Abstract
Background
Paenibacillus sp. strain VT-400, a novel spore-forming bacterium, was isolated from patients with hematological malignancies.
Methods
Paenibacillus sp. strain VT-400 was isolated from the saliva of four children with acute lymphoblastic leukemia. The genome was annotated using RAST and the NCBI Prokaryotic Genome Annotation Pipeline to characterize features of antibiotic resistance and virulence factors. Susceptibility to antibiotics was determined by the Kirby–Bauer disc diffusion method. We used a mouse model of pneumonia to study virulence in vivo. Mice were challenged with 7.5 log10–9.5 log10 CFU, and survival was monitored over 7 days. Bacterial load was measured in the lungs and spleen of surviving mice 48 h post-infection to reveal bacterial invasion and dissemination.
Results
Whole-genome sequencing revealed a large number of virulence factors such as hemolysin D and CD4+ T cell-stimulating antigen. Furthermore, the strain harbors numerous antibiotic resistance genes, including small multidrug resistance proteins, which have never been previously found in the Paenibacillus genus. We then compared the presence of antibiotic resistance genes against results from antibiotic susceptibility testing. Paenibacillus sp. strain VT-400 was found to be resistant to macrolides such as erythromycin and azithromycin, as well as to chloramphenicol and trimethoprim–sulphamethoxazole. Finally, the isolate caused mortality in mice infected with ≥8.5 log10 CFU.
Conclusions
Based on our results and on the available literature, there is yet no strong evidence that shows Paenibacillus species as an opportunistic pathogen in immunocompromised patients. However, the presence of spore-forming bacteria with virulence and antibiotic resistance genes in such patients warrants special attention because infections caused by spore-forming bacteria are poorly treatable.
Similar content being viewed by others
Background
Acute leukemia accounts for more than 10,000 deaths annually despite improved treatment regimens and novel cytostatic agents [1]. Pneumonia due to opportunistic Gram-positive Staphylococcus spp., Bacillus spp., and Enterococcus spp. is one of the leading causes of morbidity in these patients, as well as in patients with other forms of hematological malignancies, because of treatment-induced immunosuppression [2, 3].
The oral cavity, which hosts more than 700 commensal bacterial species, is the main reservoir of microorganisms that cause aspiration pneumonia [4, 5]. Thus, investigating the oral microbiome is essential to improve therapeutic strategies, especially for patients with hematological malignancies [6]. However, most commensal bacteria are not yet culturable, and molecular techniques based on cloning and sequencing the ribosomal 16S RNA have been used instead to identify species in the human microbiome [7]. Nevertheless, these techniques are prone to false negatives, such as when one bacterial species masks another, and thus underestimate bacterial diversity [8, 9]. In a previous study, we described Paenibacillus sp. strain VT-400, a novel spore-forming bacterium isolated from the saliva of patients with acute lymphoblastic leukemia [10]. The strain has never been previously detected in humans.
Notably, spore-forming bacteria are poorly studied, and only a few such bacteria have been described and are associated with the human microbiota [11, 12]. Spores tolerate high temperature, radiation, and noxious chemicals, harbor genes that confer antibiotic resistance, and allow bacteria to survive in unfavorable conditions [13, 14]. Thus, spores contribute significantly to the persistence of infection and the spread of antimicrobial resistance [15]. Indeed, prophylactic treatments like oral rinses are poorly effective against spores, and are thus not sufficiently reduce the bacterial load in the oropharynx, or prevent aspiration pneumonia in at-risk patients, especially those with underlying pathologies such as hematological malignancies [16, 17]. Therefore, identification and characterization of potentially infectious spore-forming microbial species are critical to improve the management or treatment of patients with acute leukemia.
Paenibacillus spp. was not known to cause human disease until recent reports implicated P. alvei, P. thiaminolyticus, and P. sputi in respiratory and urinary tract infection, as well as bacteremia in a patient on hemodialysis [18–20]. In this study, we describe Paenibacillus sp. strain VT-400, a novel bacterium isolated from the saliva of four children with hematological malignancies, and investigate its potential to cause pneumonia.
Methods
Bacterial strain
Paenibacillus sp. strain VT-400 was isolated from the saliva of four children with acute lymphoblastic leukemia who were hospitalized at First Pavlov State Medical University, St. Petersburg, Russia. Unless stated otherwise, the isolate was grown on Columbia agar with 5 % sheep blood (BioMerieux, France) and were stored at −80 °C in Columbia broth (BioMerieux) supplemented with 50 % glycerol. The strain was screened for hemolytic activity by cultivation at 37 °C for 48 h on agar plates supplemented with 5 % sheep blood. Clearing and greenish zones around colonies were considered to indicate β- and α-hemolytic activity, respectively. Primary morphological characterization was performed by light microscopy (Axiostar, Zeiss, Germany), and Gram staining was performed using a kit (Merck, Darmstadt, Germany).
To generate inoculum for infecting mice, the strain was grown at 37 °C for 48 h on Columbia agar with 5 % sheep blood. Colonies picked from the plate were then grown for 18 h at 37 °C in 5 mL Columbia broth. Cells were harvested by centrifugation at 3000×g for 15 min (Eppendorf 5415 C centrifuge; Eppendorf Geratgebau GmbH, Hamburg, Germany), and suspended in an isotonic phosphate buffer (0.15 mM, pH 7.2). The turbidity of the suspension was adjusted using a McFarland standard.
Genome annotation and phylogenetic analysis
Whole-genome sequences from isolates of Paenibacillus sp. strain VT-400 were aligned using MUSCLE, and phylogenetic trees were constructed based on the Tamura-Nei distance model in PHYML version 3.0, with 1000 bootstrap replicates [21–23]. The most closely related Paenibacillus genomes were included in the analysis. The genome was annotated and mined for virulence factors and antibiotic resistance genes using Rapid Annotation using Subsystems Technology (RAST) and the NCBI Prokaryotic Genome Annotation Pipeline [24, 25].
Antimicrobial susceptibility testing
Susceptibility to antibiotics was determined by the Kirby–Bauer disc diffusion method according to criteria defined by the Clinical and Laboratory Standards Institute [26]. The strain was tested for susceptibility to 30 µg amoxiclav, 10 μg ampicillin, 10 U penicillin, 30 µg vancomycin, 30 μg cefotaxime, 10 μg erythromycin, 15 μg azithromycin, 10 µg gentamicin, 30 µg amikacin, 30 μg kanamycin, 2 μg clindamycin, 30 μg doxycycline, 5 µg ciprofloxacin, 30 μg neomycin, 30 μg chloramphenicol, 30 μg tetracycline (Becton–Dickinson, USA) and 1.25 μg/23.75 μg trimethoprim-sulfamethoxazole (Oxoid, UK).
Pathogenicity in a mouse infection model
Adult C57BL/6 mice weighing approximately 20 g (Rappolovo, North-West region, Russia) were housed in individual cages in a facility free of known murine pathogens, and were provided feeding ad libitum. Animals were cared for in accordance with National Research Council recommendations, and experiments were executed in accordance with the Guide for the Care and Use of Laboratory Animals [27].
Animals were randomly designated into two groups of eight, which were used to measure overall survival and bacterial load. Mice were then anesthetized with 2 % isoflurane, and orally instilled with bacterial suspension as previously described [28]. Briefly, nares were blocked, and mice aspirated 50 µL Paenibacillus sp. strain VT400 into the lungs while being held vertically for 60 s. Mice received a total dose of 7.5 log10, 8.5 log10, or 9.5 log10 CFU/mouse. Control mice were treated with sterile 50 µL phosphate-buffered saline. Overall survival was assessed over 7 days, while bacterial load was measured in the lungs and spleen of surviving mice 48 h post infection.
Microbiological assessment of infected lung and spleen
Bacterial load in the spleen and lungs was measured 48 h post infection. Briefly, surviving animals in groups designated for this assessment were euthanized by CO2 and cervical dislocation. Lungs and spleen were collected and homogenized in 1 mL phosphate-buffered saline. As Paenibacillus sp. strain VT-400 was found to be resistant to chloramphenicol and trimethoprim, serial tenfold dilutions of tissue homogenates were plated on Columbia agar with 5 % sheep blood, 5 μg/mL chloramphenicol, and 10 μg/mL trimethoprim (Sigma Chemical Co., St Louis, MO, USA), and cultured at 37 °C. Colonies of spore-forming bacteria were counted after 48 h, and bacterial loads are reported as mean log10 CFU/g tissue ± SD. Morphology was characterized by light microscopy (Axiostar, Zeiss), and cells were Gram stained using a kit (Merck).
Ethical approval and consent
Ethical approval was granted by the First State I. P. Pavlov Medical University Ethics Committee (501/M2013). In accordance with ethical approval, consent to use human biological material was assumed following completion of consent forms.
Statistics
Survival was compared by Kaplan–Meier analysis log-rank test. Differences in bacterial load were evaluated by one-way analysis of variance in SigmaStat version 2.03 (SPSS, Inc., San Rafael, CA). A P value <0.05 was considered significant.
Results
Phylogenetic analysis
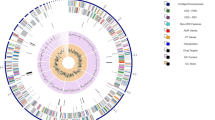
Paenibacillus sp. strain VT 400, which has never been detected in humans before, was isolated for the first time from the saliva of pediatric patients with acute lymphoblastic leukemia. In a previous study, whole-genome sequencing was performed on Illumina HiSeq 2500, with 125-fold average coverage [10]. Assembly generated 116 contigs spanning 6,986,122 bp, with G+C content 45.8 %.
On the basis of these analyses, the strain was identified as a novel species for which Paenibacillus sp. strain VT 400 was assigned, and its genome was deposited in GenBank under accession number LELF01000000. Phylogenetic analysis based on 16S rRNA demonstrated that Paenibacillus sp. strain VT 400 is clearly distinguished from other species, as well as from other strains of P. amylolyticus (Fig. 1).
Microbiological characteristics of Paenibacillus sp. strain VT 400
Paenibacillus sp. strain VT 400 is Gram-positive, aerobic, spore-forming, rod-shaped, and motile via peritrichous flagella [10]. Colonies growing on sheep blood agar are smooth, white pearl in color, and from 0.5 to 1 mm in diameter after 24 h at 37 °C in an aerobic atmosphere. β-hemolysis was observed around colonies growing on blood agar plates. The type strain is deposited in the Deutsche Sammlung fur Mikroorganismen und Zellkulturen (Braunschweig, Germany) under accession number DSM 100755.
Genes encoding virulence factors and in vivo pathogenicity
Analysis of the genome revealed a large number of genes encoding virulence factors that may contribute to pathogenicity (Table 1) [29]. Most are degradative enzymes and adhesins that may facilitate infection, including proteases, phospholipases, ureases, chitinases, and endopeptidases [30]. Significantly, we found chemotaxis proteins that were previously shown to contribute to bacterial virulence [31]. A couple of toxins or putative toxins were also detected, as well as superantigen CD4+ T-cell-stimulating antigen, which causes severe symptoms and septic shock [32].
We used a mouse model of pneumonia to study virulence in vivo. Mice were challenged with 7.5 log10–9.5 log10 CFU, and survival was monitored over 7 days (Fig. 2). All animals exhibited typical signs of acute infection within 24 h, including hypothermia, piloerection, breathing difficulty, narrowed palpebral fissures, trembling, and reduced locomotor activity. There was a direct correlation between severity of symptoms and dose. Accordingly, mortality depended on dose as well, with mortality observed within 48 h in mice exposed to 8.5 log10 and 9.5 log10 CFU Paenibacillus sp. strain VT 400.
Bacterial load was also measured in the lungs and spleen of surviving mice 48 h post infection (Table 2). To confirm the presence of Paenibacillus sp. strain VT 400, tissues were homogenized and plated on selective media. Spore-forming bacteria were identified by microscopy. There was approximately 2.47 log10 more CFU/g of infected lung tissue in the high-dose group than in the low-dose group (P < 0.05). In addition, the data indicated that Paenibacillus sp. strain VT 400 spread from the lungs to the spleen, in which bacterial load was also dose-dependent. Taken together, the data suggest that mortality is due to, at least in part, progressive bacterial invasion and dissemination.
Moreover, analysis of the Paenibacillus sp. strain VT 400 genome revealed an array of proteins involved in or essential for sporulation (Table 3). Phylogenetic analysis indicated that these genes are conserved and are closely related to other members of the Bacillaceae family [33].
Analysis of drug resistance genes and antimicrobial susceptibility testing
Genome analysis also revealed that Paenibacillus sp. strain VT 400 harbors different antibiotic resistance genes (Table 4). A total of 96 genes were major facilitator superfamily (MFS) plasma membrane transporters, 18 were multidrug ATP-binding cassette (ABC) transporters [34, 35]. Four genes were identified as multidrug ABC transporter permeases, eight as multidrug and toxic compound extrusion (MatE) transporters, and two as small multidrug resistance (SMR) proteins [36, 37]. A multidrug drug metabolite transporter (DMT) was also detected [38]. Moreover, the Paenibacillus sp. strain VT 400 genome also contains genes that confer resistance to specific antibiotics. Finally, genes encoding resistance to tellurium, tunicamycin, and bleomycin were also present. These compounds are used to treat hematological malignancies [39, 40].
The antibiotic susceptibility of Paenibacillus sp. strain VT 400 was then tested against an array of antimicrobials commonly used to treat nosocomial pneumonia [41]. As can be seen from Table 5, the strain was resistant to macrolides such as erythromycin and azithromycin, as well as to chloramphenicol and trimethoprim-sulfamethoxazole. However, it was sensitive to β-lactams, aminoglycosides, glycopeptides, tetracyclines, lincosamides, and fluoroquinolones.
Discussion
Bacteria that colonize the oral cavity are important pathogenic agents of pneumonia and other opportunistic infections, especially in immunocompromised hosts. We have now identified one such bacterium, Paenibacillus sp. strain VT 400, a novel species that was isolated from children with acute leukemia [28].
Whole-genome analysis indicated that this spore-forming bacterium harbors known virulence factors such as hemolysin, degradative enzymes, adhesins, and flagella. Moreover, CD4+ T-cell-stimulating antigen, a superantigen that causes toxic shock, is also present, along with other virulence determinants such as peptidases, ureases, lipases, and chitinases. Chemotaxis proteins were also found, suggesting that the isolate, which is motile, is capable of chemotaxis [42].
The detection of a strain such as Paenibacillus sp. strain VT 400 in patients with hematological malignancies is a critical result, especially in light of in vivo studies. In these experiments, mice intranasally challenged with at least 8.5 log10 CFU of the isolate died from pneumonia, and were found to have infected lungs as well as spleen, indicating dissemination of the infection. Taken together, the data suggest that the strain not only presents genetic features of pathogenic bacteria, but may indeed trigger a life-threatening infection.
In addition, the genome of Paenibacillus sp. strain VT 400 features numerous multidrug efflux transporters known to confer intrinsic and acquired resistance to many antibiotics used in clinical practice [43]. These proteins catalyze uptake, efflux, diffusion, solute exchange, and other mechanisms of bacterial defense against xenobiotics [44, 45]. In addition, these transporters are not drug-specific and are associated with multidrug resistance [46].
Moreover, the isolate contains two SMR efflux pumps, which are hallmarks of nosocomial infections and imply that Paenibacillus sp. strain VT 400 is most likely a circulating hospital strain, or a strain circulating among hematology patients [47]. SMR efflux pumps confer nosocomial antibiotic resistance and poor sensitivity to biocidal quaternary ammonium compounds [48, 49]. Notably, SMR proteins have never been previously found in Paenibacillus.
We detected chloramphenicol acetyltransferase, macrolide ABC transporter, vancomycin resistance protein, and FosB, which confer resistance to chloramphenicol, macrolide, vancomycin, and fosfomycin, respectively [50–52]. A bacteriocin resistance gene was also found, as were tetracycline resistance genes, including TetA [53, 54]. d-ala-d-ala ligase confers cycloserine resistance, while dihydrofolate reductase A is associated with resistance to trimethoprim and trimethoprim-sulfamethoxazole [55, 56]. In addition, the genome contains resistance genes to β-lactams, including metal-dependent hydrolases, as well as resistance genes to chemotherapeutic drugs.
Nevertheless, many resistance genes of Paenibacillus sp. strain VT 400 are not expressed, in accordance with the idea that many mutations do not lead to resistant phenotype [57]. Sporulation, such as in Paenibacillus sp. strain VT 400, preserves and disperses genetic material such as antibiotic resistance genes to overcome harsh environmental conditions [58, 59]. These spores may be particularly hazardous to immunocompromised patients.
Conclusions
This study expands the number of poorly characterized Paenibacillus spp. that may cause pulmonary disease in humans [18]. We provide virulence and antibiotic resistance data based on draft genomes and antimicrobial susceptibility testing. We also demonstrate the ability of the strain to trigger pneumonia in vivo, and to invade spleen tissue. Our data may have important implications in the clinic, as the oral microbial flora in patients with hematological malignancies could be a reservoir of pneumonia-causing agents.
Whether Paenibacillus sp. strain VT 400 is more prevalent in individuals with acute leukemia remains to be established. However, it is clear that the isolate may have direct clinical implications for patients with therapy-induced immunosuppression. We now intend to determine the prevalence of Paenibacillus sp. strain VT 400 among different groups of patients, as well as among patients beyond hematology and bone marrow transplantation units.
Availability of supporting data
The complete genome has been deposited in GenBank under the Accession No. LELF01000000. The type strain is deposited in the Deutsche Sammlung fur Mikroorganismen und Zellkulturen under Accession Number DSM 100755.
References
Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119:34–43.
Faderl S, Kantarjian H. Leukemias: principles and practice of therapy. New York: Wiley; 2011.
Rolston KV. Challenges in the treatment of infections caused by Gram-positive and Gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;Supplement 4:S246–52.
Frias-Lopez J. Targeting specific bacteria in the oral microbiome. Trends Microbiol. 2015;23:527–8.
Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:845–54.
Wang Y, Xue J, Zhou X, You M, Du Q, Yang X, Jingzhi H, Jing Z, Lei C, Mingyun L, et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS One. 2014. doi:10.1371/journal.pone.0102116.
West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, Prescott SL. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015;135:3–13.
Li K, Bihan M, Yooseph S, Methe BA. Analyses of the microbial diversity across the human microbiome. PLoS One. 2012. doi:10.1371/journal.pone.0032118.
Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol. 2008;74:4898–909.
Tetz G, Tetz V, Vecherkovskaya M. Complete genome sequence of Paenibacillus sp. strain VT 400, isolated from the saliva of a child with acute lymphoblastic leukemia. Genome Announc. 2015. doi:10.1128/genomeA.00894-15.
Hoyles L, Honda H, Logan NA, Halket G, La Ragione RM, McCartney AL. Recognition of greater diversity of Bacillus species and related bacteria in human faeces. Res Microbiol. 2012;163:3–13.
Tetz G, Tetz V. Complete genome sequence of Bacilli bacterium strain VT-13-104 isolated from the intestine of a patient with duodenal cancer. Genome Announc. 2015. doi:10.1128/genomeA.00705-15.
Setlow P. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu Rev Microbiol. 1985;49:29–54.
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol. 2000;64:548–72.
Barra-Carrasco J, Hernandez-Rocha C, Ibáñez P, Guzman-Duran AM, Álvarez-Lobos M, Paredes-Sabja D. Clostridium difficile spores and its relevance in the persistence and transmission of the infection. Rev Chilena Infectol. 2014;31:694–703.
Russell AD. Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev. 1990;3:99–119.
Neumann S, Krause SW, Maschmeyer G, Schiel X, von Lilienfeld-Toal M. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematological malignancies and solid tumors. Ann Hematol. 2013;92:433–42.
Kim K, Lee K, Yu H, Ryoo S, Park Y, Lee J. Paenibacillus sputi sp. nov., isolated from the sputum of a patient with pulmonary disease. Int J Syst Evol Microbiol. 2010;60:2371–6.
Padhi S, Dash M, Sahu R, Panda P. Urinary tract infection due to Paenibacillus alvei in a chronic kidney disease: a rare case report. J Lab Physicians. 2013;5:133.
Ouyang J, Pei Z, Lutwick L, Dalal S, Yang L, Cassai N, Sandhu K, Hanna B, Wieczorek R, Bluth M, Pincus MR. Paenibacillus thiaminolyticus: a new cause of human infection, inducing bacteremia in a patient on hemodialysis. Ann Clin Lab Sci. 2008;38:393–400.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704.
Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–63.
Pruitt KD, Tatusova T, Brown GR, Maglott DR. NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 2012;40:D130–5.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests: approved standard. M2-A11. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
Institute of Laboratory Animal Resources (US). Committee on Care, Use of Laboratory Animals, & National Institutes of Health (US). Division of Research Resources 1985. Guide for the care and use of laboratory animals. National Academies.
Crandon JL, Kuti JL, Nicolau DP. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob Agents Chemother. 2010;54:5115–9.
Clair G, Roussi S, Armengaud J, Duport C. Expanding the known repertoire of virulence factors produced by Bacillus cereus through early secretome profiling in three redox conditions. Mol Cell Proteomics. 2010;9:1486–98.
Petersen LM, Tisa LS. Influence of temperature on the physiology and virulence of the insect pathogen Serratia sp. strain SCBI. Appl Environ Microbiol. 2012;78:8840–4.
LaGier MJ, Bilokopytov I, Cockerill B, Threadgill DS. Identification and characterization of a putative chemotaxis protein, CheY, from the oral pathogen Campylobacter rectus. Internet J Microbiol. 2014. doi:10.5580/IJMB.21300.
Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, Etienne J. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis. 2005;41:771–7.
Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ Microbiol. 2012;14:2870–90.
Pao SS, Paulsen IT, Saier MH. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34.
Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–17.
Horn C, Bremer E, Schmitt L. Functional overexpression and in vitro re-association of OpuA, an osmotically regulated ABC-transport complex from Bacillus subtilis. FEBS Lett. 2005;579:5765–8.
He X, Szewczyk P, Karyakin A, Evin M, Hong WX, Zhang Q, Chang G. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature. 2010;467:991–4.
Jack DL, Yang NM, Saier H. The drug/metabolite transporter superfamily. Eur J Biochem. 2001;268:3620–39.
Calle Y, Palomares T, Castro B, del Olmo M, Bilbao P, Alonso-Varona A. Tunicamycin treatment reduces intracellular glutathione levels: effect on the metastatic potential of the rhabdomyosarcoma cell line S4MH. Chemotherapy. 2000;46:408–28.
Kang H, Kim T, Kim W, Choi C, Lee J, Kim G, Bae D. Outcome and reproductive function after cumulative high-dose combination chemotherapy with bleomycin, etoposide and cisplatin (BEP) for patients with ovarian endodermal sinus tumor. Gynecol Oncol. 2008;111:106–10.
American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol. 2003;57:249–73.
Salavert M, Calabuig E. Role of daptomycin in the treatment of infections in patients with hematological malignancies. Med Clin. 2010;135:36–47.
Jack DL, Storms ML, Tchieu JH, Paulsen IT, Saier MH. A broad-specificity multidrug efflux pump requiring a pair of homologous SMR-type proteins. J Bacteriol. 2000;182:2311–3.
Saier MH Jr, Paulsen IT Jr. Phylogeny of multidrug transporters. Semin Cell Dev Biol. 2001;12:2015–213.
Piddock LJ. Multidrug-resistance efflux pumps? Not just for resistance. Nat Rev Microbiol. 2006;4:629–36.
Fraimow HS, Tsigrelis C. Antimicrobial resistance in the intensive care unit: mechanisms, epidemiology, and management of specific resistant pathogens. Crit Care Clin. 2011;27:163–205.
Costa SS, Mourato C, Viveiros M, Melo-Cristino J, Amaral L, Couto I. Description of plasmid pSM52, harbouring the gene for the Smr efflux pump, and its involvement in resistance to biocides in a meticillin-resistant Staphylococcus aureus strain. Int J Antimicrob Agents. 2013;5:490–2.
Weinstein RA, Hooper DC. Efflux pumps and nosocomial antibiotic resistance: a primer for hospital epidemiologists. Clin Infect Dis. 2005;40:1811–7.
Kobayashi N, Nishino K, Yamaguchi A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J Bacteriol. 2001;183:5639–44.
Galopin S, Cattoir V, Leclercq R. A chromosomal chloramphenicol acetyltransferase determinant from a probiotic strain of Bacillus clausii. FEMS Microbiol Lett. 2009;296:185–9.
Thompson MK, Keithly ME, Harp J, Cook PD, Jagessar KL, Sulikowski GA, Armstrong RN. Structural and chemical aspects of resistance to the antibiotic fosfomycin conferred by FosB from Bacillus cereus. Biochemistry. 2013;52:7350–62.
Butcher BG, Helmann JD. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol Microbiol. 2006;60:765–82.
Levy SB, McMurry LM, Burdett V, Courvalin P, Hillen W, Roberts MC, Taylor DE. Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother. 1989;33:1373–4.
Caceres NE, Harris NB, Wellehan JF, Feng Z, Kapur V, Barletta RG. Overexpression of the d-alanine racemase gene confers resistance to d-cycloserine in Mycobacterium smegmatis. J Bacteriol. 1997;179:5046–55.
Eliopoulos GM, Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis. 2001;32:1608–14.
Suzuki S, Horinouchi T, Furusawa C. Prediction of antibiotic resistance by gene expression profiles. Nat Commun. 2014. doi:10.1038/ncomms6792.
Fenselau C, Havey C, Teerakulkittipong N, Swatkoski S, Laine O, Edwards N. Identification of β-lactamase in antibiotic-resistant Bacillus cereus spores. Appl Environ Microbiol. 2008;74:904–6.
Laflamme C, Gendron L, Turgeon N, Filion G, Ho J, Duchaine C. In situ detection of antibiotic-resistance elements in single Bacillus cereus spores. Syst Appl Microbiol. 2009;32:323–33.
Authors’ contributions
Conceived and designed experiments: VT, GT. Performed experiments: VT, GT, MV. Analyzed the data: VT, GT, MV. Contributed reagents, materials, and analysis tools: VT, GT. Helped draft the manuscript: GT, MV. All authors read and approved the final manuscript.
Acknowledgements
We thank Albert Tai for performing sequencing at the Genomics Core Facility of Tufts University and for his assistance in completing the project.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tetz, G., Tetz, V. & Vecherkovskaya, M. Genomic characterization and assessment of the virulence and antibiotic resistance of the novel species Paenibacillus sp. strain VT-400, a potentially pathogenic bacterium in the oral cavity of patients with hematological malignancies. Gut Pathog 8, 6 (2016). https://doi.org/10.1186/s13099-016-0089-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-016-0089-1