Abstract
Background
Insulin resistance (IR) is an important contributor to the development of hypertension (HTN), and the triglyceride-glucose (TyG) index has been proposed as a simple, reliable marker of IR. This study investigated the association between the TyG index and blood pressure (BP) elevation in a large general population.
Methods
The study enrolled 15,721 adults with no history of cardiometabolic diseases from the 2016–2019 Korea National Health and Nutrition Examination Survey. Participants were classified into quartiles based on the TyG index and BP was categorized as normal BP, elevated BP, pre-HTN, and HTN. The associations of the TyG index with BP categories were assessed using multivariate multinomial logistic regression models with normal BP as the reference group.
Results
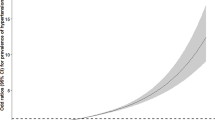
The mean systolic/diastolic BP and prevalence of HTN increased with the TyG index (P for trend < 0.001). The continuous TyG index had a strong dose-response relationship with increased odds of elevated BP, pre-HTN, and HTN. Compared with the lowest TyG index quartile, the highest TyG index quartile was significantly associated with higher odds of having elevated BP (odds ratio [OR], 1.52; 95% confidence interval [CI], 1.24–1.87; P for trend < 0.001), pre-HTN (OR, 2.22; 95% CI, 1.95–2.53; P for trend < 0.001), and HTN (OR, 4.24; 95% CI, 3.49–5.16; P for trend < 0.001).
Conclusion
We found that a higher TyG index was positively associated with the risk of increased BP in normal healthy individuals. This study suggests that the TyG index might serve as a potential predictor of HTN. However, further studies with larger sample sizes and various target populations in longitudinal designs are needed.
Similar content being viewed by others
Background
Hypertension (HTN) is an important public health problem because of its increasing prevalence and association with complications such as cardiovascular disease (CVD) [1]. According to the Korea Hypertension Fact Sheet 2020, the number of people with HTN increased from 3.0 million in 2002 to 9.7 million in 2018 [2]. Considering the impact of HTN on morbidity and mortality, there is a need for early predictors of incident HTN, especially in healthy individuals.
Insulin resistance (IR) is an important contributor to the development of HTN [3, 4]. Recently, the triglyceride-glucose (TyG) index, which is calculated using fasting triglycerides and plasma glucose (FPG), was suggested to be a reliable surrogate marker for IR [5, 6]. Several studies demonstrated that the TyG index is correlated with the homeostasis model assessment of insulin resistance (HOMA-IR) and better predicted IR than the HOMA-IR in some studies [5, 7,8,9]. Therefore, many studies have evaluated the TyG index as a predictor of diseases associated with IR, such as type 2 diabetes, metabolic syndrome, non-alcoholic fatty liver disease, and CVD [10,11,12,13]. Studies have demonstrated an association between the TyG index and HTN [14,15,16], albeit in different study populations.
No study has examined whether the TyG index can predict the risk of HTN in apparently healthy individuals. Therefore, this study evaluated the association between the quartiles of the TyG index and risk of HTN using data from the Korea National Health and Nutrition Examination Survey (KNHANES). Moreover, potential influences of obesity or insulin resistance on the association were also analyzed.
Methods
Data sources and study population
The study data were from the 7th (2016–2018) and 8th (2019) KNHANES conducted by the Korea Disease Control and Prevention Agency (KDCA). The KNHANES is a nationally representative cross-sectional survey that assesses the health and nutritional status of Koreans and monitors trends in health risk factors and the prevalence of major chronic diseases [17].
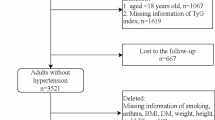
Of 25,995 adults aged 19 and over in the 2016–2019 KNHANES database, blood glucose and triglycerides levels measured after a fast of at least 8 h were available for 23,292 participants (Fig. 1). We also excluded participants whose blood pressure (BP) was not recorded (n = 76); who were taking diabetes medications (oral antihyperglycemic agents and insulins) or antihyperlipidemic agents (n = 4170); who self-reported that they had been previously diagnosed with HTN, myocardial infarction, angina, stroke, or renal failure by a physician (n = 3235); or who were pregnant women (n = 90). Finally, 15,721 participants with complete information were included in this analysis and categorized according to the quartiles of the TyG index.
Definitions of TyG index and BP categories
Blood samples of individuals for biochemical tests were collected after at least 8 h of overnight fasting at a mobile examination center according to examination protocols. FPG was measured by a hexokinase UV method and triglycerides were measured by enzymatic methods. The TyG index was calculated as ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL) / 2], and classified into quartiles to investigate the association between the TyG index and BP.
The study outcomes were the blood pressure status of participants who were not currently receiving medication for diabetes and hyperlipidemia, and had not previously been diagnosed with hypertension or cardiovascular disease. Systolic (SBP) and diastolic (DBP) blood pressure were measured three times by trained nurses with a mercury sphygmomanometer (Baumanometer Wall Unit 33 [0850], W.A. Baum, NY, USA), at 5-minute intervals in a sitting position after a 5-minute rest. The average of the second and third measurements was used in the analysis. BP categories are based on the classification recommended by the Korean Society of Hypertension [18], and were classified as normal (SBP < 120 mmHg and DBP < 80 mmHg), elevated (SBP 120–129 mmHg and DBP < 80 mmHg), pre-HTN (SBP 130–139 mmHg or DBP 80–89 mmHg), and HTN (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg).
Laboratory assessment and anthropometric measurements
All laboratory and anthropometric measurements were performed as part of a health checkup according to standard operational procedures. Hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography (HPLC); total cholesterol was measured by enzymatic methods; high-density lipoprotein (HDL) cholesterol was measured using a homogeneous enzymatic colorimetric method; low-density lipoprotein (LDL) cholesterol was measured directly using a homogeneous enzymatic colorimetric method (for the participants with triglycerides > 200) or calculated using the Friedewald equation as total cholesterol – HDL-cholesterol – (triglycerides/5) (mg/dL); aspartate (AST) and alanine (ALT) aminotransferase levels were measured using the International Federation of Clinical Chemistry and Laboratory Medicine UV method without pyridoxal-5-phosphate (P5P). During the observation period, insulin testing was performed only in 2019 and was measured using an electrochemiluminescence immunoassay (ECLIA). Therefore, the homeostasis model assessment of insulin resistance (HOMA-IR) was also calculated only in 2019 using the equation: fasting glucose (mg/dL) × fasting insulin (uIU/mL)/405 [19].
All anthropometric measurements were made by trained examiners using standardized methods. Height and weight were measured with light clothes and without shoes. Body mass index (BMI), calculated by dividing weight by height squared (kg/m2), was divided into four categories according to Asian-Pacific guidelines: < 18.5, 18.5–22.9, 23.0–24.9, and ≥ 25.0 kg/m2 [20]. Waist circumference was measured to the nearest 0.1 cm at the midpoint between the lower border of the rib cage and the iliac crest while the participants were wearing light clothes. Abdominal obesity was defined as a waist circumference ≥ 90 cm in men and ≥ 85 cm in women in accordance with the definition of the Korean Society for the Study of Obesity (KSSO) [21].
Assessing sociodemographic and lifestyle variables
The participants’ sociodemographic characteristics included age, sex, marital status, education level, and household income (quartiles). Participants were also asked about their smoking status (non-, former, or current-smoker) and frequency of heavy episodic drinking (does not drink, never in past year, once a month or less, once a week, and almost daily). Heavy episodic drinking was defined as consuming seven or more drinks on a single occasion for men, or five or more drinks on a single occasion for women. We also assessed the family history of hypertension in the parents.
Statistical analyses
Differences in general characteristics between quartile groups of the TyG index were assessed using the chi-square test for categorical variables and analysis of variance (ANOVA) with Dunnett’s post-hoc analysis for continuous variables. Average SBP and DBP according to the quartile of the TyG index were compared using a general linear model (GLM) after adjusting for survey year, age, and sex. After checking for multicollinearity among the independent variables, multiple linear regression analysis with stepwise selection was performed to identify the combination of risk factors that best explained the variance in BP. We chose the list of correlates considered for introduction in this analysis based on the literature and whether they had a P value < 0.05 in the unadjusted analysis. The following 16 variables were considered in the stepwise regression analysis: age, sex, marital status, education level, household income, smoking, alcohol drinking, BMI, FPG, triglycerides, HDL-cholesterol, LDL-cholesterol, AST, ALT, and TyG index. In the subgroup analysis of 2019 data, fasting insulin levels and insulin resistance status were also considered.
The associations between the TyG index and prevalence of elevated BP, pre-HTN, and HTN were assessed using multinomial logistic regression with normal BP as the reference group. Lipid profiles were not included as covariates in the final logistic regression model to avoid over-adjustment. Participants were assigned the median value for each category to test for trends across each quartile of the TyG index, and this variable was treated as a continuous term in the model. To evaluate the potential for effect modification of TyG index quartiles and BP categories (elevated BP, pre-HTN, or HTN), we stratified the analyses by age (< 50 and ≥ 50 years), sex, BMI (< 25 and ≥ 25 kg/m2), and insulin resistance status (HOMA-IR < 2.5 and ≥ 2.5). A cross-product interaction term was included in the multinomial logistic regression model and the statistical significance of the interactions was assessed using the Wald test. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
The analysis included 15,721 eligible subjects (6820 males, 8901 females). Table 1 presents the baseline characteristics of the subjects according to the TyG index quartiles. Compared with participants in the lowest TyG quartile, individuals in higher quartiles tend to be older, male, more prone to obesity, less educated, earn less, and have more bad habits, such as current smoking and heavy alcohol consumption (all P < 0.001). Furthermore, laboratory findings, including the glycemic markers, fasting insulin, lipid parameters, and liver function, also differed significantly among the TyG index quartiles (all P < 0.001). Dunnett’s post-hoc test revealed that subjects in the second to fourth quartiles of the TyG index had significantly higher laboratory findings than those in the lowest quartile.
Distribution of BP and prevalence of elevated BP, pre-HTN, and HTN according to TyG index quartiles
The distribution of BP according to the quartile of TyG index is shown in Fig. 2A and B. SBP and DBP differed significantly among the TyG index quartiles, and had the lowest values in the lowest TyG index quartile. Both SBP and DBP increased with the TyG index (all P for trend < 0.001).
Figure 2C shows the percentage distribution of BP categories according to the TyG index quartile. The percentage of participants defined as HTN was highest in the highest TyG quartile and lowest in the lowest TyG quartile (21.5% vs. 4.1%, P < 0.001 among groups). The percentage increased with the TyG index quartile. Similar trends were observed for the participants classified as pre-HTN (32.9% vs. 14.7%).
Similarly, in the subgroup analysis based on the insulin resistance status (Additional file 1: Figure S1), as the TyG index increased, the prevalence of pre-HTN and HTN increased in both the non-insulin and the insulin resistant groups (all P < 0.001).
Independent correlates of blood pressure variability
Table 2 shows the results of multiple regression analysis with stepwise variable selection to identify the aggregate combination of correlates making the greatest contribution to BP changes. Stepwise linear regression revealed that among the 16 entered variables, the most important correlates of SBP were the combination of age, sex, marital status, education level, household income, smoking status, frequency of heavy episodic drinking, family history of hypertension, BMI, FPG, HDL-cholesterol, AST, and TyG index, accounting for 26.43% of the SBP variance in this population (F = 429.47, P < 0.001). Age, sex, household income, smoking status, frequency of heavy episodic drinking, family history of hypertension, BMI, HDL-cholesterol, LDL-cholesterol, ALT, and the TyG index independently affected DBP, explaining 20.80% of the variance in DBP (F = 371.14, P < 0.001). In particular, higher age, BMI and TyG index, and male sex had greater correlations with BP variability. In the subgroup analysis of 2019 data, which also considered fasting insulin and HOMA-IR, similar results were observed.
Association of TyG index with elevated BP, pre-HTN and HTN
We used multinomial logistic regression analysis to evaluate the association of the TyG index with the prevalence of elevated BP, pre-HTN, and HTN (Table 3). The ORs of elevated BP, pre-HTN, and HTN increased with the TyG index quartiles (all P for trend < 0.001). Specifically, participants in the highest TyG index quartile had 1.82-fold higher odds of elevated BP (95% CI, 1.50–2.21), 2.95-fold higher odds of pre-HTN (95% CI, 2.62–3.34), and 6.46-fold higher odds of HTN (95% CI, 5.36–7.78) than the lowest TyG index quartile group in the age- and sex-adjusted model. Even after adjusting for conventional risk factors of HTN, such as demographic factors, health behavior (smoking and alcohol drinking), family history of hypertension, and BMI, participants in the highest TyG index quartile were most prominently associated with higher prevalence of elevated BP (OR, 1.52; 95% CI, 1.24–1.87), pre-HTN (OR, 2.22; 95% CI, 1.95–2.53), and HTN (OR, 4.24; 95% CI, 3.49–5.16). We also observed significant dose–response relationships between the continuous TyG index and BP categories.
In the analyses stratified by age, sex, BMI, and insulin resistance status (Table 4), age significantly modified the association between the TyG index and the prevalence of pre-HTN (P interaction < 0.001) or HTN (P interaction < 0.001), and the associations were more apparent among those who were < 50 years of age. However, the association of the TyG index with BP categories did not differ by sex, except for elevated BP; elevated BP was more prominent in women than in men (P interaction = 0.015). There were also no interactions between the TyG index and BMI or insulin resistance status.
Discussion
In this population-based cross-sectional study, the TyG index was positively associated with the increment in BP. Note that the study participants were apparently healthy individuals with no history of HTN, CVD, or renal failure and were not taking anti-diabetic or antihyperlipidemic medications. Even after adjusting for conventional risk factors, the significant association between the TyG index and BP was maintained. These findings suggest that the TyG index is independently associated with BP and may be useful for identifying and following individuals at risk of HTN.
IR has been implicated in the pathogenesis of diseases related to metabolic syndrome, including HTN, diabetes mellitus, obesity, and CVD [3, 22, 23]. The gold standard for assessing IR is hyperinsulinemic-euglycemic clamp analysis [24]. However, it is difficult to perform in real-world settings because it is time-consuming and labor-intensive. HOMA-IR has been suggested as a simpler method and its results correlated well with those assessed by the clamp analysis [19]. However, it also has limited value because serum insulin is not measured routinely in clinical settings. More recently, the TyG index has been proposed for evaluating IR [5]. In previous studies, the TyG index correlated well with HOMA-IR [5, 7,8,9]. In our study, although serum insulin was measured in a relatively small number of subjects, HOMA-IR showed a significant correlation with the quartiles of the TyG index. Compared to serum insulin, triglycerides and FPG can be assessed simply and easily; this is more suitable as a mass screening test to predict IR-related diseases, such as in our study.
Although the mechanisms underlying IR in the development of HTN have not been fully elucidated, several have been suggested. Hyperinsulinemia caused by IR may increase the activity of the renin-angiotensin-aldosterone system, which can induce renal sodium retention [25, 26]. It can indirectly cause water-sodium retention and increase vascular activity via angiotensin II, resulting in HTN [27]. IR may also stimulate sympathetic nervous system activity, inducing the secretion of adrenaline and norepinephrine, leading to increased cardiac output and peripheral vascular resistance via vascular smooth muscle cell hypertrophy and endothelial dysfunction [28,29,30].
Several studies have evaluated the relationship between the TyG index and IR-related disease, especially in HTN. In a 9-year longitudinal study, a higher TyG index was associated with an increased risk of subsequent incident HTN [14]. A large epidemiological study of the temporal relationship between BMI and the TyG index and its impact on the incidence of HTN found that a higher BMI at baseline was significantly associated with a higher TyG index and an increased risk of HTN [31]. In addition, a higher TyG index was significantly associated with a higher BMI at the 2-year follow-up and an increased risk of HTN. These results provide direct evidence for a temporal relationship between BMI and IR. A more recent study demonstrated that an increased TyG index was significantly associated with a higher risk of pre-HTN and HTN [15]. Furthermore, obesity parameters such as the waist-to-hip ratio and percent body fat have additive effects on the HTN risk with the TyG index. Our results also showed a positive correlation between the TyG index and BP and prevalence of HTN in alignment with these previous studies.
This study examined subjects who had low risks for IR. Individuals who had previously been diagnosed with HTN, CVD, or renal failure by a physician or who were receiving medication for diabetes mellitus and dyslipidemia were excluded. Nevertheless, a significant relationship was observed between the TyG index and BP; the mean SBP in the lowest and highest quartiles was 111.9 and 119.8 mmHg, respectively (P for trend < 0.001) and mean DBP in the lowest and highest quartiles was 72.7 and 79.5 mmHg (P for trend < 0.001). In the subgroup analysis according to BMI, the TyG index was significantly associated with the prevalence of elevated BP, pre-HTN, and HTN, even in the non-obese subjects (BMI < 25 kg/m2). Furthermore, the associations between the TyG index and the prevalence of pre-HTN and HTN were more prominent in younger subjects (P interaction < 0.001). Overall, these results suggest the potential of the TyG index as a predictor of HTN.
This study has several advantages. It included relatively large number of subjects from a national health survey. We were able to analyze various confounding factors potentially influencing HTN (demographic, lifestyle, and laboratory parameters). In addition, the analysis examined categories of BP and subgroups by subject characteristics. However, our study also has some limitations that need to be considered. First, because of its cross-sectional design, it cannot show a causal relationship between the TyG index and HTN. Second, as KNHANES did not usually include fasting insulin, we could analyze only a small number of subjects and compare the TyG index with the HOMA-IR as an independent risk factor. Third, although we tried to adjust for confounding risk factors, there might be some confounding factors that we did not include.
Conclusion
In conclusion, a higher TyG index significantly correlated with the risk of increased BP in healthy individuals after adjusting for conventional risk factors of HTN. Considering its simplicity of measurement and good functionality, the TyG index might be a useful marker for identifying patients at risk of HTN. However, further studies are needed to longitudinally investigate the cause-effect relationship between the TyG index and BP.
Availability of data and materials
The data of the current study are available from the Korea Disease Control and Prevention Agency (https://knhanes.kdca.go.kr/knhanes/main.do) on reasonable request.
Abbreviations
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine aminotransferase
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- HDL:
-
High‐density lipoprotein
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- HTN:
-
Hypertension
- IR:
-
Insulin resistance
- KDCA:
-
Korea Disease Control and Prevention Agency
- KNHANES:
-
Korea National Health and Nutrition Examination Survey
- LDL:
-
Low-density lipoprotein
- OR:
-
Odds ratio
- P5P:
-
Pyridoxal-5-phosphate
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- TyG:
-
Triglyceride-glucose
References
Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J. 1999;138(3 Pt 2):205–10.
Kim HC, Cho SMJ, Lee H, Lee HH, Baek J, Heo JE, et al. Korea hypertension fact sheet 2020: analysis of nationwide population-based data. Clin Hypertens. 2021;27(1):8.
Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–23.
Wang F, Han L, Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: a meta-analysis. Clin Chim Acta. 2017;464:57–63.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100.
Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul). 2019;34(2):179–86.
Kim B, Choi HY, Kim W, Ahn C, Lee J, Kim JG, et al. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES). PLoS ONE. 2018;13(11):e0206994.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Park B, Lee HS, Lee YJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl Res. 2021;228:42–51.
Shin K-A. Triglyceride and glucose (TyG) index is a clinical surrogate marker for the diagnosis of metabolic syndrome. Biomed Sci Lett. 2017;23(4):348–54.
Kitae A, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. The triglyceride and glucose index is a predictor of incident nonalcoholic fatty liver disease: A population-based cohort study. Can J Gastroenterol Hepatol. 2019;2019:5121574.
Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73.
Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16(1):175.
Wang K, He G, Zhang Y, Yin J, Yan Y, Zhang Y, et al. Association of triglyceride-glucose index and its interaction with obesity on hypertension risk in Chinese: a population-based study. J Hum Hypertens. 2021;35(3):232–9.
Zhu B, Wang J, Chen K, Yan W, Wang A, Wang W, et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: a cross-sectional survey from the Reaction Study. Cardiovasc Diabetol. 2020;19(1):112.
Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77.
Kim HC, Ihm SH, Kim GH, Kim JH, Kim KI, Lee HY, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part I-epidemiology of hypertension. Clin Hypertens. 2019;25:16.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Kim SY. The definition of obesity. Korean J Fam Med. 2016;37(6):309.
Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J Obes Metab Syndr. 2019;28(1):40–5.
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607.
Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32(2):361–6.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-23.
Saitoh S. Insulin resistance and renin-angiotensin-aldosterone system. Nihon Rinsho. 2009;67(4):729–34.
Zemel MB. Insulin resistance vs. hyperinsulinemia in hypertension: insulin regulation of Ca2 + transport and Ca(2+)-regulation of insulin sensitivity. J Nutr. 1995;125(6 Suppl):1738S-43S.
Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int. 2015;87(3):497–9.
Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87(6):2246–52.
Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediat Inflamm. 2016;2016:3634948.
Tack CJ, Smits P, Willemsen JJ, Lenders JW, Thien T, Lutterman JA. Effects of insulin on vascular tone and sympathetic nervous system in NIDDM. Diabetes. 1996;45(1):15–22.
Xie Y, Guo R, Li Z, Guo X, Sun G, Sun Z, et al. Temporal relationship between body mass index and triglyceride-glucose index and its impact on the incident of hypertension. Nutr Metab Cardiovasc Dis. 2019;29(11):1220–9.
Korea Centers for Disease Control and Prevention. The 7th (2016–2018) Korea National Health and Nutrition Examination Survey analytical guidelines. Osong: Korea Centers for Disease Control and Prevention; 2020.
Acknowledgements
Not applicable.
Funding
This research was supported by the BK21 FOUR of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 5199990614277). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C2008683).
Author information
Authors and Affiliations
Contributions
DHL, JEP, SYK, HJJ, and JHP conceived and designed the study. JEP analyzed and summarized all the results. DHL and JEP wrote the manuscript. SYK, HJJ, and JHP reviewed the manuscript and contributed to the discussion. JHP supervised the project. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study design, protocols, and data release for KNHANES were approved by the Institutional Review Board of KDCA (2018-01-03-P-A and 2018-01-03-C-A). Some work was conducted without IRB approval as research conducted directly by the government for the public welfare according to the Bioethics and Safety Act of Korea [32]. Informed consent was obtained from all participants, and all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests related to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Percentage distribution of blood pressure according to the quartile of the TyG index in (A) non-insulin resistant and (B) insulin resistant groups. P values were generated by chi-square test.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, DH., Park, J.E., Kim, S.Y. et al. Association between the triglyceride-glucose (TyG) index and increased blood pressure in normotensive subjects: a population-based study. Diabetol Metab Syndr 14, 161 (2022). https://doi.org/10.1186/s13098-022-00927-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00927-5