Abstract
Background
Hyperglycemia and insulin resistance often develop cardiovascular and nephrological dysfunction in diabetic patients. Sitagliptin is used to treat diabetes and showed potential benefit in lowering increased blood glucose level in diabetes. This investigation reports the effect of sitagliptin treatment on oxidative stress in kidney and heart of 2K1C rats.
Methods
Male Long Evans rats underwent unilateral surgical stenosis of the renal artery [2-kidney-1-clip (2K1C) method]. These animals entered a 4-weeks dosing period with sitagliptin. Blood and urine sampling and organ harvesting were finally performed. Blood plasma, heart, kidney tissues and urine were tested for the assessment of inflammation and oxidative stress in kidney and heart of 2K1C rats after 4 weeks of surgery.
Results
2K1C rats showed cardiac hypertrophy, increased left ventricular wet weight compared to sham which was not significantly altered by sitagliptin treatment. Uric acid and creatinin concentrations were also increased in 2K1C rats. Sitagliptin significantly prevented the elevation of uric acid and creatinin concentration in plasma and urine in this rat model. Oxidative stress markers in plasma such as malondialdehyde (MDA), nitric oxide (NO), and advanced protein oxidation product (APOP) concentrations were increased in the 2K1C rats as compared to sham-operated animals. Increased concentrations of these oxidative stress markers were also normalized by sitagliptin treatment. 2K1C rats also showed increased level of uric acid and creatinine both in plasma and urine; which are also reduced to normal level in sitagliptin treated rats. Moreover, 2K1C surgery initiated inflammatory cell infiltration, increased MPO activity and fibrosis in both heart and kidneys which were further ameliorated by sitagliptin treatment.
Conclusion
Our study suggests that sitagliptin treatment in 2K1C rats prevented inflammation and fibrosis of heart and kidney by ameliorating elevated oxidative stress in heart and kidney tissues.
Similar content being viewed by others
Background
Congestive heart failure and kidney dysfunction are major causes of mortality and morbidity in patient suffering diabetes worldwide [1, 2]. These secondary complications are frequently seen in most of the diabetes patients and become a public health concern now a day. Myocardial remodeling occurs during the progression of cardiomyopathy in diabetes and possesses an important role in the pathophysiology of hypertensive disease [3]. Myocardial cell apoptosis, necrosis, hypertrophy and extension have been noted during the progression of myocardial remodeling and cardiac hypertrophy [4, 5]. Moreover, myocardial extracellular matrix and collagen fiber deposition are also found in hypertrophied heart both in experimental animals [6] and in clinical experiments [7]. Free radical mediated oxidative stress is considered as a key player in cardiaomyocyte hypertrophy which can be prevented by antioxidant treatment [8, 9]. The sources of reactive free radicals in heart are mitochondrial electron transport chain, inducible nitric oxide synthase, NADPH oxidase and xanthine oxidase [10]. Oxidative stress mediated insult in the mayocardium also attract inflammatory cells [11]. Several reports also suggest that NADPH oxidase containing neutrophil infiltration in myocardium is mainly responsible for free radicals mediated oxidative stress in heart [9, 12].
Plasma renin release and Ang II activity are increased in case of kidney ischemia [13]. Type 2 diabetes patients often suffer hypertension due to the over activity of renin angiotensin system. Renin angiotensin system plays a crucial role in many pathophysiology of cardiac oxidative stress and myocardial remodeling in diabetes [14]. The two-kidney one-clip (2K1C) renovascular hypertension model animal showed elevated circulating levels of Ang II with high Ang II concentrations in the cortical tissue of the clipped and nonclipped kidneys [15, 16]. Moreover, cross talk between free radicals production and ang-II mediated hypertrophied response in heart is well documented and reviewed recently [8]. Previous studies have reported that angiotensin II (Ang II) stimulates the production of reactive oxygen species (ROS) such as superoxides [17, 18]. In experimental 2K1C hypertension, the overproduction of reactive oxygen species (ROS) may lead to oxidative stress and increased lipid peroxidation [19, 20]. Increased ANG II activity and oxidative stress further triggers the deposition of extracellular matrix and fibrosis in heart and kidney of 2K1C model animal [21, 22].
Dipeptidyl peptidase-4 (DPP-4) inhibitors are used for the treatment of the Type 2 diabetes mellitus (T2DM). Sitagliptin belongs to DPP-4 inhibitors group that prevent the degradation of insulinotropic incretin glucagon like peptide (GLP-1), without producing hypoglycemia [23]. DPP-4 inhibitors also preserves islet function in both type 1 and type 2 diabetes animal models and increases pancreatic insulin content, through an increase in proliferation, neogenesis, and apoptosis resistance of beta cells [24]. Previous studies suggest that DPP-4 inhibitors prevent cardiac diastolic dysfunction and ameliorate glomerulopathy in insulin-resistant Zucker obese rats [25, 26]. Improved endothelium-dependent relaxation function in renal arteries, restored renal blood flow and reduced systolic blood pressure was observed in spontaneously hypertensive rats by 2 weeks treatment with sitagliptin [27]. However, sitagliptin appears to limit the blood pressure lowering effect of enalapril in patients with metabolic syndrome [28]. Some other studies also demonstrated the beneficial impact of sitagliptin on diabetic nephropathy [29–31]. Therefore, it is of particular interest to examine whether DPP-4 inhibitor can suppress oxidative stress and inflammation in heart and kidney of two kidney one clip (2K1C) rats.
Methods
Chemicals and reagents
Sitagliptin were obtained from Beximco Pharmaceuticals Limited (Bangladesh) as gift sample. Thiobarbituric acid (TBA) was purchased from Sigma Chemical Company (USA). Trichloroacetic acid (TCA) was purchased from J.I. Baker (USA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and uric acid (UA) assay kits were obtained from DCI diagnostics (Budapest, Hungary). All other chemicals and reagents used were of analytical grade.
Animal’s surgery and treatment
Twelve to fourteen weeks old, 24 Long Evans male rats (170–230 g) were obtained from Animal production unit of Animal House at Department of Pharmaceutical Sciences, North South University and were kept in ordinary cages at room temperature of 25 ± 3 °C with a 12 h dark/light cycles with food water ad libitum. They have free access to food and water, according to the study protocol approved by Ethical Committee of Department of Pharmaceutical Sciences, North South University for animal care and experimentation. To study the effects of sitagliptin, rats were equally divided into four groups (six rats in each group): Sham, Sham + sitagliptin, 2K1C and 2K1C + sitagliptin. Rats were subjected to unilateral clipping of the renal artery to produce two kidney one clip (2K1C) model rats. In brief, a left kidney was exposed via laparotomy. The left renal artery was separated from the left renal vein and a silk ligature placed around the renal artery. A 23-gauge injection needle was placed into the ligature, parallel to the renal artery, the ligature tied and the needle carefully removed. Visual assessment of kidney perfusion was performed before closing the wound. Sham-operated rats underwent the same procedure, but kidneys were only mobilized and renal vessels were only separated instead of being partially ligated. All procedures were performed under intraperitoneal injection of ketamine anesthesia. Animals were transferred to the housing facility and monitored once they recovered from anesthesia. After surgery, rats were received normal food and water for 28 days. Sham + sitagliptin and 2K1C + sitagliptin groups received sitagliptin (100 mg/kg, daily) by oral gavaging. Animals were checked for the body weight and water intake on a daily basis. After 4 weeks of treatment with or without sitagliptin, the rats were placed in metabolic cages, and urine was collected for 24 h. The urine volume was measured for every rat. After 28 days of the last treatment, all the animals were weighed, sacrificed, collected the blood and organs like heart, kidney, spleen and liver. Immediately after collection of these tissues and organs, they are weighed and stored at −20 °C for further analysis.
Assessment of AST, ALT and ALP activities
Liver marker enzymes (alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were estimated in plasma by using Diatech diagnostic kits for AST, ALT and ALP (Hungary) according to the manufacturer’s protocol. Uric acid and creatinine were also measured using Diatech diagnostic kits for uric acid and creatinin (Hungary) according to the manufacturer’s protocol.
Assessment of oxidative stress markers
For determination of oxidative stress markers, heart and kidney tissue was homogenized in 10 volumes of Phosphate buffer containing (pH 7.4) and centrifuged at 12,000×g for 30 min at 4 °C. The supernatant was collected and used for the determination of protein and enzymatic studies as described below.
Estimation of lipid peroxidation product malondialdehyde (MDA)
Plasma concentrations of malondialdehyde are an index of lipid peroxidation and oxidative stress. Lipid peroxidation in heart and kidney were estimated colorimetrically measuring malondialdehyde followed by previously described method [32]. In brief, 0.1 mLentrated Acetic Acid-HCl reagent (thiobarbituric acid 0.37 %, 0.25 N HCl and 15 % TCA) and placed in water bath for 15 min and cooled. The absorbance of clear supernatant was measured against reference blank at 532 nm.
Estimation of nitric oxide (NO)
NO was determined according to the method described by Tracy et al. as nitrate and nitrite [33]. In this study, Griess-Illosvoy reagent was modified by using naphthyl ethylene diamine dihydrochloride (0.1 % w/v) instead of 1-napthylamine (5 %). The reaction mixture (3 mL) containing tissue homogenates (2 mL) and phosphate buffer saline (0.5 mL) was incubated at 25 °C for 150 min. A pink colored chromophore was formed which was measured at 540 nm.
Estimation of advanced protein oxidation products (APOP)
Determination of APOP levels was performed by modification of the method of Witko-Sarsat [34] and [35] Tiwari. Two mL of plasma was diluted 1:5 in PBS: 0.1 mL of 1.16 M potassium iodide was then added to each tube, followed by 0.2 mL acetic acid after 2 min. The absorbance of the reaction mixture was immediately read at 340 nm against a blank containing 2 mL of PBS, 0.1 mL of potassium iodide (KI), and 0.2 mL of acetic acid. The chloramine-T absorbance at 340 nm being linear within the range of 0–100 mmol/L, APOP concentrations were expressed as μmol L−1 chloramine-T equivalents.
Estimation of catalase (CAT) activity
CAT activities were determined by the method of Chance and Maehly [36, 37] with some modifications. The reaction solution of CAT activities contained: 2.5 ml of 50 mmol phosphate buffer (pH 5.0), 0.4 mL of 5.9 mmol H2O2 and 0.1 mL enzyme extract. Changes in absorbance of the reaction solution at 240 nm were determined after 1 min. One unit of CAT activity was defined as an absorbance change of 0.01 as units/min.
Estimation of myloperoxidase (MPO) activity
MPO activity was determined by a dianisidine-H2O2 method [38], modified for 96-well plates. Briefly, plasma samples (10 μg protein) were added in triplicate to 0.53 mM o-dianisidine dihydrochloride (Sigma) and 0.15 mM H2O2 in 50 mM potassium phosphate buffer (pH 6.0). The change in absorbance was measured at 460 nm. Results were expressed as units of MPO/mg protein.
Histopathalogical determination
For microscopic evaluation heart and kidney tissues were fixed in neutral buffered formalin and embedded in paraffin, sectioned at 5 μm and subsequently stained with hematoxylin and eosin (H & E) to evaluate inflammatory cell infiltration. Sirius red staining was also performed to evaluate the fibrosis in heart and kidney. Moreover, Prussian blue staining was done to determine the iron deposition in tissues. Sections were studied under light microscope at 40× magnifications.
Statistical analysis
The values are expressed as mean ± standard deviation (SD). The results were evaluated by using the Two-way ANOVA followed by Bonferroni test using Graph Pad Prism Software, USA, version 6. Statistical significance was considered as p < 0.05 in all cases.
Results
Effect of sitagliptin on body weight, food and water intake in 2K1C rats
Body weight of each rat was recorded every day during the experiment, and % change was calculated for all groups. It was found that the body weight increased consistently in sitagliptin treated rats group, which is denoted that the treatment have no effect on the body weight. On the other hand sham + sitagliptin group showed no variation in their body weight. Water intake was not changed significantly among the groups.
Effect of sitagliptin on organ wet weight in 2K1C rats
Table 1 shows the effect of various treatments on the rats’ organs wet weight. The wet weight of heart and kidney was increased in the 2K1C rats when compared with sham rats. Left ventricular wet weight was increased significantly compared to sham rats. However, sitagliptin treatment showed no significant change in the wet weight of the left ventricle of heart in the 2K1C rats. Neither 2K1C challenge and nor sitagliptin treatment in rats have changed the right ventricular wet weight significantly compared to sham rats. 2K1C rats also showed slight decrease in liver wet weight, however, sitagliptin treatment did not altered the wet weight of the liver compared to 2K1C rats. Another crucial finding in this study was that 2K1C rats showed increased kidney wet weight compared to sham rats which were unaltered by sitagliptin treatment (Table 1). Furthermore, 2K1C rats showed increased spleen wet weight compared to sham rats which were reduced by sitagliptin treatment in 2K1C rats.
Effect of sitagliptin on biochemical parameters AST, ALT and ALP activity
Biochemical measurement of 2K1C rats showed a significant increase in plasma AST, ALT, and ALP activity compared to sham rats (Table 2). Treatment with sitagliptin in 2K1C rats significantly counteracted these increased enzymes activity. In addition, treatment of animals with sitagliptin alone for 4 weeks did not show any significant changes in AST, ALT and ALP enzyme activities compared to the sham rats (Table 2).
Effect of sitagliptin on oxidative stress markers and antioxidant enzymes
To determine the oxidative stress in our study, we evaluated the malondialdehyde (MDA), nitric oxide and advanced protein oxidation product (APOP) content in plasma, heart and kidneys. 2K1C rats showed a higher concentration of lipid peroxidation product MDA in plasma, heart and kidney (Tables 2, 3). Additionally, sitagliptin treatment in 2K1C rats significantly reduced the level of lipid peroxides compared to 2K1C group in plasma, heart and kidney. 2K1C rats also showed profound effect on APOP development in plasma and kidney tissues (Tables 2, 3). 2K1C rats showed significantly increase concentration of APOP in plasma and kidney which was normalized due to sitagliptin treatment in 2K1C rats. APOP concentration was unchanged in all groups tested in this study. Nitric oxide measured as nitrate was also increased in plasma, heart and kidney compared to sham rats which were further normalized by sitagliptin treatment in 2K1C group (Tables 2, 3). 2K1C group rats showed decrease in antioxidant enzyme catalase activity compared to the sham group rats (Tables 2, 3). Treatment with sitagliptin to 2K1C significantly counteracted the oxidative stress by restoring the catalase activity to near normal compared to 2K1C group (Tables 2, 3).
Effect of sitagliptin on uric acid and creatinine concentration in plasma and urine
Uric acid concentration in plasma and urine was increased in 2K1C rats significantly compared to the sham rats. Sitagliptin treatment in these rats lowered the uric acid concentration significantly compared to 2K1C rats group. Sitagliptin treatment in sham rats did not change uric acid concentration in plasma compared to sham rats.
2K1C rats also showed increased creatinin concentration both in plasma and urine significantly compared to sham rats. Sitagliptin treatment normalized the creatinin concentration in plasma and urine of 2K1C rats.
Effect of sitagliptin on inflammation and fibrosis markers in heart and kidneys
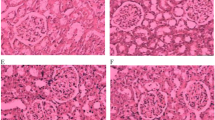
Inflammation was seen in rats of 2K1C group compared to sham rats. To determine inflammation in tissues, we measured myloperoxidase (MPO) activity in heart and kidney tissues. 2K1C group rats showed increased MPO activity both in heart and kidney compared to sham rats. Sitagliptin treatment significantly normalized the MPO activity in 2K1C rats compared to sham rats. These data are further supported by the histological assessment of tissue sections of heart and kidneys. Necrotized tissue scar and ballooning of the cardiomyocytes were also seen in heart of 2K1C rats (Fig. 1). Sitagliptin treatment significantly attenuated the inflammatory cell infiltration and necrosis in the heart tissues of 2K1C rats (Fig. 1). Moreover, sham rats and sham rats treated with sitagliptin showed no inflammatory cells infiltration in left ventricle of heart. Massive serge of inflammatory cells was found in the glomerular part of 2K1C rats kidney sections stained with H & E. Glomerulosclerosis was evident, and the interstitium showed patchy infiltrates of mononuclear cells as well as fibrosis (Fig. 2). However, no significant difference could be detected between sham rats and 2K1C rats treated with sitagliptin, demonstrating complete healing of the nephritic changes (Fig. 2). Cardiac and kidney fibrosis were evaluated histologically by visualizing the red color collagen fibers deposition using Sirius red staining process. Sham rats and sham rats treated with sitagliptin showed no/less deposition of collagen fibers in heart and kidneys. However, 2K1C rats showed massive collagen deposition both in heart and kidney which was further attenuated by sitagliptin treatment (Figs. 3, 4). Furthermore, free iron deposition was also seen in kidney sections of 2K1C rats which were ameliorated by sitagliptin treatment (Fig. 5). However, no iron deposition occurred in left ventricular section of all groups tested in this study.
Effect ofsitagliptin on cardiac inflammation in 2K1C model rats. a Sham; b Sham + Sitagliptin; c 2K1C and d 2K1C + Sitagliptin. No inflammatory process was observed in Sham animals (a) and Sham + Sitagliptin (b). Observe the increase of inflammatory cells in the left ventricle of heart of 2K1C rats (c, arrows). No/less inflammatory process can be observed in left ventricle of heart of all 2K1C animals treated with sitagliptin (d). Magnification ×40. H & E staining. ic inflammatory cells, nc necrotic site
Effect of sitagliptin on kidney inflammation in 2K1C model rats. a Sham; b Sham + Sitagliptin; c 2K1C and d 2K1C + Sitagliptin. No inflammatory process was observed in Sham animals (a) and Sham + Sitagliptin (b). Observe the increase of inflammatory cells in the kidneys of 2K1C (c arrows). Glomerular sclerosis, tubular atrophy with accompanying moderate interstitial fibrosis and infiltration by mononuclear cells was also found. No/less inflammatory process can be observed in right kidney of 2K1C treated with sitagliptin (d). Magnification ×40. H & E staining. ic inflammatory cells, gc glomerular sclerosis
Effect of sitagliptin on cardiac fibrosis in 2K1C model rats. Picrosirius red staining for fibrosis. a Sham; b Sham + Sitagliptin; c 2K1C and d 2K1C + Sitagliptin. No pathological collagen tissue deposition was observed in Sham animals (a) and Sham + Sitagliptin (b). Observe the greater collagen deposition in the left ventricle of heart of 2K1C rats (c arrows). No/less collagen tissue deposition can be observed in left ventricle of heart of all 2K1C animals treated with sitagliptin (d). Magnification ×40. fb fibrosis
Effect of sitagliptin on kidney fibrosis in 2K1C model rats. a Sham; b Sham + Sitagliptin; c 2K1C and d 2K1C + Sitagliptin. No pathological collagen tissue deposition was observed in Sham animals (a) and Sham + Sitagliptin (b). Observe the greater collagen deposition in the left kidney of 2K1C rats (c arrows). No/less collagen tissue deposition can be observed in right kidney of all 2K1C animals treated with sitagliptin (d). Magnification ×40. fb fibrosis
Effect of sitagliptin on cardiac and kidney iron deposition in 2K1C model rats. a, e Sham; b, f Sham + Sitagliptin; c, g 2K1C and d, h 2K1C + Sitagliptin. No pathological iron deposition was observed in left ventricle of heart (upper panel). Moreover, no pathological iron deposition was observed in kidneys of Sham animals (e) and Sham + Sitagliptin (f). Observe the greater iron deposition in the kidney of 2K1C rats (g arrows). No/less iron deposition can be observed in kidney of all 2K1C animals treated with sitagliptin (h). Magnification ×40. id iron deposition
Discussion
This study demonstrated that sitagliptin treatment prevented the oxidative stress, inflammatory cell infiltration and fibrosis in heart and kidney of 2 kidney one clip (2K1C) rats. Previous studies suggest that, two-kidney, one-clip (2K1C) rat model experience a decreased renal perfusion pressure which causes the kidney to overproduce renin and leads to a continual activation of the renin–angiotensin–aldosterone axis [39]. Renal artery constriction, usually from atherosclerotic or fibromuscular dysplastic renal disease may develop such condition in human [40].
Oxidative stress due to excess generation of ROS plays an important role in producing tissue damage to the organ or increased the inflammatory response which ultimately stimulates the production of pro-fibrogenic mediators and initiate fibrogenesis. Lipid peroxidation, arising from the reaction of free radicals with lipids, has been linked with altered membrane structure and enzyme inactivation. Its end products are measured as TBARS, lipid hydroperoxides and conjugated dienes [41]. In our study, all 2K1C groups showed increased serum malon dialadehyde (MDA) indicating increased production of toxic aldehydes in rats as previously reported [41]. This enhancement of lipid peroxidation products might be due to increased tissue damage, free radical production and decreased hydrolysis of lipid peroxides [41]. APOP level was also increased in our study in 2K1C rats. We explored the protective mechanisms of sitagliptin by studying markers of oxidative stress and inflammation. Furthermore, sitagliptin treatment alone significantly enhanced the antioxidant enzyme activities and inhibited lipid peroxidation as compared to the sham rats. These findings support the premise that sitagliptin can guard against the sequences of oxidative stress.
Nitric oxide (NO) is sometime considered as another mediator of the oxidative stress. Nitric oxide may convert into peroxinitrile which is much more dangerous than superoxide itself and causes more cellular damage in presence of superoxide free radicals. However, NO plays a significant role in the regulation of blood pressure and that impaired NO bioactivity is an important component of hypertension [42]. In our study, we also found that NO level increased in plasma of 2K1C rats which was normalized by sitagliptin treatment. Previous study suggest that endothelial nitric oxide synthase (eNOS) and inducible nitric oxide expression were increased in 2K1C rats [43]. Previous studies have also shown that iNOS isoforms are able to generate superoxide anions independent of NO production [44]. In our study, we also observed that AST, ALT, and ALP activities were increased significantly in 2K1C rats. Sitagliptin treatment normalized these parameters which signify the overall improvement in health condition of the studied animals.
In cardiovascular remodeling, reactive free radical species mediated oxidative stress and infiltration of inflammatory cells have been noticed in remodeled heart and implicates myocardial hypertrophy, fibrosis, conduction abnormalities and endothelial dysfunction which ultimately leading to heart failure [45, 46]. Moreover, Ang-II promotes cardiac and kidney cell apoptosis and triggers fibrosis by activating the fibroblast cells and other growth mediators like TGF-β [47, 48]. Our study showed that massive collagen was deposited in both heart and kidney tissues. Sitagliptin supplementation attenuated this collagen deposition as well as decreased the inflammation in these tissues.
Development and progression of nephropathy is another complication of diabetes which is primarily evaluated by glomerular hyperfiltration [49]. Moreover, activation of several metabolic pathways such as activation of protein kinase C [50], nonenzymatic glycosylation [51] acceleration of the polyol pathway [52], hexosamine biosynthetic pathway [53], and oxidative stress [54] are also involves in the development of diabetic nephropathy. Further evidences are also pointing to a vital role of the inflammatory process in the development and progression of diabetic nephropathy [55–57]. Diverse inflammatory cells, including macrophages, monocytes, and leukocytes, as well as other molecules, such as chemokines, adhesion molecules, and inflammatory cytokines, namely, tumor necrosis factor alpha (TNF-α) and interleukin-1β(IL-1β) [57–59] are few mediators of the inflammatory responses. If inflammation persists on, certain vascular lesions are aggravated, such as endothelial dysfunction, tissue damage, renal fibrosis, and apoptotic cell death [58, 59]. In our study, the wet weight of remnant kidney is significantly changed in treatment group when compared with sham rats. Moreover, our study suggests that sitagliptin supplementation also improved the uric acid and creatinin level in plasma of 2K1C rats. Previous report suggests that 2K1C rat model showed significant increase in rennin activity and Ang-II in circulating blood and tissues [60, 61]. Ang-II promotes cardiac and kidney growth and in pathological condition this growth may turn into hypertrophy [62]. Moreover, in this model one kidney was clipped and other one set free to serve the whole body. To facilitate the whole body blood purification, this second kidney adjusted itself and grows almost double. Previous report suggests that sitagliptin improves renal dysfunction and reduced glomerular and tubulointerstitial injury and exerts anti-oxidative, anti-apoptotic, and anti-inflammatory effects [63].
Our study revealed the anti fibrotic activity of sitagliptin in heart and kidneys of 2K1C rats. The beneficial effect of sitagliptin was mainly due to the improvement of oxidative stress and inflammation in this rat model. Further research is required to establish clinical benefit of sitagliptin in inflammation and fibrosis in diabetic hypertensive patients.
References
Zitkus BS. Update on the American Diabetes Association Standards of Medical Care. Nurse Pract. 2014;39(8):22–32.
AmericanDiabetes A. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–80.
Zhang Y, Shao L, Ma A, Guan G, Wang J, Wang Y, et al. Telmisartan delays myocardial fibrosis in rats with hypertensive left ventricular hypertrophy by TGF-beta1/Smad signal pathway. Hypertension Res. 2014;37(1):43–9.
López B, González A, Querejeta R, Larman M, Díez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol. 2006;48(1):89–96.
Shirwany A, Weber KT. Extracellular matrix remodeling in hypertensive heart disease. J Am Coll Cardiol. 2006;48(1):97–8.
Alam MA, Sernia C, Brown L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J Cardiovasc Pharmacol. 2013;61(3):240–9. doi:10.1097/FJC.0b013e31827cb600.
Olsen MH, Christensen MK, Wachtell K, Tuxen C, Fossum E, Bang LE, et al. Markers of collagen synthesis is related to blood pressure and vascular hypertrophy: a LIFE substudy. J Hum Hypertens. 2005;19(4):301–7.
Alam MA, Uddin SJ, Brown L. Mitogen-activated protein kinase and natural phenolic compounds in cardiovascular remodeling. In: Atta ur R, editor. Studies in natural products chemistry. Amsterdam: Elsevier; 2012. p. 159–90.
Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40(4):477–84.
Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93(8):903–7. doi:10.1136/hrt.2005.068270.
Sagor MAT, Tabassum N, Potol MA, Alam MA. Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxidative Med Cellular Longevity. 2015;2015:9.
Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91(6):1872–85.
Basile DP, Donohoe DL, Phillips SA, Frisbee JC. Enhanced skeletal muscle arteriolar reactivity to ANG II after recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1770–6.
Tsutsui H, Matsushima S, Kinugawa S, Ide T, Inoue N, Ohta Y, et al. Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Hypertension Res. 2007;30(5):439–49.
Helle F, Hultström M, Skogstrand T, Palm F, Iversen BM. Angiotensin II-induced contraction is attenuated by nitric oxide in afferent arterioles from the nonclipped kidney in 2K1C. Am J Physiol Renal Physiol. 2009;296(1):F78–86.
Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39(2 Pt 2):316–22.
Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302(2):148–58.
Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30(11):860–6.
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346(25):1954–62. doi:10.1056/NEJMoa013591.
Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, et al. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37(2 Pt 2):541–6.
Zhao W, Chen SS, Chen Y, Ahokas RA, Sun Y. Kidney fibrosis in hypertensive rats: role of oxidative stress. Am J Nephrol. 2008;28(4):548–54.
Rizzi E, Ceron CS, Guimaraes DA, Prado CM, Rossi MA, Gerlach RF, et al. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-beta in renovascular hypertension-induced cardiac hypertrophy. Exp Mol Pathol. 2013;94(1):1–9.
Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–43.
Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterol. 2002;122(2):531–44.
Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male zucker obese rats. J Endocrinol. 2013;154(7):2501–13.
Nistala R, Habibi J, Aroor A, Sowers JR, Hayden MR, Meuth A, et al. DPP4 inhibition attenuates filtration barrier injury and oxidant stress in the zucker obese rat. Obesity. 2014;22(10):2172–9.
Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833–41.
Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56(4):728–33.
Vaghasiya J, Sheth N, Bhalodia Y, Manek R. Sitagliptin protects renal ischemia reperfusion induced renal damage in diabetes. Regul Pept. 2011;166(1–3):48–54.
Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012;340(2):248–55.
Mega C, de Lemos ET, Vala H, Fernandes R, Oliveira J, Mascarenhas-Melo F, et al. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes Res. 2011;2011:162092.
Niehaus WG, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6(1):126–30.
Tracey WR, Tse J, Carter G. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther. 1995;272(3):1011–5.
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen A, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13.
Tiwari BK, Kumar D, Abidi AB, Rizvi SI. Efficacy of composite extract from leaves and fruits of medicinal plants used in traditional diabetic therapy against oxidative stress in alloxan-induced diabetic rats. ISRN Pharmacol. 2014;2014:7.
Chance B, Maehly A. Assay of catalase and peroxidases. Methods Enzymol. 1955;11:764–75.
Khan R. Protective effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4-induced oxidative stress in the thyroid tissue of rats. BMC Complement Altern Med. 2012;12(1):181.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Investigative Dermatol. 1982;78(3):206–9.
Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39(1):89–105.
Thurston H, Bing RF, Swales JD. Reversal of two-kidney one clip renovascular hypertension in the rat. Hypertension. 1980;2(3):256–65.
Silambarasan T, Raja B. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur J Pharmacol. 2012;679(1–3):81–9.
Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich, Conn). 2006;8(12 Suppl 4):17–29.
Santuzzi CH, Tiradentes RV, Mengal V, Claudio ERG, Mauad H, Gouvea SA, et al. Combined aliskiren and l-arginine treatment has antihypertensive effects and prevents vascular endothelial dysfunction in a model of renovascular hypertension. Braz J Med Biol Res. 2015;48:65–76.
Amaral LM, Pinheiro LC, Guimaraes DA, Palei AC, Sertorio JT, Portella RL, et al. Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J Cell Mol Med. 2013;17(10):1300–7.
Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97(16):1536–9.
Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1014–30.
Jia L, Li Y, Xiao C, Du J. Angiotensin II induces inflammation leading to cardiac remodeling. Front Biosci. 2012;17:221–31.
Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40(2):352–63.
Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–7.
Noh H, King GL. The role of protein kinase C activation in diabetic nephropathy. Kidney Int. 2007;106:S49–53.
Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11(9):1656–66.
Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000;77:S3–12.
Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101(1):160–9. doi:10.1172/jci119875.
Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–54.
Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH. Diabetic nephropathy is associated with low-grade inflammation in Type 1 diabetic patients. Diabetologia. 2003;46(10):1402–7.
DallaVestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–82.
Nelson CL, Karschimkus CS, Dragicevic G, Packham DK, Wilson AM, O’Neal D, et al. Systemic and vascular inflammation is elevated in early IgA and type 1 diabetic nephropathies and relates to vascular disease risk factors and renal function. Nephrol Dial Transpl. 2005;20(11):2420–6.
Rivero A, Mora C, Muros M, Garcia J, Herrera H, Navarro-Gonzalez JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci. 2009;116(6):479–92.
Limand AK TG. Inflammation indiabeticnephropathy. Mediat Inflamm. 2012; 2012(Article ID 146154):12.
Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39(1):89–105.
Gudbrandsen OA, Hultstrom M, Leh S, Monica Bivol L, Vagnes O, Berge RK, et al. Prevention of hypertension and organ damage in 2-kidney, 1-clip rats by tetradecylthioacetic acid. Hypertension. 2006;48(3):460–6.
Li J, Xie ZZ, Tang YB. Genistein prevents myocardial hypertrophy in 2-kidney 1-clip renal hypertensive rats by restoring eNOS pathway. J Pharmacol. 2010;86(4):240–8. doi:10.1159/000320457.
Joo KW, Kim S, Ahn SY, Chin HJ, Chae DW, Lee J, et al. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in rat remnant kidney. BMC Nephrol. 2013;14:98.
Authors’ contributions
MAA, MRHC and HMR designed the experimental protocol. MRHC and MAA carried out the animal surgery, animal care, treatment and data acquisition from the experiment. MAA, MATS and MRHC performed all the biochemical analysis. MAA, MATS and MRHC also performed the histological staining and analysis of tissues. MAA, PJ and HMR took part in data analysis and manuscript writing. MAA and HMR checked and finalized the manuscript for submission. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to the authority of Department of Pharmaceutical Sciences, North South University, Bangladesh for the providing facilities for present work.
Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Research was conducted in Department of Pharmaceutical Sciences, North South University, Bangladesh.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Md. Ashraful Alam and Mohammed Riaz Hasan Chowdhury contributed equally
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Alam, M.A., Chowdhury, M.R.H., Jain, P. et al. DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetol Metab Syndr 7, 107 (2015). https://doi.org/10.1186/s13098-015-0095-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-015-0095-3