
Results
We screened phenotypic zinc/copper tolerance of 210 isolates (including antimicrobial resistant, multi-drug resistant, and non-resistant E. coli) selected from two, independent zinc-feeding animal trials by determining a zinc/copper minimal inhibitory concentration (Merlin, Bornheim-Hersel, Germany). In both trials, groups of piglets were supplemented either with high dietary zinc (> 2000 ppm) or control (50–70 ppm, background) concentrations. Our observations showed that high concentration zinc exposure did not have an effect on either zinc or copper phenotypic tolerance of E. coli isolates from the animals. No significant association was found between antimicrobial resistance and phenotypic zinc/copper tolerance of the same isolates.
Conclusion
Our findings argue against a co-selection mechanism of antimicrobial drug-resistance and zinc tolerance after dietary zinc supplementation in weaning piglets. An explanation for an increase in multi-drug resistant isolates from piglets with high zinc dietary feeding could be that resistant bacteria to antimicrobial agents are more persistent to stresses such as zinc or copper exposure.
Similar content being viewed by others
Background
The administration of antimicrobial growth promoters in animal husbandry has been prohibited in the EU since 2006 [1]. As alternatives to the application of antimicrobials, non-antimicrobial substances including heavy metal ions like zinc and copper, prebiotics or probiotics have been suggested to improve animal health and growth rates [2,3,4,5]. Zinc is one of the compounds widely used in the pig farming industry to overcome problems during weaning of piglets, including infections caused by pathogenic E. coli [6,7,8,9]. The essential trace elements zinc and copper are both involved in numerous physiological and cellular functions in all organisms [10,11,12]. Zinc concentrations and resistance are highly regulated through uptake and efflux mechanisms in different organisms [11, 13]. However, recent studies have suggested that feeding zinc in high concentrations during weaning of piglets increases the proportion of multi-drug resistant E. coli in the gut of the piglets [14,15,16,17,18,19]. The enhancement in the spread of antimicrobial resistance by the use of zinc confounds the usefulness of zinc supplementation in piglets and raises the question as to the underlying mechanisms of this observation.
One possible mechanism could be co-selection for both heavy metal/biocide and antimicrobial resistance, either in the form of co- or cross-resistance [16, 20,21,22]. Cross-resistance occurs as a result of physiological adaptations and affects susceptibility to different compounds, for example through efflux pump regulation or changes in cell wall permeability [23]. Co-resistance phenomena include changes involving genetic linkage of different genes encoding resistance to different classes of antimicrobials [20, 24]. A number of different studies have described possible mechanisms for co-selection of antimicrobial and heavy metal (zinc) resistance [16, 25,26,27,28,29]. Physiological coupling, genetic coupling and linked/co-localized resistance genes on mobile genetic elements have been suggested as possible mechanisms of both cross- and co-resistance [19, 23, 28, 30,31,32]. Zinc dependent beta-lactamases, effects of zinc on ampicillin stability or bacterial conjugation rates, and class 1 integrons (involved in co-selection) proximity to genes coding the efflux pump CzcA have been proposed as mechanisms involved in simultaneous reduction of susceptibility to antimicrobials and zinc/copper [19, 33,34,35,36,37]. Both intrinsic and acquired resistance mechanisms including efflux pumps and cellular detoxification of high concentrations of copper in bacteria have been reported in different studies [10, 38,39,40]. In addition to zinc, copper has also been suggested to contribute to antibiotic resistance in gram-negative and positive bacteria [28, 41, 42].
In this study, we tested the hypothesis that the increased antimicrobial resistance of E. coli isolates observed in weaning piglets fed with high zinc concentrations is caused by co-selection via phenotypic zinc tolerance. For this purpose, we used selected isolates [including antimicrobial resistant, multi-drug resistant (MDR), and non-resistant/susceptible (S) E. coli] and screened the level of their phenotypic zinc tolerance by determining a zinc minimal inhibitory concentration. Isolates originated from two, independent zinc-feeding trials of piglets with two different sampling schemes performed by our group over a period of 5 years. In both trials, groups of piglets were administered either high concentrations of zinc (> 2000 ppm) or a background control (50–70 ppm). From both feeding groups, we determined whether feeding of zinc resulted in higher proportions of phenotypically zinc resistant E. coli, and whether multi-drug resistant isolates also tolerated higher zinc concentrations, indicative of a co-selection process. In addition, we also compared phenotypic zinc resistance of these isolates with their phenotypic copper resistance values to determine whether there is a correlation between phenotypic resistance/tolerance to different bivalent ionic metals.
Results
1. Phenotypic antimicrobial resistance
Out of 210 preselected isolates collected during two, independent zinc feeding trials of piglets, 114 isolates belonged to zinc feeding groups (54.3%) and 96 isolates were from control feeding groups (45.7%). From the total number of tested E. coli, 63 isolates (30%) were found to be multi-drug resistant (MDR). The resistance pattern of MDR isolates always was a combination of beta lactamases (ampicillin or cefotaxime), tetracyclines (tetracycline), aminoglycosides (streptomycin) and sulfonamides (sulfamethoxazole/trimethoprim). There was no significant difference in the number of MDR isolates between the selected isolates from zinc and control groups of the feeding trials using chi-square test (Fig. 1; P-value = 0.586). Likewise, there was no significant difference in the number of resistant isolates (R) und susceptible (S) in zinc and control groups (P-value = 0.299). The number of resistant isolates to at least one antimicrobial agent was 124 (59%) of all 210 tested isolates.
Distribution of selected multi-drug resistant (MDR) and non-multi-drug resistant (NMDR) isolates in zinc and control groups. Out of a total of 210 isolates from both zinc trials, 36/114 (31.6%) multi-drug resistant (MDR) isolates were isolated in the zinc supplemented group (54.3% of total isolates), and 27/96 (28.1%) were found in the control group (45.7% of total isolates)
2. Zinc tolerance (MIC)
All 210 E. coli isolates examined in our study were tolerant to 64 µg/ml zinc chloride (break point 128 µg/ml–1 mM) (lower cut-off). The highest tolerated zinc chloride concentration was 256 µg/ml (break point 512 µg/ml–3.7 mM). This includes only 33.3% of isolates (n = 70) (upper cutoff). The largest proportion of isolates (64.3%) showed a medium level of tolerance to zinc chloride at 128 µg/ml (break point 256 µg/ml–1.9 mM) which comprises 135 isolates.
The zinc tolerance data was not normally distributed (Kolmogorov–Smirnov test, P < 0.001). As shown in Fig. 2, there was no significant difference for the MIC of zinc between MDR and NMDR isolates (medianMDR = 256 µg/ml, mediannot-MDR = 256 µg/ml P = 0.085).
There was also no significant difference MIC values towards zinc of resistant isolates (R) compared to susceptible isolates (S) (medianresistant = 256 µg/ml, mediansusceptible = 256 µg/ml, P = 0.107) (Fig. 3).
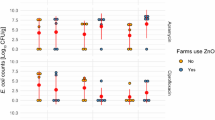
Interestingly, as shown in Fig. 4, there was also no significant difference in the MIC values for zinc comparing isolates from the high-zinc supplementation group (medianzinc = 256 µg/ml) or control group (mediancontrol = 256 µg/ml, P = 0.146).
Comparison of MIC values for zinc; high-zinc supplementation group (zinc) and the background control (control) isolates from both trials. A Mann–Whitney U test comparing 114 isolates from zinc group and 96 isolates from control group (overall 210 isolates) showed no significant difference of zinc-MIC value between considered groups (P = 0.146)
3. Copper tolerance (MIC)
All tested isolates in our experiment, with two exceptions, had MICs of 1024 µg/ml (~ 6.4 mM) for copper sulphate. No statistically significant difference in the MIC values towards copper comparing MDR (medianMDR = 1024 µg/ml) and NMDR (medianNMDR = 1024 µg/ml) isolates was observed (P = 0.540) (Fig. 5). There was also no significant difference in the MIC values for copper between resistant (R) and susceptible isolates, or isolates from the high-zinc supplementation group and control group (data not shown). There was no correlation between the zinc-MIC values and copper-MIC values (P = 0.593, correlation coefficient = − 0.037).
Discussion
During two, independent animal trials, we observed an increase in multi-drug resistant (MDR) E. coli in isolates of piglets when fed with high concentrations of zinc. One possible explanation for this effect is a co-selection for heavy metal and antimicrobial resistance, as has been previously suggested [16, 19, 20, 25, 43]. To determine whether there is an association between MDR phenotype and phenotypic zinc tolerance, we screened both MDR and non-MDR (NMDR) isolates for the level of phenotypic zinc tolerance. Out of a total of 210 isolates selected from both zinc supplementation trials, 63 isolates (30%) were multi-drug resistant.
In this study, we determined two different classifications of antibiotic resistance. We compared multi-drug resistant (MDR) to non-multi-drug resistant isolates (NMDR) according to the definition of Schwarz et al. [44], as well as resistant isolates (R), defined as resistance to at least one antimicrobial agent, and susceptible (S) isolates, defined as not resistant to any antimicrobial agents. For both definitions of antimicrobial resistance, we obtained the same result. Isolates tested in this study are not the whole set of isolates derived from two previous studies. We also did not want to show differences in the number of multi-drug resistant strains. In contrary, we chose almost identical number of strains for this experiment to compare their zinc resistance and whether it correlates with their original MDR phenotype. Therefore, it should not necessarily be a significant difference between the number of MDR isolates from zinc and control-feeding groups as was determined in our previous studies.
When comparing susceptible isolates (S) to isolates harboring at least one (or more) resistances (R), we observed no significant difference (P = 0.107) in their zinc MIC values. In addition, the zinc MIC values for zinc of MDR E. coli and NMDR isolates also showed no significant difference, suggesting that there is no association between antimicrobial resistance and phenotypic zinc tolerance of these isolates.
The observed increase in MDR—E. coli during the zinc feeding trials is therefore not likely a result of co-selection of zinc and antimicrobial resistance. As proposed by Ciesinski et al. [18], the increase of multi-drug resistant isolates in swine treated with a high dietary zinc, is likely due to formation of a persistent population of resistant bacteria already present in the gut. Furthermore, we found no difference in zinc tolerance levels of isolates from zinc-treated groups in comparison to the control groups, suggesting that the overall zinc tolerance of E. coli in the gut of piglets therefore seems not be affected by zinc feeding.
There are no universal interpretative criteria for classification of E. coli resistance towards zinc, and studies determining the MIC values for heavy metal ions are scarce [45,46,47,48]. In this study, we used a custom-made plate for phenotypic zinc tolerance levels in E. coli and which include all inhibitory concentrations mentioned in previous studies in MIC assays [45].
Despite variations in the testing methods used in previous studies and our study, such as use of agar plates or broth micro-dilution, the medium used for growing bacteria, and the formulation of zinc used in the experiments, the biological upper cut-off of phenotypic zinc tolerance for the most of E. coli isolates in these studies were around 2–2.5 mM of zinc ion. This is in accordance with the reported MIC of 2.2 mM Zn 2+ for E. coli TG1 in LB-medium [47]. The highest reported concentration of zinc (Zn 2+) which could be tolerated by E. coli isolates in the literature was 5 mM [48].
To determine whether our findings were similar for other heavy metals, we also compared our isolates for copper tolerance. All tested isolates in our experiment, with two exceptions, had the same MIC values of 1024 µg/ml for copper sulphate (~ 6.4 mM). The highest MIC concentration of copper (Cu 2+) detected for E. coli isolates in prior studies was 10.5 mM. Our results indicated no difference between copper MIC values of MDR and NMDR isolates, suggesting that there is no association between phenotypic antimicrobial resistance and phenotypic copper tolerance of the isolates. Interestingly, we also observed no correlation between the zinc and copper MIC values of the same isolates.
Co-selection for antimicrobial and metal-resistance has been suggested in many studies [11, 39,40,41]. In many of these studies, co-resistance was not shown, but a co-existence of resistance was reported in the same bacteria. Nevertheless, it is believed that some metal and antimicrobial resistance genes are linked and co-resistance of antimicrobial and heavy metal resistant bacteria have been discussed in several studies as likely to arise through co-selection [22, 29,30,31,32,33,34].
These studies are mostly on genome level and several of studied antimicrobial and metal ion resistance genes are on plasmids [16, 32, 43, 49,50,51]. For example, the plasmids of Salmonella abortus equi were found to co-transfer antimicrobial resistance (ampicillin-resistance) and heavy metal resistance (As, Cr, Cd, Hg) genes in mating experiments with E. coli strains. Salmonella strains cured of the plasmids were found to be sensitive towards ampicillin and heavy metals [32]. In a genomic transcriptional study, Lee et al. [30] found up-regulation of the mdtABC operon after exposure to high levels of zinc which suggested a potential influence of metal stresses on bacterial resistance to antibiotics.
In a recent genomic study by Pal et al. [28], a total of 2522 fully sequenced bacterial genomes and 4582 plasmids were analyzed. The authors concluded from their large-scale study that plasmids have only a limited potential for horizontal transfer of biocides and metals resistance by co-selection.
Prior studies have also tested isolates at both the genomic and phenotypic levels. One such study showed co-regulation of resistance to heavy metals and carbapenems through the CzcR–CzcS system in Pseudomonas aeruginosa strain PT5. In that study, it was shown that a mutation in the CzcS sensor protein found in zinc and imipenem resistant isolates led to efflux pump CzcCBA overexpression and down-regulation of the OprD porin resulting in a co-selection for both increased zinc and carbapenem resistance [52]. In a series of retrospective studies screening E. faecium isolated from different species, it was found that tcrB (transfer copper resistance) and ermB (transfer macrolide resistance) genes were present on the same conjugative plasmid. However, the data did not demonstrate a co-selection between these two phenomena and the strong correlation between copper and macrolide resistance was found only in pig isolates. In addition, while the prevalence of macrolide resistance in isolates decreased during the years covered in the study, the prevalence of copper resistance among pig E. faecium isolates remained more or less the same. The authors argued that the reduction in the antimicrobial usage during this period lead to a decrease in antimicrobial resistance, whereas in the same time period the use of copper derivatives remained unchanged. Therefore, they concluded that copper exposure might not alone be sufficient to induce antimicrobial resistance and a strong selective pressure of macrolide administration should be present to select the antimicrobial resistance [40, 53, 54].
There are few experimental studies available evaluating the induction of antimicrobial resistance following metal exposure. Peltier et al. [55] investigated antimicrobial resistance to ciprofloxacin, oxytetracycline, and tylosin in zinc-activated sludge bioreactors. Zinc application alone did not affect zinc and antimicrobial resistance to ciprofloxacin and oxytetracycline. Increased antimicrobial resistance could be the result of co-exposure of zinc and antimicrobial agents. Berg et al. [56] found that strains isolated from soil treated with copper for 21 months were more resistant to both copper and indirectly antimicrobials compared to control plots.
In contrast to the above-mentioned studies in which co-selection was the subject of discussion, there are also studies reporting counter-selection of heavy metal and antimicrobial resistance [57]. Hölzel et al. [26] found that while exposure to zinc and copper increased the rate of β-lactam resistance in E. coli, the presence of mercury was associated with a lower rate of antimicrobial resistance.
Conclusions
In summary, our results do not indicate a co-selection process of antimicrobial resistance and higher zinc tolerance in the MDR isolates of our feeding trials. An increase of E. coli more tolerant to zinc due to the feeding of high zinc concentrations as an explanation for the increase of multi-drug resistant isolates via co-selection can therefore be excluded. This seems to be also true for copper tolerance levels. These results would appear to argue against a co-selection mechanism for drug-resistance after zinc supplementation, since we did not find an association between antimicrobial resistance and phenotypic zinc/copper tolerance for the same isolates. We also found that zinc exposure did not have an effect on either zinc or copper phenotypic tolerance of the isolates.
An explanation for an increase in MDR isolates from piglets with high zinc dietary feeding in our previous studies could be that resistant bacteria to antimicrobial agents are more persistent to stresses such as zinc or copper exposure. Ciesinski et al. have argued that the increase in multi-drug resistant E. coli populations is associated with persistence of the resistant population under the influence of high dietary zinc, while in that study the total number of E. coli population had been decreased.
Another explanation might be that in the zinc-fed groups, zinc activates genes involved in metal ion resistance to deal with the metal ion load, and which might also be involved in antibiotic resistance, but this is a transient phenotypic zinc resistance. In accordance to this argumentation, Peltier et al. also found that zinc exposure increases resistance to antibiotics but had a minimal effect on zinc resistance [55]. In addition, the duration of experiments, co-exposure to both metal and antimicrobial agents and concentration of the substances could play role in either in vivo or in vitro-resistance studies.
Interpretation and analysis of resistance data based only on genetic data should be made carefully, a combination of both genetic and phenotypic resistance determinations is required, and it will also be important to show whether resistance could be developed in non-resistant isolates. The result of these types of studies could have implications for the prophylactic use of zinc in the field, i.e. pigs daily fed zinc to prevent infections.
Methods
Sample origin
A total of 210 E. coli isolates originally collected during two independent zinc feeding trials (S1 and S2) in 36 and 32 piglets respectively were used in this study (S1 = 105, S2 = 105) [18, 58]. All the experimental trials of these studies were approved by the local state office of occupational health and technical safety ‘Landesamt für Gesundheit und Soziales, Berlin’ (LaGeSo Reg. Nr. 0347/09 and LaGeSo Reg. Nr. 0296/13). The E. coli isolates were isolated from intestinal contents (digesta) on the 1st, 2nd and 4th weeks of both feeding trials. The first trial (S1) was a clonal study concentrating on the diversity of the E. coli analyzed via PFGE, which identified 105 clones from 1481 isolates in either only control or only zinc feeding groups independent of sampling time. In this study, one isolate from each of the 105 clones was tested. To obtain a comparable number of samples from the second feeding trial (S2), we randomly chose 105 isolates using representative random sampling method out of a total of 550 samples isolated from digesta [59]. The second feeding trial was performed with a selective culturing approach using CHROMagar Orientation plates supplemented with one of nine different antimicrobials as well as CHROMagar Orientation plates without supplementation to select resistant E. coli populations during the zinc treatment. Antibiotic concentrations in media plates were adapted from Guenther et al. [60] or are derived from the breakpoint concentrations of the Clinical and Laboratory Standards Institute [61, 62]. The schematic workflow of E. coli analyses (Fig. 6) shows the study design of previous and current studies.
In both trials, zinc oxide (Sigma Aldrich, Taufkirchen, Germany) was applied as a feed supplement to a high zinc feeding group (2000–2500 ppm) and background control (50–70 ppm). Further details of the animal trials can be found in the original publications [18, 58].
Phenotypic antimicrobial resistance
All isolates were initially screened for their resistance profiles against ampicillin, chloramphenicol, gentamicin, streptomycin, tetracycline, cefotaxime, enrofloxacin, sulfamethoxazole/trimethoprim and imipenem (BD BBL Sensi-Disc Antimicrobial Susceptibility Test Discs, Becton-Dickinson, United States) according to the standards of the Clinical and Laboratory Standards Institute [63]. The results from the agar disc diffusion tests were confirmed using minimum inhibitory concentration (MIC) microdilution using cation adjusted Mueller Hinton II medium (Micronaut breakpoint plates, Genzyme Diagnostics, Rüsselsheim, Germany) according to CLSI standards (CLSI, 2008). Based on their resistance patterns these strains were stratified as multi-drug resistant or non-multi-drug resistant according to the definition of Schwarz et al. [44], as resistant (resistant to at least one antimicrobial agent) or susceptible (completely sensitive to the tested antimicrobials).
Phenotypic zinc/copper resistance testing
Overnight cultures of all E. coli isolates were adjusted to McFarland Standard 0.5 (1.5 × 108 CFU) and 50 µl of 1:200 dilution of adjusted suspensions in Mueller–Hinton broth (Roth, Karlsruhe, Germany) were used as inocula for incubations for 16 to 20 h at 35 °C in biocide and heavy metal microtiter-plates (Merlin, Bornheim-Hersel, Germany). The plates contained a wide range of concentrations of biocides/heavy metals in twofold dilution steps including 32 to 8192 μg/ml copper sulfate (COP) and 4 to 8192 μg/ml zinc chloride (ZKC) [45]. In our study, the minimal inhibitory concentration data of two heavy metals including copper sulfate and zinc chloride were collected. To prevent drying of the plates during incubation a sealing tape was used to seal the surface of the plate. After the incubation, the MIC for zinc was determined visually and reported as the growth breakpoint. E. coli ATCC25922 and ATCC10536 strains were used as reference strains for internal quality control.
Statistical analysis
Statistical analysis was performed based on the combined datasets from both zinc feeding trials. Isolates were stratified irrespective of the zinc feeding either as multi-drug resistant (MDR) or non- multi-drug resistant (NMDR) isolates, as well as resistant (R) (at least one resistance) or susceptible (S) isolates. In addition, the isolates were subsequently grouped based on their origin from either high-zinc supplementation group (zinc) or the background control (control). Statistical analyses were performed using the SPSS software, version 25.0 (IBM, New York, NY, USA). The normal distribution of data was evaluated by a 1-sample Kolmogorov–Smirnov test. Mann–Whitney (non-parametric test) and chi-square tests were used for the analysis of data [64, 65]. The correlation between zinc tolerance and copper tolerance was calculated using Spearman rank correlation test (non-parametric correlation) [66]. The non-normally distributed data are shown as the median ± standard deviation (SD), and P < 0.05 was considered statistically significant.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Casewell M, Friis C, Marco E, McMullin P, Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. 2003;52(2):159–61.
de Lange CFM, Pluske J, Gong J, Nyachoti CM. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci. 2010;134(1–3):124–34.
Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 2013;97(2):207–37.
Lallès JP, Bosi P, Smidt H, Stokes CR. Nutritional management of gut health in pigs around weaning. Proc Nutr Soc. 2007;66(2):260–8.
Gadde U, Kim WH, Oh ST, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18(1):26–45.
Crane JK, Naeher TM, Shulgina I, Zhu C, Boedeker EC. Effect of zinc in enteropathogenic Escherichia coli infection. Infect Immun. 2007;75(12):5974–84.
Crane JK, Broome JE, Reddinger RM, Werth BB. Zinc protects against shiga-toxigenic Escherichia coli by acting on host tissues as well as on bacteria. BMC Microbiol. 2014;14(1):145.
Melin L, Wallgren P. Aspects on feed related prophylactic measures aiming to prevent post weaning diarrhoea in pigs. Acta Vet Scand. 2002;43(4):231–45.
Kwon CH, Lee CY, Han SJ, Kim SJ, Park BC, Jang I, et al. Effects of dietary supplementation of lipid-encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim Sci J. 2014;85(8):805–13.
Rademacher C, Masepohl B. Copper-responsive gene regulation in bacteria. Microbiology. 2012;158(Pt 10):2451–64.
Coleman JE. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946.
Vallee BL, Auld DS. Functional zinc-binding motifs in enzymes and DNA-binding proteins. Faraday Discuss. 1992;93:47–65.
Aarestrup FM, Hasman H, Jensen LB, Moreno M, Herrero IA, Dominguez L, et al. Antimicrobial resistance among enterococci from pigs in three European countries. Appl Environ Microbiol. 2002;68(8):4127–9.
Vahjen W, Pietruszynska D, Starke IC, Zentek J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015;7:23.
Bednorz C, Oelgeschlager K, Kinnemann B, Hartmann S, Neumann K, Pieper R, et al. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. IJMM. 2013;303(6–7):396–403.
Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14(4):176–82.
Starke IC, Pieper R, Neumann K, Zentek J, Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol Ecol. 2014;87(2):416–27.
Ciesinski L, Guenther S, Pieper R, Kalisch M, Bednorz C, Wieler LH. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE. 2018;13(1):e0191660.
Seiler C, Berendonk TU. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol. 2012;3:399.
Wales A, Davies R. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4(4):567.
Chapman JS. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int Biodeterior Biodegrad. 2003;51(4):271–6.
Nguyen CC, Hugie CN, Kile ML, Navab-Daneshmand T. Association between heavy metals and antibiotic-resistant human pathogens in environmental reservoirs: a review. Front Environ Sci Eng. 2019;13(3):46.
Nishino K, Nikaido E, Yamaguchi A. Regulation of multidrug efflux systems involved in multidrug and metal resistance of Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189(24):9066–75.
Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016. https://doi.org/10.1128/microbiolspec.VMBF-0016-2015.
Cavaco LM, Hasman H, Aarestrup FM. Zinc resistance of Staphylococcus aureus of animal origin is strongly associated with methicillin resistance. Vet Microbiol. 2011;150(3–4):344–8.
Hölzel CS, Müller C, Harms KS, Mikolajewski S, Schäfer S, Schwaiger K, et al. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ Res. 2012;113:21–7.
Lloyd NA, Janssen SE, Reinfelder JR, Barkay T. Co-selection of mercury and multiple antibiotic resistances in bacteria exposed to mercury in the Fundulus heteroclitus gut microbiome. Curr Microbiol. 2016;73(6):834–42.
Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DGJ. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015;16(1):964.
Song J, Rensing C, Holm PE, Virta M, Brandt KK. Comparison of metals and tetracycline as selective agents for development of tetracycline resistant bacterial communities in agricultural soil. Environ Sci Technol. 2017;51(5):3040–7.
Lee LJ, Barrett JA, Poole RK. Genome-wide transcriptional response of chemostat-cultured Escherichia coli to zinc. J Bacteriol. 2005;187(3):1124–34.
Bass L, Liebert CA, Lee MD, Summers AO, White DG, Thayer SG, et al. Incidence and characterization of integrons, genetic elements mediating multiple-drug resistance, in avian Escherichia coli. Antimicrob Agents Chemother. 1999;43(12):2925–9.
Ghosh A, Singh A, Ramteke PW, Singh VP. Characterization of large plasmids encoding resistance to toxic heavy metals in Salmonella abortus equi. Biochem Biophys Res Commun. 2000;272(1):6–11.
Mukherjee G, Ghosh T. Metal ion interaction with penicillins—part VII: mixed-ligand complex formation of cobalt(II), nickel(II), copper(II), and zinc(II) with amplicillin and nucleic bases. J Inorg Biochem. 1995;59(4):827–33.
Cooper GL, Louie A, Baltch AL, Chu RC, Smith RP, Ritz WJ, et al. Influence of zinc on Pseudomonas aeruginosa susceptibilities to imipenem. J Clin Microbiol. 1993;31(9):2366–70.
Ou JT, Anderson TF. Effect of Zn2+ on bacterial conjugation: inhibition of mating pair formation. J Bacteriol. 1972;111(1):177–85.
Ou JT. Effect of Zn2+ on bacterial conjugation: increase in ability of F-cells to form mating pairs. J Bacteriol. 1973;115(2):648–54.
Stokes HW, Nesbø CL, Holley M, Bahl MI, Gillings MR, Boucher Y. Class 1 integrons potentially predating the association with Tn402-like transposition genes are present in a sediment microbial community. J Bacteriol. 2006;188(16):5722–30.
Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287(17):13549–55.
Rensing C, Grass G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev. 2003;27(2–3):197–21313.
Hasman H, Aarestrup FM. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob Agents Chemother. 2002;46(5):1410–6.
Becerra-Castro C, Machado RA, Vaz-Moreira I, Manaia CM. Assessment of copper and zinc salts as selectors of antibiotic resistance in Gram-negative bacteria. Sci Total Environ. 2015;530–531:367–72.
Silveira E, Freitas AR, Antunes P, Barros M, Campos J, Coque TM, et al. Co-transfer of resistance to high concentrations of copper and first-line antibiotics among Enterococcus from different origins (humans, animals, the environment and foods) and clonal lineages. J Antimicrob Chemother. 2014;69(4):899–906.
Gullberg E, Albrecht LM, Karlsson C, Sandegren L, Andersson DI. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio. 2014;5(5):e01918-14.
Schwarz S, Silley P, Simjee S, Woodford N, van Duijkeren E, Johnson AP, et al. Editorial: assessing the antimicrobial susceptibility of bacteria obtained from animals. J Antimicrob Chemother. 2010;65(4):601–4.
Deus D, Krischek C, Pfeifer Y, Sharifi AR, Fiegen U, Reich F, et al. Comparative analysis of the susceptibility to biocides and heavy metals of extended-spectrum beta-lactamase-producing Escherichia coli isolates of human and avian origin Germany. Diagn Microbiol Infect Dis. 2017;88(1):88–92.
Aarestrup FM, Hasman H. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Vet Microbiol. 2004;100(1–2):83–9.
Brocklehurst KR, Morby AP. Metal-ion tolerance in Escherichia coli: analysis of transcriptional profiles by gene-array technology. Microbiology. 2000;146(Pt 9):2277–82.
Liedtke J, Vahjen W. In vitro antibacterial activity of zinc oxide on a broad range of reference strains of intestinal origin. Vet Microbiol. 2012;160(1–2):251–5.
Humphrey B, Thomson NR, Thomas CM, Brooks K, Sanders M, Delsol AA, et al. Fitness of Escherichia coli strains carrying expressed and partially silent IncN and IncP1 plasmids. BMC Microbiol. 2012;12:53.
Foster TJ. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983;47(3):361.
Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Cloeckaert A, et al. Comparative analysis of extended-spectrum-{beta}-lactamase-carrying plasmids from different members of Enterobacteriaceae isolated from poultry, pigs and humans: evidence for a shared {beta}-lactam resistance gene pool? J Antimicrob Chemother. 2009;63(6):1286–8.
Perron K, Caille O, Rossier C, Van Delden C, Dumas JL, Köhler T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J Biol Chem. 2004;279(10):8761–8.
Aarestrup FM. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J Clin Microbiol. 2000;38(7):2774–7.
Hasman H, Aarestrup FM. Relationship between copper, glycopeptide, and macrolide resistance among Enterococcus faecium strains isolated from pigs in Denmark between 1997 and 2003. Antimicrob Agents Chemother. 2005;49(1):454–6.
Peltier E, Vincent J, Finn C, Graham DW. Zinc-induced antibiotic resistance in activated sludge bioreactors. Water Res. 2010;44(13):3829–36.
Berg J, Tom-Petersen A, Nybroe O. Copper amendment of agricultural soil selects for bacterial antibiotic resistance in the field. Lett Appl Microbiol. 2005;40(2):146–51.
Agga GE, Scott HM, Amachawadi RG, Nagaraja TG, Vinasco J, Bai J, et al. Effects of chlortetracycline and copper supplementation on antimicrobial resistance of fecal Escherichia coli from weaned pigs. Prev Vet Med. 2014;114(3–4):231–46.
Bednorz C, Guenther S, Oelgeschlager K, Kinnemann B, Pieper R, Hartmann S, et al. Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli pathotypes that adhere to the gut mucosa. Appl Environ Microbiol. 2013;79(24):7896–904.
EpiTools. EpiTools epidemiological calculators: the Australian biosecurity cooperative research centre for emerging infectious disease; 2019. https://epitools.ausvet.com.au/.
Guenther S, Grobbel M, Lübke-Becker A, Goedecke A, Friedrich ND, Wieler LH, et al. Antimicrobial resistance profiles of Escherichia coli from common European wild bird species. Vet Microbiol. 2010;144(1):219–25.
NCCLS CaLSIf. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard—second edition. NCCLS document M31-A2; 2002.
Institute CaLS. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. CLSI document M100-S23; 2013.
CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard—CLSI document M31-A3, third edition. CLSI, Wayne, PA, USA; 2008.
Pearson KX. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philos Mag. 1900;50(302):157–75.
Wilcoxon F. Individual comparisons by ranking methods. Biometr Bull. 1945;1(6):80–3.
Hotelling H, Pabst MR. Rank correlation and tests of significance involving no assumption of normality. Ann Math Stat. 1936;7:29–43.
Acknowledgements
We thank Dr. Birgit Walther for support and helpful discussions. We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 393148499) and the Open Access Publication Fund of the University of Greifswald.
Funding
This work was supported by DFG research grant WI 1436/12-1.
Author information
Authors and Affiliations
Contributions
LHW and SG provided funding and supervision of this study. FG, LHW and SG developed the design and concept of the study. FG, LC and CB obtained the data. FG, VJ, LP and KT were involved in analysis and interpretation of the data. FG, LC, CB, VJ, KT, LHW and SG have drafted the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghazisaeedi, F., Ciesinski, L., Bednorz, C. et al. Phenotypic zinc resistance does not correlate with antimicrobial multi-resistance in fecal E. coli isolates of piglets. Gut Pathog 12, 4 (2020). https://doi.org/10.1186/s13099-019-0342-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-019-0342-5