Abstract
Background
To introduce a prospective cohort for rheumatoid arthritis (RA) patients with interstitial lung disease (ILD) and to identify their clinical features in comparison with RA patients without ILD.
Methods
Using a multidisciplinary collaborative approach, a single-center cohort for RA patients with ILD (RA-ILD) was established in May 2017, and enrolment data from May 2017 to March 2021 were used to compare the clinical features of RA patients without ILD (RA-non ILD). Multivariable logistic regression analysis was used to identify factors associated with ILD in RA patients.
Results
Among 148 RA-ILD and 410 RA-non ILD patients, participants in the RA-ILD group were older (65.8 ± 9.9 vs. 58.0 ± 10.4 years, P < 0.001) and included more males (35.8% vs. 14.6%, P < 0.001) than in the RA-non ILD group. The RA-ILD group had a higher proportion of late-onset RA patients (age ≥ 60 years) than in the comparator group (43.9% vs. 14.2%, P < 0.001). Multivariable logistic regression analysis showed that higher age at RA onset (OR 1.056, 95% CI 1.021–1.091), higher body mass index (BMI; OR 1.65, 95% CI 1.036–2.629), smoking history (OR 2.484, 95% CI 1.071–5.764), and oral glucocorticoid use (OR 3.562, 95% CI 2.160–5.874) were associated with ILD in RA patients, whereas methotrexate use was less likely to be associated with ILD (OR 0.253, 95% CI 0.155–0.412).
Conclusions
Higher age at RA onset, smoking history, and higher BMI were associated with the presence of ILD among RA patients. Oral glucocorticoids were more frequently used whereas methotrexate was less likely to be used in RA-ILD patients.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is an autoimmune disease that predominantly affects the musculoskeletal system and leads to significant joint destruction [1]. Interstitial lung disease (ILD) is one of the most common extra-articular manifestations of RA, and RA-ILD is associated with significant morbidity and mortality [2,3,4]. The prevalence of ILD among RA patients is 1–67.3% and varies by the study design, study population, and definition of ILD [2, 4,5,6,7,8,9,10]. One of the challenges in treating RA-ILD is that many of the therapeutic options for RA, such as conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and biologic agents potentially induce pulmonary toxicity [11,12,13,14,15]. Although csDMARDs and biologic agents are widely used for RA-related manifestations in joints, their potential therapeutic benefits for RA-ILD remain controversial [16,17,18,19,20]. Therefore, joint and lung involvement should be evaluated independently of each other for treatment-related decision-making. However, some similarities between RA-ILD and idiopathic pulmonary fibrosis and the recent clinical trials suggest a possible future role for treatment with antifibrotic agents [21].
ILD usually appears after symptomatic joint involvement, but sometimes it might precede the articular manifestations [22]. As the clinical manifestations usually appear in only advanced-stage lung disease, early diagnosis of RA-ILD proves challenging. Furthermore, the progression and the severity of lung involvement are the two major factors to consider for treatment-related decision-making. Though dyspnea and cough are the most common symptoms in patients with ILD, up to 30% of RA patients with or without respiratory symptoms had subclinical ILD on high-resolution computerized tomography (HRCT) scanning of the chest [23, 24]. Due to the large variation in the clinical course of ILD in RA patients, ILD is difficult to diagnose without a radiologic evaluation in this population. The diagnostic approach to patients with ILD in the setting of known or suspected RA requires a collaborative multidisciplinary approach, with expert radiology, rheumatology, and pulmonology inputs, whereas evaluating for other potential causes of ILD, such as hypersensitivity pneumonitis, pneumoconiosis, connective tissue diseases (CTD) other than RA, or iatrogenic causes, such as drug toxicity [25].
This study was conducted to introduce a Korean RA-ILD registry that was established with a multidisciplinary collaborative approach with an emphasis on the study design and, by using the baseline data, to provide clinical characteristics of the RA-ILD patients who are currently registered in this prospective cohort.
Materials and methods
Establishment of a prospective cohort for RA-ILD patients
Study purpose and outcomes
We designed and established a prospective cohort study of RA patients at a single center to compare the long-term prognosis between RA-ILD patients and RA patients without ILD (RA-non ILD) and to explore prognostic factors among RA-ILD patients.
Study population
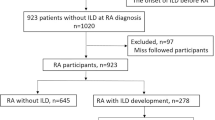
Patients were eligible for inclusion if they were 19 years or older, met the 1987 American College of Rheumatology (ACR) [26] or 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for RA [27], underwent a chest CT scan within the 2 years preceding enrolment, and provided written consent to participate in this study. The RA-ILD group was selected based on the chest CT scan after re-evaluation by radiologists and rheumatologists if they had interstitial lung abnormalities that were indicative of RA-ILD. We excluded patients in whom it was difficult to evaluate the extent and type of ILD, such as those with a history of radiation therapy (n = 1), asbestosis (n = 2), or pulmonary lobectomy (n = 4). Among the RA patients who underwent CT within the previous 2 years, only those who did not have ILD or other pulmonary comorbidities, such as malignancy, pneumonia, or active pulmonary tuberculosis, were included in the RA-non ILD group (Fig. 1).
Enrolment and follow-up
After enrolment, the demographics and clinical information were collected, and the RA disease activity was evaluated by rheumatologists. We assessed the functional disability and quality of life by using a self-reported questionnaire that was completed by the participants with the assistance of a well-trained healthcare professional. We planned to conduct annual follow-up with patients and to collect their comprehensive clinical information. The underlying lung function of patients with ILD at the time of enrolment was assessed through a pulmonary function test (PFT) and echocardiography, if possible (Additional Table 1), and the performance of those tests could be adjusted according to the clinical situation of each patient and were scheduled at every 2-year follow-up visit.
Clinical characteristics and associated factors of RA-ILD patients
We collected data on demographic features, such as age at enrolment, age at RA diagnosis, sex, body mass index (BMI), smoking, income, and education level; clinical characteristics, such as disease activity score in 28 joints (DAS28) based on the erythrocyte sedimentation rate (ESR) or CPR (C-reactive protein) and comorbidities based on the Charlson Comorbidity Index, the Health Assessment Questionnaire-Disability Index (HAQ-DI), and the EuroQol-5 Dimension Index (EQ-5D); laboratory tests, including rheumatoid factor (RF), anti-citrullinated peptide antibody (ACPA), ESR, and CRP; and medication use, including DMARDs and glucocorticoid. In this study, we compared the demographics and clinical characteristics at enrolment between RA-ILD and RA-non ILD patients.
Statistical analysis
Categorical variables are presented as numbers and percentages (%), and continuous variables are presented as means with standard deviations (SD). We employed the independent t-test and the chi-square test to compare the two groups. Factors related to the presence of ILD among RA patients were analyzed using multivariable logistic regression analysis. All analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC, USA), and results were considered statistically significant when P-values were less than 0.05.
Results
Demographic and clinical characteristics of RA-ILD and RA-non ILD patients
In total, 148 RA-ILD and 410 RA-non ILD patients were enrolled between May 2017 and March 2021 (Fig. 1). The mean age at enrolment and at RA diagnosis was significantly higher in the RA-ILD group than in the RA-non ILD group (65.8 ± 9.9 vs. 58.0 ± 10.4 and 57.4 ± 11.3 vs. 47.1 ± 12.0, respectively; P < 0.001 for both). The disease duration of ILD of RA-ILD group was 2.1 ± 1.8 years on average. The proportion of patients with late-onset RA (age > 60 years) was higher in the RA-ILD group than in the comparator (43.9% vs. 14.2%, P < 0.001). The RA-ILD group included more male patients (35.8 vs. 14.5%, P < 0.001) and comprised more smokers compared to the RA-non ILD group (38.5% vs. 18.5%, P < 0.001). The RF positivity was higher in the RA-ILD group compared to that in the RA-non ILD group whereas the prevalence of anti-CCP antibody was comparable between the two groups. At enrolment, the disease activity as estimated by DAS28-ESR was significantly higher in the RA-ILD group (3.7 ± 1.4 vs. 3.4 ± 1.2, P = 0.007). Inflammatory markers were elevated more in the RA-ILD group than in the comparators (ESR 38.5 ± 27.5 vs. 26.5 ± 20.4 mm/h, P < 0.001 and CRP 1.0 ± 1.8 vs. 0.5 ± 0.8 mg/dL, P < 0.001; Table 1).
The presence of ILD among RA patients may affect their medication use for controlling the RA disease activity. Table 1 shows that oral glucocorticoids were used more frequently in the RA-ILD group (54.7 vs. 23.4%, P < 0.001), with an approximately 1.4-times higher daily dose compared to the RA-non ILD group. The use of MTX and leflunomide was less frequent in the RA-ILD group (54.1% vs. 82.7% and 5.4% vs. 24.4%, respectively; P < 0.001 for both), whereas hydroxychloroquine and sulfasalazine were more frequently used in the RA-ILD group than in the RA-non ILD group (42.6 vs. 18.1% and 40.5 vs. 18.3%, respectively, P < 0.001 for both). Despite a difference in the use of each agent, there was no significant difference in the use of biological agents between the two groups (16.9 vs. 20.2%, P = 0.376); non-tumor necrosis factor (TNF) inhibitors were used more frequently in the RA-ILD group (10.8% vs. 4.4%, P = 0.005), whereas TNF-inhibitor use was more prevalent in the RA-non ILD group (6.1% vs. 15.9%, P = 0.003).
Factors related to the presence of ILD among RA patients
In a multivariable analysis, a higher BMI (OR 1.65, 95% CI 1.036–2.629), history of smoking (OR 2.484, 95% CI 1.071–5.764), and higher age at RA diagnosis (OR 1.056, 95% CI 1.021–1.091) were significantly associated with ILD in RA patients. Oral glucocorticoid use was more likely in RA-ILD patients (OR 3.853, 95% CI 2.32–6.4), whereas MTX (OR 0.253, 95% CI 0.155–0.412) was less likely to be used in RA-ILD patients (Table 2).
Respiratory symptoms and pulmonary function test results of RA-ILD patients
Among the 148 RA-ILD patients who had initial CT, usual interstitial pneumonia (UIP) was the most common subtype of ILD (n = 107, 72.8%), followed by non-specific interstitial pneumonia (NSIP; n = 35, 23.6%), organizing pneumonia (OP; n = 1, 0.7%), and respiratory bronchiolitis-interstitial lung diseases (n = 1, 0.7%; Table 3).
The most common symptom of ILD was dyspnea, followed by sputum and cough (Supplementary Table 2). The RA patients with ILD in this cohort had relatively well-preserved pulmonary function in accordance with 81.4 ± 15.1% predicted value in forced vital capacity (FVC). A moderate degree of reduction in diffusion capacity was noted in the diffusion capacity of the lungs for carbon monoxide (DLco; 57.1 ± 13.8% predicted). These are summarized in Supplementary Table 2.
Discussion
Based on a multidisciplinary collaborative approach that was implemented according to a systematic protocol, we established a prospective cohort for RA-ILD patients. In the cross-sectional study which used the enrolment data of this prospective cohort, we demonstrated that higher age at RA diagnosis, a higher BMI, and smoking were associated with accompanying ILD in RA patients. In addition, we found that RA-ILD patients tended to use glucocorticoids more frequently and less frequently used methotrexate as compared with RA-non ILD patients.
There are no definitive guidelines for screening patients with RA for the presence of ILD at present; therefore, screening is usually performed according to the clinical needs of each patient. In our study, though we included definite RA-ILD patients, 28.4% of these participants had no definite respiratory symptoms and 27.3% did not have any indicative findings of ILD on simple chest X-ray. Instead, most RA-non ILD patients were evaluated using a chest CT scan for screening for lung diseases before starting targeted therapies or to identify the underlying pulmonary pathology. According to our previous study, the prevalence of old pulmonary tuberculosis (18.2%) or bronchiectasis (27.5%) in South Korea was relatively high as compared to that in Western countries [28,29,30]. In addition, our study might suggest that associated factors for RA-ILD, such as a higher BMI, history of smoking, and higher age at RA diagnosis, are worth considering when planning chest CT scan to screen for the presence of ILD in RA patients.
Sometimes, a problem that is faced when studying ILD that it is clinically complex to capture the exact timing of occurrence of ILD because chest CT scanning is usually considered only if a patient is suffering from any respiratory symptoms, rather than for screening purposes. Therefore, many clinical studies could not include patients with subclinical ILD which might affect the natural history of RA. The sequence in which both RA and ILD occurred was uncertain, and RA occurrence was sometimes followed by ILD. In clinical practice, physicians are concerned that ILD might progress or become exacerbated on exposure to certain DMARDs. This concern usually affects the treatment decision-making for RA-ILD patients. Insufficient medication use could result in higher disease activities of RA, and it is hard to assess causality whether a certain drug or uncontrolled RA disease activity affected the occurrence of ILD.
Previous studies identified traditional risk factors for ILD including smoking [18], older age [31], male sex [32], longer RA duration [31], elevated inflammatory markers [33], and the RA serologic status [34]. Except for smoking, these risk factors were non-modifiable. A higher disease activity of RA was associated with an increased risk of developing RA-ILD [35] as well as a poor prognosis for RA-ILD patients [36]. The disease activity of RA was a modifiable risk factor; however, the study population already had longstanding RA at baseline, which might have affected the occurrence of ILD. Though the confounders were adjusted, it is unclear whether RA itself or uncontrolled disease activity was associated with an increased risk of ILD.
In this study, we demonstrated the distinct characteristics of RA-ILD patients in comparison with RA-non ILD patients. The results of our study consistently match those of previous studies which showed that a history of smoking was an associated factor for the presence of ILD among RA patients. Smoking itself may contribute to the citrullination of proteins and induce ACPA by promoting autoimmune responses [37]. However, RF or ACPA positivity was not associated with ILD in RA patients in our study, though previous studies reported that concentrations of RF or ACPA were associated with prevalent RA-ILD [38] or increased the risk of disease progression and mortality [39]. In our study, the positive rate of RF was higher in RA-ILD group compared to RA-non ILD group, but this was not associated with ILD after adjusting confounders. This suggests that, regardless of seropositivity, smoking may be independently associated with ILD. Being male and older were not significant factors whereas the age at RA onset was an independent associated factor for ILD in the multivariable analysis.
Higher BMI was another associated factor for ILD in RA patients in our study. However, it is unclear whether obesity is a cause of ILD development or rather the sequelae of glucocorticoid treatment or limitation of physical activities [40]. Through our prospective cohort study, we expected to investigate whether BMI constitutes an aggravating factor in RA-ILD patients or a risk factor for ILD occurrence in RA-non ILD patients.
With regard to medication, there were apparently different patterns of DMARD use based on the presence of ILD. For example, RA-ILD patients used MTX and leflunomide less frequently but used hydroxychloroquine and sulfasalazine more frequently than those without ILD. Though the causative association between MTX use and ILD development remains inconclusive, which is similar to the conclusion of previous studies [41,42,43], the presence of ILD could guide clinicians to avoid specific medications for their patients. The use of oral glucocorticoids was related to the presence of RA-ILD in our analysis. This indicates that clinicians tend to choose glucocorticoids to treat both RA and ILD because of concerns with regard to inducing or exacerbating ILD with DMARDs. Using cumulative clinical information, we expect that the results of our prospective cohort study would facilitate an assessment of the relationship between certain medications and the occurrence or progression of ILD.
This study has some limitations. We could not investigate causal relationships between clinical factors and ILD in RA patients because of the cross-sectional study design. Thus, our results are not conclusive. Instead, prospective data based on our cohort which includes RA-non ILD RA patients will provide a better understanding of the natural course of ILD development in RA patients. The advantage of our prospective cohort is the simultaneous enrolment of RA-non ILD patients as comparators based on a multidisciplinary approach.
Conclusion
In conclusion, the presence of RA-ILD was associated with a higher RA-onset age, smoking history, and a higher BMI. Oral glucocorticoid use was more likely in RA-ILD patients, but MTX was less likely to be used.
Availability of data and materials
The data analyzed in this article cannot be shared publicly owing to the requirements for protecting the privacy and confidentiality of the participants. The research data will be shared on reasonable request made to the corresponding author.
Abbreviations
- RA:
-
Rheumatoid arthritis
- ILD:
-
Interstitial lung disease
- csDMARDs:
-
Conventional synthetic disease-modifying anti-rheumatic drugs
- HRCT:
-
High-resolution computerized tomography
- CTD:
-
Connective tissue disease
- RA-non ILD:
-
RA patients without ILD
- ACR:
-
American College of Rheumatology
- EULAR:
-
European League Against Rheumatism
- PFT:
-
Pulmonary function test
- BMI:
-
Body mass index
- DAS28:
-
Disease Activity Score in 28 Joints
- ESR:
-
Erythrocyte sedimentation rate
- CPR:
-
C-reactive protein
- HAQ-DI:
-
Health Assessment Questionnaire-Disability Index
- EQ-5D:
-
EuroQol-5 Dimension Index
- RF:
-
Rheumatoid factor
- SD:
-
Standard deviation
- UIP:
-
Interstitial pneumonia
- NSIP:
-
Nonspecific interstitial pneumonia
- LIP:
-
Lymphocytic interstitial pneumonia
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Kim D, Cho SK, Choi CB, Choe JY, Chung WT, Hong SJ, et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol Int. 2017;37:1735–45.
Brito Y, Glassberg MK, Ascherman DP. Rheumatoid arthritis-associated interstitial lung disease: current concepts. Curr Rheumatol Rep. 2017;19:79.
Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–91.
Zou YQ, Li YS, Ding XN, Ying ZH. The clinical significance of HRCT in evaluation of patients with rheumatoid arthritis-associated interstitial lung disease: a report from China. Rheumatol Int. 2012;32:669–73.
Richman NC, Yazdany J, Graf J, Chernitskiy V, Imboden JB. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore). 2013;92:92–7.
Norton S, Koduri G, Nikiphorou E, Dixey J, Williams P, Young A. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford). 2013;52:99–110.
Salaffi F, Carotti M, Di Carlo M, Tardella M, Giovagnoni A. High-resolution computed tomography of the lung in patients with rheumatoid arthritis: prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine (Baltimore). 2019;98:e17088.
Carmona L, González-Alvaro I, Balsa A, Angel Belmonte M, Tena X, Sanmartí R. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis. 2003;62:897–900.
Kim H, Sung Y-K. Epidemiology of rheumatoid arthritis in Korea. J Rheumatic Dis. 2021;28:60–7.
Hozumi H, Nakamura Y, Johkoh T, Sumikawa H, Colby TV, Kono M, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. 2013;3:e003132.
Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41:256–64.
Hagiwara K, Sato T, Takagi-Kobayashi S, Hasegawa S, Shigihara N, Akiyama O. Acute exacerbation of preexisting interstitial lung disease after administration of etanercept for rheumatoid arthritis. J Rheumatol. 2007;34:1151–4.
Ostör AJ, Chilvers ER, Somerville MF, Lim AY, Lane SE, Crisp AJ, et al. Pulmonary complications of infliximab therapy in patients with rheumatoid arthritis. J Rheumatol. 2006;33:622–8.
Schoe A, van der Laan-Baalbergen NE, Huizinga TW, Breedveld FC, van Laar JM. Pulmonary fibrosis in a patient with rheumatoid arthritis treated with adalimumab. Arthritis Rheum. 2006;55:157–9.
Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2014;66:803–12.
Sawada T, Inokuma S, Sato T, Otsuka T, Saeki Y, Takeuchi T, et al. Leflunomide-induced interstitial lung disease: prevalence and risk factors in Japanese patients with rheumatoid arthritis. Rheumatology (Oxford). 2009;48:1069–72.
Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology (Oxford). 2014;53:1676–82.
Kur-Zalewska J, Kisiel B, Kania-Pudło M, Tłustochowicz M, Chciałowski A, Tłustochowicz W. A dose-dependent beneficial effect of methotrexate on the risk of interstitial lung disease in rheumatoid arthritis patients. PLoS One. 2021;16:e0250339.
Li L, Liu R, Zhang Y, Zhou J, Li Y, Xu Y, et al. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2020;39:1457–70.
Wells AU, Flaherty KR, Brown KK, Inoue Y, Devaraj A, Richeldi L, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med. 2020;8:453–60.
Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheum. 2018;70:1544–54.
Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156:528–35.
Habib HM, Eisa AA, Arafat WR, Marie MA. Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol. 2011;30:217–21.
Kadura S, Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev. 2021;30:210011.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford). 2019;58:2031–8.
Huang S, Doyle TJ, Hammer MM, Byrne SC, Huang W, Marshall AA, et al. Rheumatoid arthritis-related lung disease detected on clinical chest computed tomography imaging: prevalence, risk factors, and impact on mortality. Semin Arthritis Rheum. 2020;50:1216–25.
World Health Organization. Global tuberculosis report 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports. Accessed 24 January 2023.
Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106:1591–9.
Shidara K, Hoshi D, Inoue E, Yamada T, Nakajima A, Taniguchi A, et al. Incidence of and risk factors for interstitial pneumonia in patients with rheumatoid arthritis in a large Japanese observational cohort, IORRA. Mod Rheumatol. 2010;20:280–6.
Yang JA, Lee JS, Park JK, Lee EB, Song YW, Lee EY. Clinical characteristics associated with occurrence and poor prognosis of interstitial lung disease in rheumatoid arthritis. Korean J Intern Med. 2019;34:434–41.
Restrepo JF, del Rincón I, Battafarano DF, Haas RW, Doria M, Escalante A. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol. 2015;34:1529–36.
Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheum. 2019;71:1472–82.
Rojas-Serrano J, Mejía M, Rivera-Matias PA, Herrera-Bringas D, Pérez-Román DI, Pérez-Dorame R, et al. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): a possible association between disease activity and prognosis. Clin Rheumatol. 2022;41:1741–7.
Deane KD, Nicolls MR. Developing better biomarkers for connective tissue disease-associated interstitial lung disease: citrullinated hsp90 autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2013;65:864–8.
Natalini JG, Baker JF, Singh N, Mahajan TD, Roul P, Thiele GM, et al. Autoantibody seropositivity and risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of U.S. Vveterans. Ann Am Thorac Soc. 2021;18:598–605.
Tyker A, Ventura IB, Lee CT, Strykowski R, Garcia N, Guzy R, et al. High-titer rheumatoid factor seropositivity predicts mediastinal lymphadenopathy and mortality in rheumatoid arthritis-related interstitial lung disease. Sci Rep. 2021;11:22821.
Kronzer VL, Huang W, Dellaripa PF, Huang S, Feathers V, Lu B, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol. 2021;48:656–63.
Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open. 2019;9:e028466.
Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford). 2010;49:1483–9.
Rojas-Serrano J, Herrera-Bringas D, Pérez-Román DI, Pérez-Dorame R, Mateos-Toledo H, Mejía M. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol. 2017;36:1493–500.
Acknowledgements
Not applicable.
Funding
This work was funded by Bristol-Myers Squibb, JW Pharmaceuticals, and the Basic Science Research Program of the National Research Foundation of Korea [grant number NRF-2021R1A6A1A03038899], which is funded by the Ministry of Education.
Author information
Authors and Affiliations
Contributions
YK Sung and SK Cho contributed to conceptualization and methodology of the study. H Kim, SK Cho, YJ Song, J Kang, SA Jeong, CB Choi, TH Kim, JB Jun, SC Bae, DH Yoo, H Lee, DW Park, JW Sohn, HJ Yoon, SJ Hong, SJ Yoo, YW Choi, HK Lee, SH Kim, YK Sung contributed to the data aquisition. HW Kim and H Kim performed the statistical analysis. H Kim performed data interpretation and wrote the main manuscript. All authors read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki; the institutional review board (IRB) of Hanyang University Hospital for Rheumatic Diseases [IRB file no. HYUH 2016-06-019] has approved the research protocol; and written informed consent has been obtained from the participants (or their legally authorized representatives). This study has been registered in the U.S. ClinicalTrials.gov database (no. NCT03099525).
Consent for publication
Not applicable.
Competing interests
YK Sung has received research grants from Bristol-Myers Squibb, Eisai, Pfizer, and JW Pharmaceuticals. The other authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Variables assessed at enrolment and follow-up in the cohort. Supplementary Table 2. Pulmonary symptoms and test results of RA-ILD patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, H., Cho, SK., Song, YJ. et al. Clinical characteristics of rheumatoid arthritis patients with interstitial lung disease: baseline data of a single-center prospective cohort. Arthritis Res Ther 25, 43 (2023). https://doi.org/10.1186/s13075-023-03024-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-023-03024-8