Abstract
Background
Periarticular osteopenia is an early sign of incipient joint injury in rheumatoid arthritis (RA), but cannot be accurately quantified using conventional radiography. Digital X-ray radiogrammetry (DXR) is a computerized technique to estimate bone mineral density (BMD) from hand radiographs. The aim of this study was to evaluate whether decrease in BMD of the hands (BMD loss), as determined by DXR 3 months after diagnosis, predicts radiographic joint damage after 1 and 2 years in patients with early RA.
Methods
Patients (n = 176) with early RA (<12 months after onset of symptoms) from three different Swedish rheumatology centers were consecutively included in the study, and 167 of these patients were included in the analysis. Medication was given in accordance with Swedish guidelines, and the patients were followed for 2 years. Rheumatoid factor and antibodies to cyclic citrullinated peptides (anti-CCP) were measured at baseline, and 28-joint Disease Activity Score (DAS28) was assessed at each visit. Radiographs of the hands and feet were obtained at baseline, 3 months (hands only) and 1 and 2 years. Baseline and 1-year and 2-year radiographs were evaluated by the Larsen score. Radiographic progression was defined as a difference in Larsen score above the smallest detectable change. DXR-BMD was measured at baseline and after 3 months. BMD loss was defined as moderate when the decrease in BMD was between 0.25 and 2.5 mg/cm2/month and as severe when the decrease was greater than 2.5 mg/cm2/month. Multivariate regression was applied to test the association between DXR-BMD loss and radiographic damage, including adjustments for possible confounders.
Results
DXR-BMD loss during the initial 3 months occurred in 59% of the patients (44% moderate, 15% severe): 32 patients (19%) had radiographic progression at 1 year and 45 (35%) at 2 years. In multiple regression analyses, the magnitude of DXR-BMD loss was significantly associated with increase in Larsen score between baseline and 1 year (p = 0.033, adjusted R-squared = 0.069).
Conclusion
DXR-BMD loss during the initial 3 months independently predicted radiographic joint damage at 1 year in patients with early RA. Thus, DXR-BMD may be a useful tool to detect ongoing joint damage and thereby to improve individualization of therapy in early RA.
Similar content being viewed by others
Background
Early stages of rheumatoid arthritis (RA) are characterized by gradually developing joint swelling, stiffness and pain, and the patients often have a history of several months of symptoms when first presenting to the rheumatologist. Periarticular bone loss may already be present at this stage, representing an early radiologic manifestation visible on plain radiographs [1, 2]. The disease course in RA shows considerable inter-individual variation, ranging from mild and self-limiting to severe erosive disease, sometimes with extra-articular manifestations. Early treatment with disease-modifying anti-rheumatic drugs (DMARDs) is known to improve disease outcome [3,4,5], and may limit disease-associated bone loss [6]. However, further improved individual prediction of the disease course and outcome remains an important issue in order to optimize anti-rheumatic therapy.
Digital X-ray radiogrammetry (DXR) is a technique that uses computerized analyses of standard hand radiographs to estimate peripheral bone mineral density (BMD) of the three middle metacarpal bones (DXR-BMD) [7, 8]. DXR-BMD loss has repeatedly been shown to predict radiographic joint progression in early RA [9,10,11,12,13,14]. However, the majority of previous DXR-BMD studies have been based on 12-month change, and by that time, conventional X-ray assessments of joint damage are at least as informative about disease progression [9, 11,12,13,14]. A Dutch study addressing DXR-BMD change after 4 months, reported an independent association between DXR-BMD loss and subsequent radiographic damage [10]. This study was part of a clinical trial with selected patients, and the treatment regimens were slightly different from standard care in Sweden. Therefore, we wished to investigate whether 3-month change in DXR-BMD predicts radiographic joint damage after 1 and 2 years in “real-world” patients with recent-onset RA.
Methods
Patients
Patients (n = 176) with early RA (64% women, symptom duration < 12 months), fulfilling the inclusion criteria (see subsequent text) and giving their informed consent, were consecutively included from three Swedish regions (one in Northern and two in Southern Sweden) in 2008–2014 and were followed for 2 years. All patients fulfilled the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) [15] and/or the 1987 ACR [16] classification criteria. Pharmacotherapy was prescribed as found appropriate by the treating rheumatologist, according to Swedish guidelines. Baseline characteristics are detailed in Table 1.
At baseline, 83% of patients were prescribed oral prednisolone, 49% received osteoporosis prophylaxis with low-dose calcium phosphate and vitamin D, and 6% with bisphosphonates. 91% received conventional synthetic DMARDs (csDMARD) (88% methotrexate, 2.4% other csDMARDs and 0.6% combination therapy). One patient (0.6%) was started on a tumor necrosis factor (TNF) inhibitor at baseline. During the follow-up period 14.4% received biologic therapy (bDMARDs) as displayed in Table 2.
Radiographic assessment and digital X-ray radiogrammetry (DXR)
Radiographs (posterior-anterior projection) of the hands, wrists and forefeet were performed at baseline, 3 months (hands only) and 1 and 2 years. The baseline and 1-year and 2-year radiographs were read in chronological order and evaluated according to the Larsen score [17] by one investigator at each center (MZ, KF and EB). The scoring system included 32 areas; metacarpal-phalangeal joints II − V, all proximal interphalangeal joints, the wrists divided into four areas and the metatarsophalangeal joints II–V. Each joint and joint area was graded 0–5. The maximum total score was 160. The smallest detectable change (SDC) was calculated for the three readers individually (EB, 2; KF, 1; MZ, 3) according to the method of Bruynesteyn [18]. Radiographic progression was defined as a difference in Larsen score above the SDC of the corresponding reader. The intra-rater and inter-rater reliability of the readers was assessed by calculating the intraclass correlation coefficient (ICC). The ICC was 0.903.
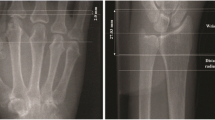
BMD was estimated on hand radiographs of the second, third and fourth metacarpal bones using DXR (the online Pronosco X-posure System, SECTRA, Linköping, Sweden), a computerized version of the traditional technique of radiogrammetry measuring cortical bone thickness [8, 19]. DXR-BMD was assessed at inclusion and after 3 months. The mean value of both hands was used in all analyses. DXR-BMD values are given in mg/cm2. DXR-BMD loss was categorized either as a moderate decrease in DXR-BMD (≥ 0.25 but < 2.5 mg/cm2 per month) or a severe decrease (≥ 2.5 mg/cm2 per month), as defined by the provider (Sectra) [20]. To ensure consistent image acquisition, the images for each patient were always taken in a frontal position of the hands using the same X-ray machine. The images were sent unprocessed to Sectra for DXR analysis.
Clinical and laboratory assessments
The erythrocyte sedimentation rate (ESR, mm/1 h) and C-reactive protein (CRP, mg/L) were determined at baseline and after 3, 6, 12 and 24 months. At the same time points, the 28-joint Disease Activity Score (DAS28) was calculated by the patient’s regular physician [21]. Therapy response was determined according to EULAR response criteria [22]. Functional status was evaluated using the Swedish version of the Stanford Health Assessment Questionnaire (HAQ) [23]. Rheumatoid factor (RF) and antibodies to cyclic citrullinated peptides (anti-CCP2) were analyzed in baseline serum samples at the clinical immunology units of the local hospitals.
Statistics
Statistical calculations were performed using SPSS software (version 23, IBM Corporation, Armonk, USA). Linear regression analyses were used to explore the effect of DXR-BMD alone, and in combination with various clinical and laboratory variables, chosen with respect to clinical assumptions, for associations with change in Larsen score after 1 and 2 years. After testing each variable in a simple regression analysis with change in Larsen score at 1 and 2 years as the dependent variable, multiple regression analyses were performed including variables with p < 0.2. Radiographic progression was defined as a difference in Larsen score above the SDC between baseline and 1 and 2 years, respectively. Thus, for example, radiographs assessed by KF with a difference > 1 between the two time points were graded as the actual difference, otherwise as 0. As sensitivity analyses, we also performed linear regression with the same variables, but with absolute changes in Larsen, i.e. not considering the SDC. In addition, logistic regression analysis was performed, in which radiographic progression was defined as change in Larsen score greater than SDC. The Pearson chi2 test or Fisher’s exact test were used for categorical variables, and the independent samples t test was used for continuous variables. All p values are two-sided, and p values less than 0.05 were considered statistically significant.
Results
In total, 176 patients without previous DMARD exposure were included in the study. Table 1 shows the patient characteristics and Table 2 the anti-rheumatic therapy including glucocorticoids (GC), and treatments that influence BMD, initiated at baseline. There were no significant differences in these background characteristics between the participating sites (data not shown). Nine patients were lost because of missing radiographs at 3 months. Thus, the evaluation included 167 patients with radiographs at baseline, 3 months and 1 year (Helsingborg, n = 38; Linköping, n = 65; Umeå, n = 64). Of the 167 patients, 129 also underwent radiography at 2 years (Fig. 1). Compared with the patients with 2-year radiographs available, patients without 2-year radiographs had lower mean Larsen score at baseline (2.1 (SD = 3.17) vs 4. 7 (SD = 5.17); p < 0.001) and at 1 year (2.6 (SD = 3.34) vs 5.8 (SD = 5.66); p < 0.001), and were to a lesser extent RF positive (48.6% vs 67.5%; p = 0.037). Other baseline characteristics, as detailed in Tables 1 and 2 did not significantly differ from those in patients with radiographs available at 2 years.
Availability of radiographs at baseline and follow up. There were 176 patients included in the study. aNine patients were lost because of missing radiographs at 3 months, and thus, the evaluation included 167 patients with radiographs at baseline, 3 months and 1 year. bOf the 167 patients included in the evaluation, 129 also underwent radiography at 2 years
At 3 months, 105 (63%) patients had low disease activity (DAS28 ≤ 3.2) and 80 (48%) had reached EULAR remission (DAS28 ≤ 2.6): 46 (28%) of the patients had moderate and 6 (4%) high disease activity (DAS28 > 5.2). DAS28 values from the 3-month visits were missing for 10 (6%) patients. After 1 year, 108 (65%) of the patients had low disease activity, 36 (22%) patients had moderate and 7 (4%) high disease activity: 88 (53%) of the patients had reached EULAR remission. DAS28 values from the 1-year visit were missing in 16 patients (10%).
The mean age of the male patients (n = 60) at baseline was 61 years (SD = 14.5) and the mean age of the female patients (n = 107) was 57 years (SD = 14.3). Comparing our DXR-BMD values with a Danish reference cohort of healthy individuals [24], 92 patients (55.1%) in our cohort had bone loss in the hand exceeding the age-related bone loss in the hand among the Danish controls.
Of the 167 patients, 32 (19%) had radiographic progression at 1 year and 45 of 129 patients (35%) had radiographic progression at 2 years. The change in DXR-BMD over 3 months showed BMD loss in 98 patients (59%). The DXR-BMD loss was moderate in 73/167 patients (44%) and severe in 25/167 patients (15%). Radiographic joint damage was significantly different across the three categories of DXR-BMD loss at baseline and at 1 year (p = 0.039 and p = 0.024, respectively) and there was a trend towards statistical significance after 2 years (p = 0.056) (Table 3). Categorizing DXR-BMD loss according to the age-related reference material presented by Ornbjerg et al. [24] yielded very similar results (data not shown).
Patients with change in Larsen score greater than the SDC after 1 year had significantly higher Larsen scores (mean) at baseline (3.6 vs 6.2; p = 0.005). Compared with patients without DXR-BMD loss, patients with DXR-BMD loss after 3 months were significantly older (60.3 years vs 55.7; p = 0.042), had significantly higher baseline DAS28 (5.1 vs 4.6; p = 0.023) and significantly higher Larsen scores at baseline (4.7 vs 3.2; p = 0.034). Also, the proportion of women was significantly higher (70.4% vs 55.1%; p = 0.05) among patients with BMD loss (Table 2). There was no significant difference in the DXR value at baseline in the anti-CCP2-positive compared with the anti-CCP-negative patients (576 vs 587 mg/cm2; p = 0.426).
Simple regression analyses with change in Larsen score greater than the SDC at 1 year as the dependent variable were performed, including the following covariates: age, sex, oral corticosteroid treatment, DXR-BMD loss/month, baseline DAS28, CRP, ESR, Larsen score, anti-CCP2 status, and RF status. Also, DAS28 > 2.6 at 3 months (yes/no) was included. Covariates with a p value < 0.2 in these analyses were included in a multiple regression model (Table 4). This model, adjusting for sex and baseline values of ESR, DAS28, Larsen score and anti-CCP2 status, showed a significant association between 3-month BMD loss and increase in Larsen score above the SDC after 1 year (p = 0.033, adjusted R-squared = 0.069) (Table 4). No significant association was observed between early bone loss and increase in the Larsen score above the SDC at 2 years (p = 0.604). When using the same covariates but with change in Larsen score without considering the SDC as the dependent variable, DXR-BMD loss was significantly associated with the 1-year Larsen score (p = 0.048), but not the Larsen score at 2 years (p = 0.491). In logistic regression analysis, there was no significant association between DXR-BMD loss and 1-year radiographic progression defined as a change in Larsen score above the SDC (p = 0.158). Treatment with bisphosphonates, calcium and vitamin D did not influence the DXR-BMD loss (data not shown).
When analyzing only patients without erosions at baseline (n = 123), using the same multivariate model (adjusted for the same variables but not for the Larsen score at baseline), 3-month DXR-BMD loss remained associated with radiographic progression after 1 year (p = 0.021, R-squared = 0.07). Also, when analyzing the 1-year outcome among the 129 patients with 2-year radiographs available, the association between DXR-BMD loss and radiographic progression remained statistically significant (p = 0.039, adjusted R-squared = 0.08).
Discussion
To our knowledge, this is the first study addressing the predictive value of 3-month DXR-BMD in patients with recent-onset RA compared with radiography and clinical data. In clinical practice, evaluation of prescribed DMARDs is commonly performed 3 months after initiation. At this occasion, particularly in early disease, additional information on the patient’s radiographic prognosis would be highly valuable in order to optimize therapy decisions. In this study, we found DXR-BMD loss during the first 3 months to independently predict radiographic joint damage at 1 year and the 1-year progression from baseline.
Our results on metacarpal bone loss among patients with early RA are in line with previous reports [9,10,11,12,13,14]. The shortest interval of DXR-BMD assessments among previous studies was 3 months in the study from Bøyesen et al. [25], addressing 3-month change in DXR-BMD as a predictive factor for erosive progression identified on magnetic resonance imaging (MRI) in patients with early RA. In the 53 patients completing that study there was only a trend towards higher MRI synovitis score and 3-month DXR BMD loss in patients developing MRI erosions, and no significant changes. Wevers-de Boer and coworkers [10] presented 4-month data with similar findings on the 1-year radiographic outcome as in the current study. Thus, early DXR-BMD assessments seem to be of clinical value, in order to optimize early institution of anti-rheumatic pharmacotherapy and thereby diminish the risk of future disability [4, 26,27,28,29,30]. However, we found no predictive value of DXR-BMD loss in relation to the 2-year radiographic outcome. This was somewhat surprising, since existing radiographic damage often predicts radiographic progression. One possible explanation for the disparate 1-year and 2-year findings in our study could be that potent instituted pharmacotherapy attenuated radiographic differences over time. Also, missing data from 2-year radiographs (n = 38) need to be considered, but the association between DXR-BMD loss and radiographic damage at 1 year remained statistically significant, also after excluding the 38 patients without 2-year radiographs. Thus, difference in statistical power appears to be an unlikely explanation for the discordance between 1-year and 2-year data. A previous study by Forslind et al. [9] showed that patients with early RA, who were on prednisolone 7.5 mg per day in addition to conventional DMARDS, had significantly less DXR-BMD loss as compared with patients with RA who were not receiving corticosteroids. This finding was attributed to the anti-inflammatory effect of prednisolone, hampering osteopenia induced by inflammation. Although not primarily designed to address this, our study did not identify a significant impact of oral corticosteroid on BMD loss or radiographic progression. Similarly, treatment with bisphosphonates, calcium and vitamin D did not significantly impact BMD loss.
In our study we did not observe a significant difference in the DXR value at baseline in the anti-CCP2-positive compared with the anti-CCP-negative patients. This fact is contrary to the findings in other studies [31,32,33] in which BMD loss was significantly more widespread in anti-CCP-positive patients. Different methods for estimating BMD loss (comparative micro computed tomography (micro-CT) analysis and dual-energy X-ray absorptiometry) may influence the differing results. Also, it needs to be pointed out that our study was not primarily designed to address the influence of anti-CCP on BMD loss.
Whenever a patient with RA presents with erosions at the time of diagnosis, an aggressive disease course is assumed, and treatment is chosen accordingly. Thus, information on the radiographic prognosis is even more important in the large category of patients without evidence of erosive disease at diagnosis. Interestingly, we found that 3-month DXR-BMD loss also predicted 1-year radiographic damage in this subgroup of patients. However, the comparative value of DXR-BMD compared with other imaging modalities needs to be assessed.
Limitations of this study are the missing 2-year radiographs in 38 patients (23%), and the fact that the baseline Larsen score and RF status differed between patients with radiographs available at 2 years and those without. Nevertheless, analyzing the 129 patients with all radiographs available up to 2 years did not substantially alter the findings.
Conclusion
In this real-world study of patients with early RA, we found that DXR-BMD loss during the initial 3 months independently predicted radiologic damage at 1 year. However, DXR-BMD loss predicts only a minor part of the variation in radiographic damage, and an association was not established after 2 years of disease. Future studies should compare the value of DXR-BMD with other imaging modalities.
Abbreviations
- ACR:
-
American College of Rheumatology
- bDMARD:
-
Biologic disease modifying anti-rheumatic drug
- BMD:
-
Bone mineral density
- CCP:
-
Cyclic citrullinated peptides
- csDMARD:
-
Conventional synthetic disease modifying anti-rheumatic drug
- CRP:
-
C-reactive protein
- DAS:
-
Disease Activity Score
- DAS28:
-
DAS with 28 joints
- DXR:
-
Digital X-ray radiogrammetry
- DXR-BMD:
-
Bone mineral density measured by digital X-ray radiogrammetry
- ESR:
-
Erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- GC:
-
Glucocorticoid
- HAQ:
-
Health Assessment Questionnaire
- ICC:
-
Intraclass correlation coefficient
- MRI:
-
Magnetic resonance imaging
- MTX:
-
Methotrexate
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SDC:
-
Smallest detectable change
- TNF:
-
Tumor necrosis factor
References
Brook A, Corbett M. Radiographic changes in early rheumatoid disease. Ann Rheum Dis. 1977;36(1):71–3.
Grassi W, et al. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998;27 Suppl 1:S18–24.
Darawankul B, et al. The good EULAR response at the first year is strongly predictive of clinical remission in rheumatoid arthritis: results from the TARAC cohort. Clin Rheumatol. 2015;34(1):43–9.
Markusse IM, et al. Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med. 2016;164(8):523–31.
Schneider M, Kruger K. Rheumatoid arthritis–early diagnosis and disease management. Dtsch Arztebl Int. 2013;110(27-28):477–84.
Dolan AL, et al. Does active treatment of rheumatoid arthritis limit disease-associated bone loss? Rheumatology (Oxford). 2002;41(9):1047–51.
Jorgensen JT, et al. Digital X-ray radiogrammetry: a new appendicular bone densitometric method with high precision. Clin Physiol. 2000;20(5):330–5.
Rosholm A, et al. Estimation of bone mineral density by digital X-ray radiogrammetry: theoretical background and clinical testing. Osteoporos Int. 2001;12(11):961–9.
Forslind K, et al. Hand bone loss measured by digital X-ray radiogrammetry is a predictor of joint damage in early rheumatoid arthritis. Scand J Rheumatol. 2009;38(6):431–8.
Wevers-de Boer KV, et al. Four-month metacarpal bone mineral density loss predicts radiological joint damage progression after 1 year in patients with early rheumatoid arthritis: exploratory analyses from the IMPROVED study. Ann Rheum Dis. 2015;74(2):341–6.
Guler-Yuksel M, et al. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2009;68(3):330–6.
Hoff M, et al. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis. 2009;68(3):324–9.
Rezaei H, et al. Evaluation of hand bone loss by digital X-ray radiogrammetry as a complement to clinical and radiographic assessment in early rheumatoid arthritis: results from the SWEFOT trial. BMC Musculoskelet Disord. 2013;14:79.
Forslind K, et al. Does digital X-ray radiogrammetry have a role in identifying patients at increased risk for joint destruction in early rheumatoid arthritis? Arthritis Res Ther. 2012;14(5):R219.
Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Larsen A. How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies. J Rheumatol. 1995;22(10):1974–5.
Bruynesteyn K, et al. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis. 2005;64(2):179–82.
Barnett E, Nordin BE. The radiological diagnosis of osteoporosis: a new approach. Clin Radiol. 1960;11:166–74.
de Rooy DP, et al. Loss of metacarpal bone density predicts RA development in recent-onset arthritis. Rheumatology (Oxford). 2012;51(6):1037–41.
Prevoo ML, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8.
van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41(10):1845–50.
Ekdahl C, et al. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol. 1988;17(4):263–71.
Ornbjerg LM, et al. Establishment of age- and sex-adjusted reference data for hand bone mass and investigation of hand bone loss in patients with rheumatoid arthritis treated in clinical practice: an observational study from the DANBIO registry and the Copenhagen Osteoarthritis Study. Arthritis Res Ther. 2016;18:53.
Boyesen P, et al. Prediction of MRI erosive progression: a comparison of modern imaging modalities in early rheumatoid arthritis patients. Ann Rheum Dis. 2011;70(1):176–9.
Goekoop-Ruiterman YP, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2008;58(2 Suppl):S126–35.
Breedveld F. The value of early intervention in RA − a window of opportunity. Clin Rheumatol. 2011;30 Suppl 1:S33–9.
Ahlstrand I, et al. Pain and activity limitations in women and men with contemporary treated early RA compared to 10 years ago: the Swedish TIRA project. Scand J Rheumatol. 2015;44(4):259–64.
Kudo-Tanaka E, et al. Early therapeutic intervention with methotrexate prevents the development of rheumatoid arthritis in patients with recent-onset undifferentiated arthritis: A prospective cohort study. Mod Rheumatol. 2015;25(6):831–6.
Kyburz D, Finckh A. The importance of early treatment for the prognosis of rheumatoid arthritis. Swiss Med Wkly. 2013;143:w13865.
Kleyer A, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73(5):854–60.
Bugatti S, et al. Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther. 2016;18(1):226.
Orsolini G, et al. Titer-dependent effect of anti-citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif Tissue Int. 2017;101(1):17–23.
Acknowledgements
This work was financially supported by the Swedish Rheumatism Association, the Norrbacka-Eugenia foundation, the King Gustav V 80-year Foundation, the Swedish Medical Society, ALF Grants from Region Östergötland, the Linköping University Hospital Research Fund and the Foundation for Assistance to Disabled People in Skane (Stiftelsen för Bistånd åt Rörelsehindrade i Skåne).
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
KF, AK, TS, EB and MZ designed the study and participated in patient enrollment and characterization. MZ, KF and EB scored radiographs. MZ and EB analyzed the data. All authors contributed to the interpretation of data, drafting the article and revising it critically. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients gave their written informed consent. The regional ethics committees in Linköping (DNR 2012/273-31), Lund (DNR Lu 464/2008), and Umeå (DNR 09-095 M) approved the study protocol, which was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ziegelasch, M., Forslind, K., Skogh, T. et al. Decrease in bone mineral density during three months after diagnosis of early rheumatoid arthritis measured by digital X-ray radiogrammetry predicts radiographic joint damage after one year. Arthritis Res Ther 19, 195 (2017). https://doi.org/10.1186/s13075-017-1403-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-017-1403-0