Abstract
Background
Chickens are regarded as the main reservoir for human campylobacteriosis. Little is known about the interaction between Campylobacter jejuni (C. jejuni) and chickens. This interaction may be influenced by the stage of maturation of the immune system, developing gut microbiota composition and other factors including breed and diet. Our aim was to investigate the impact of breed, and diet on C. jejuni colonization and host immune responses in chickens. Birds were inoculated with 104 colony forming units (CFU) of C. jejuni or diluent at one (Exp. 1) or 22 (Exp. 2) days post hatch. We compared local immune cell subpopulations, cytokine expression levels, and gut microbiota composition between broiler-type (BT) and layer-type (LT) birds fed with either commercial broiler feed (bf) or layer feed (lf).
Results
Lower colonization rates were observed in the older age group independent of breed and diet. Independent of breed, birds fed with bf showed higher CFU of C. jejuni compared to lf-fed groups. Campylobacter jejuni-inoculation had a significant effect on lymphocyte numbers and cytokine expression levels in BT birds independent of feeding strategy (p < 0.05). These effects were not detected in LT birds, only LT birds fed with bf showed a significant increase in IL-8-expression at 7 days post C. jejuni inoculation compared to LT-control birds (p < 0.05). Diet influenced gut microbiota composition in a comparable manner between BT and LT birds, but changes in microbiota composition associated with C. jejuni inoculation varied between breeds.
Conclusions
Diet and breed influenced C. jejuni colonization, immune responses and microbiota composition to a different extent comparing between LT and BT birds. The mechanisms behind these differences have to be elucidated further. Our results suggest that selection for more resistant breeds in combination with adapted feeding strategies may help to reduce Campylobacter colonization levels in commercial poultry in the future.
Similar content being viewed by others
Background
Campylobacter species, in particular Campylobacter jejuni (C. jejuni), cause the majority of human food-borne bacterial gastroenteritis in the industrialized world [1, 2]. Campylobacter jejuni is found in a range of domesticated animals, and chickens are the predominant reservoir for C. jejuni [3]. So far no suitable strategies have been implemented which allow a reliable prevention of C. jejuni colonization of chickens in the field [4]. The reduction of C. jejuni colonization rates may be a reachable goal [5, 6]. While pro- and prebiotics have led to inconsistent results [7–9], other control measures including feeding strategies and the use of more resistant breeds may allow significant reduction of C. jejuni colonization [10–12].
The induction of local and systemic humoral immune responses [13] have been described after C. jejuni-inoculation suggesting that C. jejuni may be not only a commensal bacteria of chickens [14]. Campylobacter jejuni induced innate immune responses in vitro in different avian cell lines, including HD 11 macrophages, primary chicken kidney cells and primary chicken embryo intestinal cells [15, 16]. In vivo studies demonstrated an increase of proinflammatory cytokines following C. jejuni colonization. Campylobacter jejuni-inoculated birds showed an increase in the mRNA expression of interleukin (IL)-6 and the chicken IL-8-homolog in ileal and caecal tissues [17]. This may be associated directly with colonizing Campylobacter or indirectly with a C. jejuni mediated changes in the microbiota composition, including bacterial species such as Staphylococci, Enterococci, Enterobactericeae or Escherichia coli [18] and subsequently a modified immune response, which has not become clear so far. However, the immune responses in vivo may be affected and modified by many factors. Most studies are difficult to compare because different C. jejuni strains and dosages, different breeds of birds and age groups were used [14, 17, 19–21]. Li et al. demonstrated by caecal transcriptome and gene expression profiling that one broiler line may be more resistant than another line to C. jejuni infection [20, 21]. Mainly meat type birds were investigated and different breeds compared [14, 20–22], but field observations also described the disease in layer-type birds [23], which is associated with the sole isolation of Campylobacter in affected tissue.
Often only one time point post C. jejuni inoculation was investigated not considering the dynamics of colonization [17, 24].
The role of T cells in the control of C. jejuni in mice and human beings was demonstrated, but little is known about T cell responses in chickens [15, 25, 26]. It has been suggested that C. jejuni infections in avian species are associated with Th1 polarization of the immune response [27, 28].
It can be speculated that changes in the poultry diets may modify the caecal microbiota and gut health of chickens, and therefore pertinently affect the presence of C. jejuni in the chicken gut. Recent studies have shown that the feeding strategy can alter the viscosity of gut content as well as the histomorphology of the chicken gut [11], and modify goblet cell glycoconjugates in the intestinal tract in vitro [29]. It is not fully clear how different feeding strategies may alter the colonization pattern of the intestinal bacteria, the development of local immunity and subsequently modulate local immune reactions in response to C. jejuni colonization. Most of these studies were conducted in broilers and focused on the relationship between C. jejuni colonization and nutritional changes [10, 30–32].
The goal of this study was to investigate the interaction between breed and feeding strategy on C. jejuni colonization in commercial hybrid layer and broiler type birds with a cross-over study. Campylobacter jejuni-inoculated and non-inoculated groups of both breeds were either fed with commercial broiler-feed (bf) or layer-feed (lf). We investigated local and systemic immune reactions as well as possible differences in gut microbiota composition. Our data clearly demonstrate that feed composition, as well as breed, influenced the outcome of C. jejuni colonization, immunity development and the gut microbiota, providing the basis for follow-up studies on the possibility of a reduction of C. jejuni colonization by the selection for more resistant breeds in combination with protective feeding strategies.
Methods
Animals
Embryonated eggs from commercial layer-type (LT) hybrids (Lohmann Selected Leghorn, LSL) chickens were provided by the KG Geflügelzuchtbetriebe Gudendorf-Ankum GmbH & Co. KG, Ankum, Germany and eggs from the commercial Ross-308 broiler-type (BT) chickens were obtained from the BWE Hatchery Weser-Ems GmbH & Co. KG, Visbek, Rechterfeld, Germany. Eggs were incubated and hatched at the Clinic for Poultry, University of Veterinary Medicine Hannover, Germany. Chickens were housed and raised at the Clinic of Poultry, University of Veterinary Medicine Hannover.
All BT or LT birds were kept in the same room on wood shavings until the age of inoculation. Afterwards, inoculated and non-inoculated experimental groups were moved to different isolation rooms (one for inoculated and one for non-inoculated control birds) with units with wire floors. All groups received feed from the same source (broiler-type or layer-type feed fed to either BT or LT birds).
Commercial broiler and layer feed (Table 1) as well as water were provided ad libitum. Birds were fed a standard starter diet up to 14 days of age and then received a grower diet until the end of the experiment. Birds were distributed randomly to different groups based on SRS (simple random sample), and observed daily for the presence of clinical signs. All birds tested were negative for C. jejuni by cloacal swabs on the day of C. jejuni inoculation. The animals did not receive any vaccination.
Bacterial strains and C. jejuni inoculum preparation
The C. jejuni strain of serogroup Lior6 had been isolated from a chicken at the Clinic of Poultry, University of Veterinary Medicine Hannover, Germany and was stored in skim milk at −70 °C [26].
The cryopreserved bacteria were thawed and plated on charcoal cefoperazone dexoxycholate agar (CCDA, Oxoid, Basingstoke, England). The plates were incubated for 48 h under microaerophilic conditions (10% CO2, 5% O2, 85% hydrogen) at 38 °C. After 2 days, one C. jejuni colony was transferred into 3 ml Standard-I-Bouillon (Merck, Damstadt, Germany) and incubated for another 48 h under microaerophilic conditions at 38 °C.
One milliliter of the bacterial suspension was diluted with sterile phosphate buffered saline (PBS) to achieve approximately 104 CFU/ml (Colony Forming Units) for oral inoculation. To confirm the CFU of C. jejuni in the inocula, the bacterial suspension was serially diluted in a 10-fold dilution series, spread on CCDA plates and incubated for 48 h at 38 °C. After incubation the colonies were counted to calculate the CFU [17].
Isolation of intraepithelial lymphocytes and flow cytometric analysis
Single cell suspensions of intraepithelial lymphocytes (IEL) were prepared as previously described in detail [33].
106 IEL of the caecum were triplestained with a combination of the following antibodies (final concentrations per ml): mouse-anti-chicken-CD3 (2 µg) [34] conjugated to phycoerythrin (R-PE), biotinylated mouse-anti-chicken-CD8β (5 µg) [35] used in conjunction with streptavidin conjugated to SpectralRed™ (SPRD) (5 µg) and fluorescein (FITC)-conjugated mouse-anti-chicken CD4 (5 µg). All antibodies were obtained from Southern Biotech, provided by Biozol, Eching, Germany. The lymphocyte population was gated for CD3+ IELs according to size and granularity and 200,000 events per caecum sample were measured for R-PE, FITC or SPRD positive staining with a Beckman Coulter Epics XL© flow cytometer. The stained cells were analyzed by using the EXPO 32 ADC software program (Beckman Coulter Company, Miami, FL). CD3+ IELs were then analyzed for positive staining with anti-CD4-FITC and anti-CD8-SPRD. Presented is the percentage of CD4+ and CD8+ T lymphocytes within the CD3+ cell population.
Histology
Samples of liver and middle-caecum were collected, fixed in phosphate-buffered formalin (4%) for 24 h and further processed for histological examination following standard procedures. The different tissue sections of 2 µm were investigated microscopically for histopathological lesions such as oedema in the lamina propria of caecum or crypt abscesses and cell ballooning as previously described in mice and chicken after C. jejuni inoculation [36, 37].
Immunohistochemistry
Frozen sections of middle caecum were processed as previously described [33, 38]. Sections were stained with one of the following mouse-anti-chicken unlabeled monoclonal antibodies: anti-CD4, anti-CD8β and anti-Bu1 (0.05 µg/ml) (Southern Biotech, provided by Biozol, Eching, Germany). The secondary anti-mouse IgG biotinylated antibodies and ABC reagent (Vectastain® Elite® ABC Kit, Vector Laboratories Inc., provided by Linaris, Wertheim-Bettingen, Germany) were applied according to the manufacturer’s instructions. The enzyme-linked ABC complex was visualized by the reaction with 3.3′-diaminobenzidine (DAB) chromagen substrate and hydrogen peroxide (DAB peroxidase substrate Kit, Vector Laboratories Inc.). Sections were examined with light microscopy. The different lymphocyte populations were evaluated by counting the number of positive stained cells per 3 crypts in the lamina propria of 5 representative microscopic fields at 200× optical magnification per animal [33].
Real-time quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from caecum samples with 1000 µl Trifast®-GOLD reagent (PeqLab, Biotechnologie GmbH, Erlangen, Germany) according to the manufacturer’s instructions. RNA-quality and concentrations were determined using the NanoDrop ND-1000 (PeqLab, Biotechnologie GmbH).
All details of the specific primers and probes for the detection of expressed cytokines IL-6 and the chicken IL-8 homolog as well as the house-keeping gene 28S have been described previously [15, 39]. Real-time quantitative RT-PCR was performed using the AgPath-ID One-Step RT-PCR Kit (Applied Biosystems, Ambion, USA). Amplification and quantification of the specific products was carried out by using the Mx3005P™ thermal cycle system and Mx3005P™ Q PCR Software (STRATAGENE, Agilent Technologies Company, USA), respectively. The following cycle profile was applied: one cycle at 50 °C for 30 min and 95 °C for 10 min, and 40 cycles at 95 °C for 20 s and 60 °C for 1 min.
The results were normalized with the house-keeping gene 28S [40], the expression of which was comparable between birds of C. jejuni-inoculated and non-inoculated ones, and were expressed as 40-Ct in mRNA expression in the tissues of C. jejuni inoculated birds and C. jejuni-free controls.
DNA purification and pyrosequencing
Microbiota was characterized by the next generation sequencing of the V3/V4 variable region of 16S rRNA genes. Caecal samples were homogenized using zirconia silica beads (BioSpec Products) in a Mag NALyzer (Roche Diagnostics). Following homogenization, the DNA was extracted using the QIAamp DNA Stool Mini Kit according to the manufacturer’s instructions (Qiagen). The DNA concentration and quality was determined spectrophotometrically and the DNA was stored at −20 °C until use. Prior to PCR, DNA samples were diluted to 5 ng/μl and used as a template with forward primer 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG -MID- GT-CCTACGGGNGGCWGCAG-3′ and reverse primer 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG -MID- GT-GACTACHVGGGTATCTAATCC-3′. The sequences in italics served as an index and adapter ligation while underlined sequences allowed an amplification over the V3/V4 region of 16S rRNA genes. MIDs represent different sequences of 5, 6, 9 or 12 bp in length designed to differentiate samples. PCR amplification and clean- up were performed using KAPA Taq HotStart PCR kit (Kapa Biosystems). In the next step the DNA concentration was determined fluorometrically and the DNA was diluted to 100 ng/µl. Groups of 14 PCR products with the same MID sequences were indexed with a Nextera XT Index Kit following the manufacturer’s instructions (Illumina). Prior to sequencing, the concentration of differently indexed samples was determined using a KAPA Library Quantification Complete kit (Kapa Biosystems). All indexed samples were diluted to 4 ng/µl and 20% of phiX DNA was added. Sequencing was performed using MiSeq Reagent Kit v3 and MiSEQ 2000 apparatus according to the manufacturer’s instructions (Illumina).
Sequence analysis
Fasta and qual files generated after Illumina sequencing were uploaded into Qiime software [41]. Reverse reads were shortened to a length of 250 bp and forward and reverse sequences were joined. Quality trimming criteria were set to a value of 19 and no mismatch in the MID sequences. In the next step, chimeric sequences were predicted by slayer algorithm and excluded from subsequent analysis. The resulting sequences were then classified by RDP Seqmatch with an OTU (operational taxonomic units) discrimination level set to 97% followed by UniFrac analysis [42]. Principal coordinate analysis (PCoA) was used for data visualization.
Experimental design
Experiment 1 (Exp. 1)
72 commercial broilers and 72 commercial layer pullets were divided into two subgroups. Subgroups of C. jejuni-free commercial broilers and layer pullets were fed either with broiler feed (bf) or layer feed (lf). 18 birds per subgroup were orally inoculated with C. jejuni strain Lior 6 at 1 day post hatch (dph) by crop inoculation with a dose of approximately 104 colony-forming units (CFU) or C. jejuni-free medium. Six birds of each subgroup were randomly selected at a specific point of time and necropsied at 1, 7 and 14 days post inoculation (dpi). Individual body weight and pathological lesions were determined. To avoid cross contamination, liver samples were collected under sterile conditions at the first step of necropsy, and subsequently were investigated for C. jejuni by taking direct swabs from the depth of the parenchym. Additionally, caecal content was analyzed for the number of CFU of C. jejuni/g caecal content. Samples of middle caecum were taken for immunohistochemical staining of local lamina propria lymphocyte (LPL) populations. IELs of caecum were isolated for the flow cytometric analysis of T cell subpopulations. Middle caecum from birds was also evaluated for cytokine expression levels at 1 and 7 dpi by using quantitative real-time RT-PCR (qRT-PCR). In addition, the caecal content of birds was collected at 7 dpi and investigated for gut microbiota composition.
Experiment 2 (Exp. 2)
Exp. 1 was repeated with 22-days-old birds. 72 commercial broilers and 72 commercial layer pullets were divided into two subgroups. 18 birds per subgroup were orally inoculated with either C. jejuni-free medium or approximately 104 CFU of C. jejuni at 22 dph. Most parameters were investigated as described in Exp. 1. Cytokine expression levels and gut microbiota composition were not determined in this experiment.
Statistical analysis
Statistical analyses were carried out with the Statistix version 10.0 (Analytical software, Thallahassee, FL, USA). For the statistical analysis of differences in the CFU numbers of C. jejuni of different C. jejuni-inoculated subgroups of the same age at the indicated dpi, Kruskal–Wallis all-pairwise comparisons test was used. The differences in the IEL T cell subsets and the number of LPL immune cell populations between C. jejuni-inoculated and non-inoculated controls were determined by Two-Sample T test or Wilcoxon Rank sum T test, respectively. The difference in cytokine expression level between C. jejuni-inoculated and C. jejuni-free control groups was determined by Two-Sample T test. Statistical significance was designated as p < 0.05.
Results
Clinical signs and tissue lesions
No clinical signs, macroscopical or microscopical lesions were observed in the caecum of either C. jejuni-free control or C. jejuni-inoculated groups in both experiments. No significant differences in body weight were detected between C. jejuni-inoculated and non-inoculated control birds within each breed and diet-group at the different necropsy days (data not shown, p > 0.05). BT and LT birds fed with layer-feed (lf) had significantly lower body weight on all necropsy days compared to broiler-feed (bf) fed groups of the respective breed (data not shown, p < 0.05).
Qualitative detection of C. jejuni in liver
Campylobacter jejuni positive-liver samples were only observed in LT-bf birds, which had been inoculated with C. jejuni at 1 dph, with one of six and two of six chickens having a C. jejuni positive liver at 1 and 7 dpi respectively.
Quantitative detection of C. jejuni in caecal content
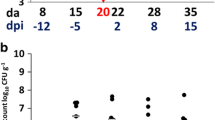
Caecal content was evaluated for C. jejuni quantitatively at 1, 7 and 14 dpi. Lower CFU numbers as well as a lower colonization rate were observed in birds, which had been inoculated at 22 dph compared to birds inoculated at 1 dph at all investigated time points. Feed and breed influenced the colonization pattern of C. jejuni. LT birds fed with bf, which had been inoculated at 1 dph, showed significantly higher numbers of CFU of C. jejuni compared to LT birds fed with lf at 1, 7 and 14 dpi indicating the effect of feed (Table 2, p < 0.05). This observation was confirmed also with LT birds, which had been inoculated at 22 dph, at 1 and 7 dpi, but the difference was not significant due to higher individual variation.
In addition in Exp. 1, LT birds fed with bf showed higher CFU numbers of C. jejuni compared to BT birds on most of the investigated points of time. This effect was only detected in Exp. 2 at 1 dpi, while at later points of time BT birds had higher colonization rates than LT birds.
Detection of local intestinal lymphocyte populations
Immunohistochemical detection of T and B lymphocytes in the lamina propria of caecum
As expected based on previous studies [17], the numbers of T- and B-lymphocyte populations in the lamina propria were influenced by age. Caecal CD4+ , CD8+ and Bu1+ LPL gradually increased in control birds over time (Figs. 1, 2).
Immunohistochemical detection of T and B lymphocytes in caecum of 1-day-old birds. Immunohistochemical detection of CD4+ (a, b), CD8β+ (c, d) and Bu1+ (e, f) lymphocytes in caecal lamina propria of birds, which had been inoculated with either C. jejuni or C. jejuni-free medium at 1 day post hatch (Experiment 1). Broiler-type (BT) (a, c, e) and layer-type (LT) (b, d, f) birds, which were fed with either broiler feed (bf) or layer feed (lf). Asterisk letters indicate significant differences between C. jejuni-inoculated (C. jejuni) and C. jejuni-free control (con) groups at the indicated days post C. jejuni inoculation (n = 6/group, p < 0.05)
Immunohistochemical detection of T and B lymphocytes in caecum of 22-days-old birds. Immunohistochemical detection of CD4+ (a, b), CD8β+ (c, d) and Bu1+ (e, f) lymphocytes in caecal lamina propria of birds, which had been inoculated with either C. jejuni or C. jejuni-free medium at 22 day post hatch (Experiment 2). Broiler-type (BT) (a, c, e) and layer-type (LT) (b, d, f) birds, which were fed with either broiler feed (bf) or layer feed (lf). Asterisk letters indicate significant differences between C. jejuni-inoculated (C. jejuni) and C. jejuni-free control (con) groups at the indicated days post C. jejuni inoculation (n = 6/group, p < 0.05)
Breed influenced the number of T- and B-lymphocyte populations in LPL. Independent of feeding strategy and age, higher numbers of caecal CD4+ and CD8+ cells were observed in C. jejuni-free LT birds compared to C. jejuni-free BT birds (Figs. 1, 2).
A significant increase in B lymphocytes as well as CD4+ and CD8+ T lymphocytes in caecal lamina propria was observed after C. jejuni inoculation of 1-day-old as well as 22-days-old BT but not in LT chickens. Campylobacter jejuni-inoculated BT-bf birds showed an increase in the number of caecal CD4+ and CD8+ T cells at 7 and 14 dpi (Figs. 1, 2). B lymphocytes increased at 1 and 7 dpi, which was significant for the younger age group (p < 0.05). Fewer changes in lymphocyte numbers were observed in BT-lf chickens in which a significant increase was recorded only in CD8+ T lymphocytes at 7 dpi for both age groups and B lymphocytes at 1 dpi for the younger age group (Figs. 1, 2). In LT birds, a transient increase in numbers of CD8+ T lymphocytes and B lymphocytes was observed also in the caecum of LT-bf chickens at 1 dpi (p > 0.05).
Overall, there was a significant effect of C. jejuni colonization and breed on LPL populations in caecum (p < 0.05).
Detection of IEL in caecum
Campylobacter jejuni inoculation affected IEL cell numbers only in BT birds, but not in LT chickens, indicating a breed effect (Fig. 3) (p < 0.05). A significant upregulation of CD4+ intraepithelial T cells (Fig. 3a, c) was observed in C. jejuni-inoculated BT-bf birds at 7 dpi for both age groups compared to C. jejuni-free control groups (p < 0.05). Campylobacter jejuni-inoculated BT-lf birds showed up-regulation in CD4+ IEL numbers only at 14 dpi in birds, which had been inoculated at 1 dph (Fig. 3a). Numbers of CD8+ IEL were not affected in either experiment.
Flow cytometric analysis of T lymphocytes. Flow cytometric analysis of the percentage of CD4+ intraepithelial lymphocytes (IEL) in caecum of broiler-type (BT) (a, c) and layer-type (LT) birds (b, d) fed with either broiler feed (bf) or layer feed (lf). Birds had been C. jejuni-inoculated at 1 (Exp. 1) (a, b) and 22 (Exp. 2) (c, d) days post hatch. CD4+ T cells were gated within the CD3+ IEL population. Asterisk letters indicate significant differences between C. jejuni-inoculated (C. jejuni) and C. jejuni-free control (con) groups at the indicated days post C. jejuni inoculation (n = 6/group, p < 0.05)
Detection of cytokine mRNA expression levels
For this investigation we selected birds, which had been inoculated at 1 dph and focused on 1 and 7 dpi, because most prominent changes in immune cell numbers were observed in this age group [15, 43].
We observed a breed and C. jejuni effect. Independent of C. jejuni-infection and feeding strategy, C. jejuni-free layers showed significant higher IL-6 mRNA expression levels only at 1 dpi, as well as IL-8 mRNA expression levels at both 1 and 7 dpi compared to C. jejuni-free broilers (Fig. 4), indicating a breed effect (p < 0.05).
mRNA expression levels in caecum of 1-day-old birds. IL-6 (a, b) and IL-8 (c–f) mRNA expression levels in caecum samples of broiler-type (BT; a, c, e) and layer-type (LT; b, d, f) birds fed with either broiler feed (bf) or layer feed (lf). Birds had been C. jejuni-inoculated at 1 day post hatch. Comparison of cytokine mRNA expression between C. jejuni-free control and C. jejuni-inoculated birds at 1 dpi (a–d). Comparison of cytokine mRNA expression between C. jejuni-free control and C. jejuni-inoculated birds at 7 dpi (e, f). Data are presented as the mean mRNA expression (40-Ct) normalized to 28S. Asterisk letters indicate significant differences between C. jejuni-inoculated (C. jejuni) and C. jejuni-free control (con) groups at the indicated days post C. jejuni inoculation (n = 6/group, p < 0.05)
Campylobacter jejuni colonization modified the expression level of the chicken IL-8-homolog and IL-6 significantly in BT birds (p < 0.05). Independent of feeding strategy, significant up-regulations were detected in C. jejuni-inoculated BT birds for both IL-6 and IL-8 at 1 dpi (Fig. 4a, c) as well as for IL-8 at 7 dpi (Fig. 4e) compared to C. jejuni-free groups (p < 0.05). A significant up-regulation of IL-8 was also observed at 7 dpi in the caecum of C. jejuni-inoculated LT-bf birds. IL-6 mRNA expression levels were not significantly different between C. jejuni-inoculated and non-inoculated groups in both BT and LT birds at 7 dpi (data not shown).
Gut microbiota composition
UniFrac analysis followed by PCoA indicated effects of genetic background and feeding strategy using both unweighted and weighted analyses since clear separations were observed between subgroups (Fig. 5). This analysis also indicated a difference in gut microbiota composition between C. jejuni-inoculated and control groups (Fig. 5). Non-inoculated broilers fed with bf diet had a higher abundance of Enterobacteriaceae and Clostridiaceae, and a lower abundance of Lachnospiraceae, in comparison to the non-infected birds fed with lf diet. In the absence of C. jejuni infection, chicken breed had a less significant impact on microbiota composition than feed composition (Fig. 6). After the C. jejuni inoculation, more significant differences in microbiota composition were observed in chickens fed bf than in chickens provided with lf diet. Irrespective of diet, microbiota composition of LT chickens changed to a greater extent after C. jejuni inoculation than the microbiota of BT chickens (Fig. 6).
Microbiota diversity. Microbiota diversity in caecum of broiler-type (BT) and layer-type (LT) birds fed with either broiler feed (bf) or layer feed (lf). UniFrac analysis followed by PCoA indicates variability in the caecal microbiota composition based on different feeding strategy (a, b) and genetic background (c, d). “con” = C. jejuni-free control, “C. jejuni” = C. jejuni-inoculated. (Figures were generated from raw data but when we produced the figures from normalized data, these were essentially the same. We therefore used maximal data available for each sample in this figure)
Gut microbiota composition. The composition of the main families present caecal microbiota in chicken. The sum of the appropriate families as indicated in the figure legend provides information on the microbiota distribution at the phylum level. Taxonomy summary and microbial diversity of the operational taxonomic units (OTU) from caecal samples collected at 7 days post inoculation from broiler-type (BT) and layer-type (LT) birds fed with either broiler feed (bf) or layer feed (lf), which had been C. jejuni-inoculated at 1 day post hatch (n = 6 per group). “con” = C. jejuni-free control, “C. jejuni” = C. jejuni-inoculation. (Figures were generated from raw data but when we produced the figures from normalized data, these were essentially the same. We therefore used maximal data available for each sample in this figure)
Discussion
No studies have been conducted so far that combined a comparison of genetically different chickens and different diets. We selected two commercially commonly applied breeds of broiler- and layer-type birds to be as close as possible to the field situation. Both were fed with commercially available broiler or layer feed in each combination. Two age groups of 1 (Exp. 1) and 22 (Exp. 2) days old chicken were inoculated with C. jejuni and investigated for C. jejuni-colonization pattern, local T and B cell numbers, and for the younger age group for IL-6 as well as IL-8-homolog mRNA expression in caecum and gut microbiota composition. Consistent with previous investigations [27, 36, 44], none of the C. jejuni-inoculated subgroups showed any clinical signs, pathological or histopathological lesions. We observed different immune reactions and colonization patterns between BT and LT birds, age groups, as well as different feeding groups.
Breed has been considered to be one of the key factors influencing the host response and outcome of C. jejuni colonization [14, 20]. To the best of our knowledge, no investigations were conducted to demonstrate breed effects between BT and LT birds. Other studies compared mainly between different broiler lines [14, 20, 21]. Some broiler lines were shown to be more susceptible to C. jejuni infection, which were characterized by diarrhea, a prolonged inflammatory response and induction of lymphocyte activation, compared to less susceptible breeds [14, 20]. The mechanisms behind these differences are not known. Possible modification of breed differences by feed composition has not been investigated before.
In our experiment we observed differences in host immune responses between BT and LT chickens. Comparing C. jejuni-free groups, LT birds showed higher numbers of caecal CD4+ and CD8+ LPL than BT chickens independent of the investigated age. mRNA expression levels of IL-6 and IL-8 were also higher in the caecum of non-inoculated LT chickens, compared to C. jejuni-free broilers independent of feeding strategy and microbiota composition (Exp. 1). It was demonstrated before that the breed may significantly affect early cytokine mRNA expression in the caecum of 1-day-old chickens with and without Salmonella enteritidis infection [45]. These differences in immune cell numbers and cytokine expression pattern in C. jejuni-free BT and LT birds may explain why also differences in the immune response were detected after C. jejuni inoculation of these different breeds. We observed that C. jejuni-inoculated broilers showed an upregulation of CD4+ and CD8+ T cells in the lamina propria independent of feeding strategy, which was significant for CD4+ T cells for both age groups compared to C. jejuni-free controls (p < 0.05). While a clear upregulation of immune cell numbers and cytokine expression was observed in C. jejuni-inoculated BT birds, C. jejuni-inoculated LT birds did not show any significant changes, which suggests that C. jejuni-related changes in microbiota composition alone are not sufficient to modify cytokine expression pattern. Differences in the magnitude of immune responses between LT- and BT-birds were also detected after the infection with other avian pathogens such as Salmonella spp., infectious bursal disease virus, or Marek’s disease virus [46–50].
To further understand the role of cellular immunity in C. jejuni infection in chicken, we investigated the mRNA expression level of IL-6 and IL-8. It is evident that IL-6 plays an important role in the maintenance of the intestinal epithelium and also in transiting innate to the adaptive immunity [51]. IL-8 is a potent chemokine and an inducer of local inflammatory responses, which attracts and activates macrophages and leukocytes [52, 53]. It has been reported that IL-8 can contribute to the clearance of C. jejuni by inducing the activation of neutrophils and T cell subpopulations [54]. Consistent with previous studies [14, 17], mRNA expression levels of IL-6 and IL-8 were increased in C. jejuni-inoculated birds compared to C. jejuni-free controls. The difference was significant for both cytokines in BT at 1 and 7 dpi independent of diet, but only for IL-8 in LT-bf birds at 7 dpi, indicating that BT birds might mount a more vigorous immune response after C. jejuni inoculation compared to LT chickens. The difference in background expression levels between BT and LT may contribute to this observation.
Changing poultry diet may modify the caecal microbiota and gut health of chicken, and therefore may affect the colonization pattern of C. jejuni [11, 12]. Recent studies have shown that maize- or wheat-based diets, which contain different levels of crude protein, can alter the viscosity of gut content and histomorphology of the chicken gut [11], and subsequently reduce C. jejuni colonization in broilers [9]. Changes in diet due to different protein sources or non-antibiotic feed additive may modify the gastrointestinal environment creating disturbances in the resident microbiota thus allowing—directly or indirectly—either a proliferation or reduction of bacterial pathogens [10, 55]. We observed that LT-bf birds, which were inoculated with C. jejuni at 1 dph, showed a significantly higher numbers of CFU of C. jejuni compared to LT-lf chickens (p < 0.05). Crude protein and fat levels in broiler feed were higher than in layer feed in both starter and grower diet. Other studies have shown that the numbers of caecal CFU of C. jejuni were significantly lower in birds fed with protein derived from plants compared to groups which received animal-protein-based feed [56]. Evidence for the role of protein and fat in C. jejuni colonization is also given from investigations of mice [57]. Mice were fed with either murine diet or “human cafeteria diet” such as curry sausages, fried chicken nuggets, French fries, and others, which have a higher level of protein and fat. Obesity-induced mice were more susceptible to C. jejuni infection compared to the standard diet group [57]. This observation was also confirmed in dogs [58]. This diet influence however, was not significant between the two BT subgroups possibly due to the more vigorous immune response compensating feed effects.
In poultry, it was demonstrated that higher fiber levels in diet may enhance short chain fatty acid production, gut metabolism, and intestinal immunity [59, 60]. We observed a higher fiber content in the starter layer diet with 47% compared to 31% in broiler feed, which may also have contributed to the differences observed between feeding groups.
Different C. jejuni strains may have different potential for systemic invasion [61]. On the other side host factors, feed or other so far undefined factors may contribute to the evasion of C. jejuni from the gut. C. jejuni strain Lior6 was not detected in the liver of BT and LT birds in previous studies [26]. Interestingly, in this study C. jejuni invaded the liver of LT-bf birds which also showed the highest level of CFU of C. jejuni in the caecum. It may be speculated that there may be a correlation between CFU in the gut and intestinal permeability allowing evasion of Campylobacter to other tissues [62].
Overall, our results provide circumstantial evidence that the colonization pattern of commensal bacteria and development of local immunity may be influenced by the breed. Independent of feeding strategy, BT chickens mounted a more vigorous immune response compared to LT birds following C. jejuni inoculation. Feeding strategy affected the caecal microbiota composition of both BT and LT birds, and significantly influenced the outcome of C. jejuni colonization in LT birds. Further studies should be carried out to understand which components of gut microbiota may influence local immune development and the outcome of C. jejuni infection. This should be investigated by considering possible breed differences and may open up new strategies to reduce C. jejuni on the poultry flock level and subsequently may reduce the risk of human infections.
Abbreviations
- C. jejuni :
-
Campylobacter jejuni
- BT:
-
broiler-type
- LT:
-
layer-type
- bf:
-
broiler feed
- lf:
-
layer feed
- CFU:
-
colony forming units
- dph:
-
days post hatch
- IL:
-
interleukin
- CCDA:
-
charcoal cefoperazone dexoxycholate agar
- PBS:
-
phosphate buffered saline
- IEL:
-
intraepithelial lymphocyte
- OTU:
-
operational taxonomic units
- PCoA:
-
principal coordinate analysis
- dpi:
-
days post inoculation
- LPL:
-
lamina propria lymphocyte
References
Frost JA. Current epidemiological issues in human campylobacteriosis. Symp Ser Soc Appl Microbiol. 2001;30:85S–95S.
Bronzwaer S, Hugas M, Collins JD, Newell DG, Robinson T, Makela P, Havelaar A. EFSA’s 12th Scientific Colloquium—assessing health benefits of controlling Campylobacter in the food chain. Int J Food Microbiol. 2009;131:284–5.
Hermans D, Van Deun K, Martel A, Van Immerseel F, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet Res. 2011;42:82.
Wagenaar JA, French NP, Havelaar AH. Preventing Campylobacter at the source: why is it so difficult? Clin Infect Dis. 2013;57:1600–6.
Lin J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog Dis. 2009;6:755–65.
Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl Environ Microbiol. 2011;77:8605–14.
Arsi K, Donoghue AM, Woo-Ming A, Blore PJ, Donoghue DJ. Intracloacal inoculation, an effective screening method for determining the efficacy of probiotic bacterial isolates against Campylobacter colonization in broiler chickens. J Food Prot. 2015;78:209–13.
Robyn J, Rasschaert G, Hermans D, Pasmans F, Heyndrickx M. In vivo broiler experiments to assess anti-Campylobacter jejuni activity of a live Enterococcus faecalis strain. Poult Sci. 2013;92:265–71.
Van Deun K, Haesebrouck F, Van Immerseel F, Ducatelle R, Pasmans F. Short-chain fatty acids and l-lactate as feed additives to control Campylobacter jejuni infections in broilers. Avian Pathol. 2008;37:379–83.
Thibodeau A, Fravalo P, Yergeau E, Arsenault J, Lahaye L, Letellier A. Chicken caecal microbiome modifications induced by Campylobacter jejuni colonization and by a non-antibiotic feed additive. Plos ONE. 2015;10(7):e0131978.
Molnar A, Hess C, Pal L, Wagner L, Awad WA, Husveth F, Hess M, Dublecz K. Composition of diet modifies colonization dynamics of Campylobacter jejuni in broiler chickens. J Appl Microbiol. 2015;118:245–54.
Grilli E, Vitari F, Domeneghini C, Palmonari A, Tosi G, Fantinati P, Massi P, Piva A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: from in vitro to in vivo, a proof of concept. J Appl Microbiol. 2013;114:308–17.
Cawthraw S, Ayling R, Nuijten P, Wassenaar T, Newell DG. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 1994;38:341–9.
Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio. 2014;5:e01364.
Smith CK, Kaiser P, Rothwell L, Humphrey T, Barrow PA, Jones MA. Campylobacter jejuni-induced cytokine responses in avian cells. Infect Immun. 2005;73:2094–100.
Li YP, Ingmer H, Madsen M, Bang DD. Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence-associated genes. BMC Microbiol. 2008;8:107.
Smith CK, Abuoun M, Cawthraw SA, Humphrey TJ, Rothwell L, Kaiser P, Barrow PA, Jones MA. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol Med Microbiol. 2008;54:114–21.
Sofka D, Pfeifer A, Gleiss B, Paulsen P, Hilbert F. Changes within the intestinal flora of broilers by colonisation with Campylobacter jejuni. Berl Munch Tierarztl Wochenschr. 2015;128:104–10.
Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, Karlyshev AV, Dorrell N, Wren BW, Barrow PA. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect Immun. 2004;72:3769–76.
Li X, Swaggerty CL, Kogut MH, Chiang HI, Wang Y, Genovese KJ, He H, Zhou H. Gene expression profiling of the local cecal response of genetic chicken lines that differ in their susceptibility to Campylobacter jejuni colonization. Plos ONE. 2010;5(7):e11827.
Li XY, Swaggerty CL, Kogut MH, Chiang HI, Wang Y, Genovese KJ, He H, Pevzner IY, Zhou HJ. Caecal transcriptome analysis of colonized and non-colonized chickens within two genetic lines that differ in caecal colonization by Campylobacter jejuni. Anim Genet. 2011;42:491–500.
Williams LK, Sait LC, Trantham EK, Cogan TA, Humphrey TJ. Campylobacter infection has different outcomes in fast- and slow-growing broiler chickens. Avian Dis. 2013;57:238–41.
Ruiz-Palacios GM, Escamilla E, Torres N. Experimental Campylobacter diarrhea in chickens. Infect Immun. 1981;34:250–5.
Chaloner G, Wigley P, Humphrey S, Kemmett K, Lacharme-Lora L, Humphrey T, Williams N. Dynamics of dual infection with Campylobacter jejuni strains in chickens reveals distinct strain-to-strain variation in infection ecology. Appl Environ Microbiol. 2014;80:6366–72.
Jennings JL, Sait LC, Perrett CA, Foster C, Williams LK, Humphrey TJ, Cogan TA. Campylobacter jejuni is associated with, but not sufficient to cause vibrionic hepatitis in chickens. Vet Microbiol. 2011;149:193–9.
Pielsticker C, Glünder G, Rautenschlein S. Colonization pattern of C. jejuni isolates of human and avian origin and differences in the induction of immune responses in chicken. Vet Immunol Immunopathol. 2016;169:1–9.
Shaughnessy RG, Meade KG, Cahalane S, Allan B, Reiman C, Callanan JJ, O’Farrelly C. Innate immune gene expression differentiates the early avian intestinal response between Salmonella and Campylobacter. Vet Immunol Immunopathol. 2009;132:191–8.
Hermans D, Pasmans F, Heyndrickx M, Van Immerseel F, Martel A, Van Deun K, Haesebrouck F. A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut. Crit Rev Microbiol. 2012;38:17–29.
Fernandez F, Sharma R, Hinton M, Bedford MR. Diet influences the colonisation of Campylobacter jejuni and distribution of mucin carbohydrates in the chick intestinal tract. Cell Mol Life Sci. 2000;57:1793–801.
van Gerwe T, Bouma A, Klinkenberg D, Wagenaar JA, Jacobs-Reitsma WF, Stegeman A. Medium chain fatty acid feed supplementation reduces the probability of Campylobacter jejuni colonization in broilers. Vet Microbiol. 2010;143:314–8.
Hermans D, Martel A, Van Deun K, Verlinden M, Van Immerseel F, Garmyn A, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult Sci. 2010;89:1144–55.
Skanseng B, Kaldhusdal M, Moen B, Gjevre AG, Johannessen GS, Sekelja M, Trosvik P, Rudi K. Prevention of intestinal Campylobacter jejuni colonization in broilers by combinations of in-feed organic acids. J Appl Microbiol. 2010;109:1265–73.
Schwarz A, Gauly M, Abel H, Das G, Humburg J, Rohn K, Breves G, Rautenschlein S. Immunopathogenesis of Ascaridia galli infection in layer chicken. Dev Comp Immunol. 2011;35:774–84.
Chen CL, Ager LL, Gartland GL, Cooper MD. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986;164:375–80.
Chan MM, Chen CL, Ager LL, Cooper MD. Identification of the avian homologues of mammalian CD4 and CD8 antigens. J Immunol. 1988;140:2133–8.
Beery JT, Hugdahl MB, Doyle MP. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl Environ Microbiol. 1988;54:2365–70.
Murphy H, Cogan T, Humphrey T. Direction of neutrophil movements by Campylobacter-infected intestinal epithelium. Microbes Infect. 2011;13:42–8.
Berndt A, Wilhelm A, Jugert C, Pieper J, Sachse K, Methner U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect Immun. 2007;75:5993–6007.
Rautenschlein S, von Samson-Himmelstjerna G, Haase C. A comparison of immune responses to infection with virulent infectious bursal disease virus (IBDV) between specific-pathogen-free chickens infected at 12 and 28 days of age. Vet Immunol Immunopathol. 2007;115:251–60.
Powell FL, Rothwell L, Clarkson MJ, Kaiser P. The turkey, compared to the chicken, fails to mount an effective early immune response to Histomonas meleagridis in the gut. Parasite Immunol. 2009;31:312–27.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–72.
Edwards LA, Nistala K, Mills DC, Stephenson HN, Zilbauer M, Wren BW, Dorrell N, Lindley KJ, Wedderburn LR, Bajaj-Elliott M. Delineation of the innate and adaptive T-cell immune outcome in the human host in response to Campylobacter jejuni infection. Plos ONE. 2010;5:e15398.
Dhillon AS, Shivaprasad HL, Schaberg D, Wier F, Weber S, Bandli D. Campylobacter jejuni infection in broiler chickens. Avian Dis. 2006;50:55–8.
Cheeseman JH, Kaiser MG, Ciraci C, Kaiser P, Lamont SJ. Breed effect on early cytokine mRNA expression in spleen and cecum of chickens with and without Salmonella enteritidis infection. Dev Comp Immunol. 2007;31:52–60.
Kaiser MG, Lamont SJ. Genetic line differences in survival and pathogen load in young layer chicks after Salmonella enterica serovar enteritidis exposure. Poult Sci. 2001;80:1105–8.
Abasht B, Kaiser MG, van der Poel J, Lamont SJ. Genetic lines differ in toll-like receptor gene expression in spleens of chicks inoculated with Salmonella enterica serovar Enteritidis. Poult Sci. 2009;88:744–9.
Aricibasi M, Jung A, Heller ED, Rautenschlein S. Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain. Vet Immunol Immunopathol. 2010;135:79–92.
Tippenhauer M, Heller DE, Weigend S, Rautenschlein S. The host breed influences infectious bursal disease virus pathogenesis in chickens by modulation of T cells responses and cytokine gene expression. Dev Comp Immunol. 2013;40:1–10.
Sarson AJ, Parvizi P, Lepp D, Quinton M, Sharif S. Transcriptional analysis of host responses to Marek’s disease virus infection in genetically resistant and susceptible chickens. Anim Genet. 2008;39:232–40.
Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–8.
Fleckenstein JM, Kopecko DJ. Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J Clin Invest. 2001;107:27–30.
Larson CL, Shah DH, Dhillon AS, Call DR, Ahn S, Haldorson GJ, Davitt C, Konkel ME. Campylobacter jejuni invade chicken LMH cells inefficiently and stimulate differential expression of the chicken CXCLi1 and CXCLi2 cytokines. Microbiology. 2008;154:3835–47.
Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Gross U, Zautner AE. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. 2013;2013:526860.
Stanley D, Wu SB, Rodgers N, Swick RA, Moore RJ. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. Plos ONE. 2014;9:e104739.
Udayamputhoor RS, Hariharan H, Van Lunen TA, Lewis PJ, Heaney S, Price L, Woodward D. Effects of diet formulations containing proteins from different sources on intestinal colonization by Campylobacter jejuni in broiler chickens. Can J Vet Res. 2003;67:204–12.
Bereswill S, Plickert R, Fischer A, Kuhl AA, Loddenkemper C, Batra A, Siegmund B, Gobel UB, Heimesaat MM. What you eat is what you get: novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Eur J Microbiol Immunol. 2011;1:237–48.
Stavisky J, Radford AD, Gaskell R, Dawson S, German A, Parsons B, Clegg S, Newman J, Pinchbeck G. A case-control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev Vet Med. 2011;99:185–92.
Schley PD, Field CJ. The immune-enhancing effects of dietary fibres and prebiotics. Br J Nutr. 2002;87(Suppl 2):S221–30.
Wils-Plotz E, Jenkins M, Dilger R. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult Sci. 2013;92:735–45.
Knudsen KN, Bang DD, Andresen LO, Madsen M. Campylobacter jejuni strains of human and chicken origin are invasive in chickens after oral challenge. Avian Dis. 2006;50:10–4.
Awad WA, Molnar A, Aschenbach JR, Ghareeb K, Khayal B, Hess C, Liebhart D, Dublecz K, Hess M. Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immun. 2015;21:151–60.
Authors’ contributions
ZH, CP, and SR conceived and designed the experiments; ZH performed the experiments with the help of TW; ZH analyzed the data; LG performed and IR analyzed the data of gut microbiota composition; ZH, IR and SR wrote or helped to draft the paper. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Christine Haase, Sonja Bernhardt and Katja Stolpe for their excellent technical assistance and support. In addition we would like to thank Christine and Hermann Meyeringh for help with proofreading the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The data supporting the findings of this study are contained within the manuscript.
Ethics approval and consent to participate
All animal experiments were conducted in accordance to the Animal Welfare Regulation of Lower Saxony and were approved by the Lower Saxony State Office for Customer Protection and Food Safety (LAVES: 33.12-42505-04-13/1215).
Funding
This work was supported by EMIDA ERANet, DIFAGH: Development of immune function and avian gut health’, funded by the Federal Ministry of Education and Research (031A097A to S. R.). In addition, Zifeng Han was supported by the Chinese Scholar Council and Ivan Rychlik was partially supported by AdmireVet project CZ.1.05/2.1.00/01.0006 – ED0006/01/01 from the Czech Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Han, Z., Willer, T., Pielsticker, C. et al. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog 8, 56 (2016). https://doi.org/10.1186/s13099-016-0133-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-016-0133-1