Abstract
Background
Systemic lupus erythematosus (SLE) is associated with B cell hyperactivity, and lupus nephritis (LN), in particular, is promoted by the production of autoantibodies and immune complex deposition. Bruton’s tyrosine kinase (BTK) plays critical roles in B cell receptor-related and Fc receptor-related signaling. We aimed to investigate the impact of therapeutic intervention with HM71224 (LY3337641), a selective BTK inhibitor, on the development of murine SLE-like disease features.
Methods
We examined the therapeutic effects of HM71224 on SLE-like disease features in MRL/lpr and NZB/W F1 mice. The disease-related skin lesion was macroscopically observed in MRL/lpr mice, and the impact on splenomegaly and lymphadenopathy was determined by the weight of the spleen and cervical lymph node. The renal function was evaluated by measuring blood urea nitrogen, serum creatinine, and urine protein, and the renal damage was assessed by histopathological grading. Survival rate was observed during the administration period. The impact of B cell inhibition was investigated in splenocytes from both mice using flow cytometry. Autoantibody was measured in serum by ELISA.
Results
HM71224 effectively suppressed splenic B220+GL7+, B220+CD138+, and B220+CD69+ B cell counts, and anti-dsDNA IgG and reduced splenomegaly and lymph node enlargement. The compound also prevented skin lesions caused by lupus development, ameliorated renal inflammation and damage with increased blood urea nitrogen and creatinine, and decreased proteinuria. Furthermore, HM71224 also decreased mortality from lupus development in both mouse models.
Conclusion
Our results indicate that inhibition of BTK by HM71224 effectively reduced B cell hyperactivity and significantly attenuated the development of SLE and LN in rodent SLE models.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease [1, 2], and lupus nephritis (LN) is one of the most severe SLE-associated organ complications [3, 4]. The etiology of SLE has been still unclear, however, abnormalities of genetic variants, that of molecular pathways, and dysregulation of several immune cells have been previously reported as disease pathogenesis in SLE. Dysregulation of B cell receptor (BCR) signaling that can lead to autoimmunity including autoantigen presentation to activate T cells and pro-inflammatory signaling by cytokines secretion and complement activation have been implicated in pathogenesis of SLE and LN [5, 6]. Especially, several pathogenic autoantibodies secreted by abnormalities of B cells play crucial roles in pathogenesis of SLE. Moreover, autoantibodies and intrarenal complement activation contribute to the renal immune-pathogenesis of LN [7,8,9]. Given the crucial role of B cells in SLE pathogenesis, they have emerged as promising new targets for SLE and LN treatment in the last decade. Clinical trials of several B cell-targeted biological treatments, such as the anti-CD20 antibody rituximab [10,11,12], B cell-activating factor inhibitor belimumab [13], and anti-CD22 antibody epratuzumab [14, 15], have been conducted, but only belimumab has demonstrated a clinical benefit, leading to US Food and Drug Administration approval [16, 17].
Bruton’s tyrosine kinase (BTK) is a non-receptor tyrosine kinase belonging to the tec protein tyrosine kinase (TEC) kinase family that is encoded by the TEC gene. BTK plays critical roles in activation mediated by the B cell receptor (BCR), Fc receptor (FcR), Toll-like receptor (TLR), and chemokine receptor [18,19,20]. In humans, BTK deficiency causes inherited X-linked agammaglobulinemia [21]. In mice, it leads to X-linked immunodeficiency (xid) [21] and BTK-knockout mice carrying the 56R anti-DNA Ig transgene do not produce anti-DNA antibodies [22]. Moreover, macrophages in BTK-deficient xid mice have impaired proinflammatory cytokine generation because BTK plays a major role in immune complex-mediated activation via FcγR in macrophages [21]. BTK is closely associated with SLE and LN development via both BCR and Fc receptor signaling, and therapeutic potential of BTK inhibitors have been demonstrated in several SLE animal models. For example, it have been reported that the therapeutic effects for glomerular nephritis of RN486 and PF-06250112, selective BTK inhibitors, by inhibition of effector cells and target autoantibodies in NZB/W F1 mice with Fc receptor dependent and antibody mediated LN [23, 24]. Ibrutinib also ameliorated humoral and cellular autoimmunity by partial crippling of cell signaling in both B cells and antigen presenting cells in lupus–prone B6.Sle1 and B6.Sle1.Sle3 mice [25]. Thus, targeting the BTK signaling pathway may provide an effective therapeutic strategy [26].
HM71224, orally active and irreversible small-molecule BTK inhibitor, may covalently bind to the active site (cysteine 481 residue) of BTK. HM71224 inhibited BTK, BMX, TEC and TXK which carry a conserved cysteine in the binding pocket in more than 85-kinase assays. HM71224 showed a highly selective inhibition for BTK with IC50 of 1.95 nM. The selectivity toward other BMX, TEC and TXK were 0.3, 2.3 and 2.4 fold, respectively. Moreover, HM71224 completely occupied to BTK and revealed potent inhibition of BCR, FcR and TLR mediated signaling [27].
In this study, to determine whether HM71224 could attenuate SLE and LN by suppressing BTK activation, the MRL/lpr and New Zealand Black/White F1 (NZB/W F1) mouse models, which have physiological relevance for human lupus, were used. MRL/lpr mice are homozygous for the lympho-proliferation spontaneous mutation (Faslpr) and have impaired central tolerance [28, 29], and NZB/W F1 mice co-express several sle loci that are associated with increased risk of SLE development [30].
Methods
Immunoblotting
Ramos cells (American Type Culture Collection, Manassas, VA, USA), the human Burkitt’s lymphoma cell line, were cultured in suspension using Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Rockville, MD, USA) with 10% fetal bovine serum (Gibco) at 37 °C in 5% CO2 in air. Cells were starved of serum for 1 h and then treated with HM71224 for 1 h. Cells were washed with phosphate-buffered saline (Welgene, Korea) and then were stimulated with 1 μg/ml of goat F(ab’)2 anti-human IgM (Southern Biotech, Birmingham, AL, USA) for 10 min on ice. Cells were then lysed in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, St. Louis, MO, USA) and the proteins were separated by SDS-PAGE (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). Proteins were detected with anti-BTK Y223 (Cell Signaling Technology, Danvers, MA, USA), anti-BTK (53/BTK, Cell Signaling Technology), anti-PLCγ2 Y1217 (Cell Signaling Technology), anti-PLCγ2 (Cell Signaling Technology), and anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. Protein signals were visualized by chemiluminescence using ECL western blotting detection reagent (EMD Millipore), and scanned blots were quantified using a Multi-Image analyzer (Fujifilm, Japan). The percentage of phosphorylation of each lane was determined using Multi-Gauge software (Fujifilm). The IC50 value was calculated using control IgM+ set at 100% and control IgM- set at 0%.
FcγR-stimulated cytokines
THP-1 cells (American Type Culture Collection) were differentiated to macrophages by stimulation with interferon-γ (IFN-γ) at 10 ng/ml in RPMI 1640 medium (Gibco) with 10% fetal bovine serum (Gibco) for 6 days at 37 °C in 5% CO2 in air. Monocyte-derived macrophages were pre-treated with HM71224 for 30 min. Cells were stimulated with a high concentration of plate-bound human IgG. The supernatant was collected after 24 h, and TNF-α and IL-6 were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA).
Experimental protocol for MRL/lpr mice and NZB/W F1 mouse models
Female MRL/MpJ-Fas lpr/J mice (20 ± 5 g, 8 weeks old) and female NZB/W F1 mice (30 ± 5 g, 18 weeks old) were obtained from SLC Inc. (Japan). Animals were housed and handled in a temperature-controlled environment with a 12-h light/12-h dark cycle. They had free access to standard pelleted food (Picolab Rodent diet 5053, St. Louis, MO, USA) and water ad libitum. In MRL/lpr mice, 12 animals per group identified by their urine protein score [31] were treated with HM71224 orally once per day from 8 weeks through 28 weeks of age, and in NZB/W F1 mice, 12 animals per group were administered HM71224 orally once daily from 18 weeks through 40 weeks of age. All animal experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Hanmi Research Center and performed according to the approved guideline.
Skin lesion scores
Macroscopic lupus-erythematosus-like skin lesions were evaluated only in MRL/lpr mice with the spontaneous development of skin lesions similar to those seen in human SLE [32]. Skin lesions were scored every week and were expressed using a scoring system from 0 to 3 (0, none; 1, mild; 2, moderate (<2 cm); 3, severe (≥ 2 cm)) for changes in the nose, ears, and intrascapular region.
Urine protein
Urine protein was individually evaluated weekly or biweekly using a urine test strip (URiSCAN strips, YD Diagnostics, Korea). Scores for protein concentration were graded from 0 to 4: 0, none; 1, 30–99 mg/dl; 2, 100–299 mg/dl; 3, 300–1999 mg/dl; 4, ≥ 2000 mg/dl or death.
Blood urea nitrogen, creatinine, and autoantibody measurements
Blood samples were obtained from mice via the caudal vena cava under anesthesia with ether. Blood was centrifuged at 13,400 g for 2 min at 4 °C to collect serum. Blood urea nitrogen (BUN) and creatinine (CRE) were measured using a Hitachi 7020 automatic chemical analyzer (Hitachi, Japan). Serum mouse anti-dsDNA antibody levels were determined using a commercially available ELISA kit (Alpha Diagnostic, San Antonio, TX, USA) according to the manufacturer’s instructions.
Kidney histopathological analysis
The kidneys were surgically removed under anesthesia with ether. They were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections of 4-μm thickness were stained with hematoxylin and eosin and Periodic acid Schiff. The histopathological score was evaluated microscopically in a blinded manner. The membranous glomerulonephritis score (GN score) was evaluated using scoring from 0 to 4: 0, normal; 1, mild, focal or early proliferation; 2, moderate or definite proliferation and increased matrix; 3, diffuse and focal or diffuse proliferation; 4, severe diffuse proliferation with crescent/sclerosis. The renal interstitial nephritis score (IN score) was graded from 0 to 4 for inflammation and necrosis: 0, normal; 1, occasional, focal or small pockets of mononuclear cells (MNCs, 10–14 cells); 2, focal infiltration of MNCs (15–30 cells); 3, multifocal and extensive infiltration of MNCs; 4, severe infiltration of MNCs with extensive necrosis. Vasculitis was scored from 0 to 4: 0, normal; 1, occasional perivascular infiltration of MNCs; 2, several foci of perivascular infiltration of MNCs without necrosis; 3, multifocal perivascular infiltration of MNCs with/without necrosis; 4, multifocal or diffuse perivascular infiltration of MNCs; extensive with necrosis.
Splenomegaly and lymphadenopathy
Spleen and cervical lymph nodes were removed and their weights were measured before fixation.
Flow cytometry
A single cell suspension from the spleen was prepared by homogenizing the spleen through a 40-μm mesh nylon cell strainer (BD Falcon 352340, BD Biosciences, San Jose, CA, USA). Red blood cells were lysed using ACK lysing buffer (Gibco). Splenocytes were stained with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD45R/B220 (BD Biosciences), phycoerythrin (PE)-conjugated hamster anti-mouse CD69 (BD Biosciences), PE-conjugated rat anti-mouse GL7 (BD Biosciences), and PE-conjugated rat anti-mouse CD138 (BD Biosciences). At least 10,000 stained cells were counted and were analyzed with FACSCalibur™ flow cytometry (BD Biosciences). All splenocytes were primarily gated on live lymphocytes based on forward scatter (FCS) and side scatter (SSC). Germinal center B cells were identified as B220+ and GL7+. Activated B cells were identified as B220+ and CD69+, and plasma B cells were identified as B220+ and CD138+.
Statistical analysis
Data are expressed as the mean ± SEM. Analysis of the significance of differences was performed using one-way analysis of variance (ANOVA) (for parametric data) or the Kruskal–Wallis test (for non-parametric data) using GraphPad Prism (version 5.0, La Jolla, CA, USA). In Kaplan-Meier survival curve, the curve comparison was resulted of the log-rank test using GraphPad Prism.
Results
HM71224 regulates BCR signaling in B cells and FcγR signaling in monocytes
To characterize the inhibitory effect of HM71224 on the BCR signaling pathway, the activation of BTK and PLCγ2, the physiological substrate of BTK, following stimulation with anti-IgM was examined in human Ramos B lymphoma cells. HM71224 blocked both autophosphorylation of BTK and phosphorylation of PLCγ2 with IC50 values of less than 10 nM (Fig. 1a). To explore the inhibitory effect of HM71224 on the FcγR signaling cascade, the production of the inflammatory cytokines TNF-α and IL-6 was evaluated following stimulation with anti-IgG in human monocyte THP-1 cells. Production of both TNF-α and IL-6 effectively decreased in a dose-dependent manner (Fig. 1b and c).
Inhibitory effects of HM71224 on B cell antigen receptor (BCR) and Fc receptor (FcR) signaling. a HM71224 dose-dependently inhibited BCR stimulation (1 μM of goat F(ab’)2 anti-human IgM)-induced phosphorylation of both Bruton’s tyrosine kinase (BTK) Y223 and phosphoinositide phospholipase C-γ2 (PLCγ2) Y1217 in Ramos cells. The western blots were reproduced three times and the representative one was chosen. b, c THP-1 cells (n = 2) were differentiated to macrophages by 10 ng/ml of interferon-γ. HM71224 treatment resulted in a trend toward reduction of IgG-induced TNF-α and IL-6 production by THP-1 cells. Data are shown as the mean ± SEM. GAPH glyceraldehyde 3-phosphate dehydrogenase
HM71224 inhibits B cell activation in animal models of lupus
To determine whether HM71224 effectively inhibits B cells, splenic B cell subpopulations were evaluated using flow cytometric analysis. In MRL/lpr mice, the proportion of B220+CD69+ activated B cells dose-dependently decreased (P < 0.01 at 30 mg/kg, Fig. 2a) and that of B220+GL7+ germinal center B cells decreased without reaching statistical significance (Fig. 2b). In NZB/W F1 mice, both activated B cells and plasma B cells decreased in a dose-dependent manner. In addition, B220+CD69+ activated B cells (P < 0.01, Fig. 2c) and B220+CD138+ plasma B cells (P < 0.001, Fig. 2d) were significantly reduced at 30 mg/kg of HM71224 compared with those in vehicle-treated mice.
HM71224 inhibits B cells and autoantibody. Quantitative analysis of splenic B cell populations was performed by flow cytometry. HM71224 dose-dependently reduced activated B cells (a), germinal center B cells (b) and showed a significant ameliorative effect on activated B cells at 30 mg/kg after treatment for 20 weeks in MRL/lpr mice (n = 11 per vehicle-treated group, n = 12 per HM71224-treated group). Treatment with HM71224 for 24 weeks in NZB/W F1 mice resulted in a significant decrease in both activated (c) and plasma B cells (d) (n = 8 per vehicle-treated group, n = 9 per 3 mg/kg of HM71224-treated group, n = 12 per 10 and 30 mg/kg of HM71224-treated group). Serum anti-dsDNA IgG was measured by ELISA at the end of the study. HM71224 dose-dependently inhibited autoantibody in both mouse models (e, f). Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle control, analyzed by the Kruskal–Wallis test
Moreover, HM71224 dose-dependently suppressed serum anti-dsDNA IgG in both MRL/lpr (P < 0.01 at 30 mg/kg, Fig. 2e) and NZB/W F1 mice (P not significant, Fig. 2f).
HM71224 reduces spleen and lymph node enlargement
To investigate the inhibitory effects for splenomegaly and lymphadenopathy as manifestations of SLE, the weights of the spleen and lymph node were measured after 20 and 22 weeks of HM71224 treatment in MRL/lpr and NZB/W F1 mice, respectively. Spleen weights decreased (P < 0.001, Fig. 3a and b) in HM71224-treated MRL/lpr and NZB/W F1 mice, respectively. Cervical lymph node weight was reduced (P < 0.001, Fig. 3a and P = not significant, Fig. 3b) in HM71224-treated MRL/lpr and NZB/W F1 mice, respectively, compared with those of vehicle-treated mice.
HM71224 inhibits enlargement of the spleen and lymph nodes resulting from disease development. a In MRL/lpr mice, the organ weights of the spleen and cervical lymph nodes (LN) were significantly reduced by treatment with 10 and 30 mg/kg of HM71224 for 20 weeks (n = 11 per vehicle-treated group, n = 12 per HM71224-treated group). b In NZB/W F1 mice, spleen weight was significantly reduced by HM71224 treatment, whereas the weight of cervical lymph nodes showed a nonsignificant trend toward decrease after treatment for 24 weeks (n = 8 per vehicle-treated group, n = 9 per 3 mg/kg of HM71224-treated group, n = 12 per 10 and 30 mg/kg of HM71224-treated group). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle control, analyzed by the Kruskal–Wallis test
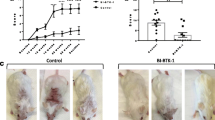
HM71224 prevents skin lesions
To investigate the ameliorative effects of HM71224 for cutaneous lupus erythematosus, macroscopic skin lesions were observed in MRL/lpr mice with the development of spontaneous lupus-erythematosus-like skin lesions. Alopecia and scab formation were markedly developed in aged MRL/lpr mice. These typical skin lesions caused by progression of lupus were observed around the nose, ears, and intrascapular area of vehicle-treated mice (Fig. 4a and b), with lesion scores of 1.4 ± 0.5 (Fig. 4e), whereas no skin lesions developed in the mice treated with HM71224, irrespective of the dose levels, up to 28 weeks of age (Fig. 4c-e).
HM71224 prevents skin lesion progression. Skin lesions were observed in vehicle-treated mice around the nose or eyes (arrows show representative skin lesions) (a, b) compared with HM71224-treated MRL/lpr mice (c, d). e Skin lesion severity score was 1.4 ± 0.5 (mean ± SEM) in vehicle-treated mice, whereas no skin lesions developed in HM71224-treated MRL/lpr mice (P < 0.001) at 28 weeks of age (n = 11 per vehicle-treated group, n = 12 per HM71224-treated group)
HM71224 limits renal dysfunction in animal models of lupus
To determine the effects of HM71224 on renal impairment, we measured BUN and CRE at the end of the study, and to assess glomerular dysfunction, we measured urine protein once weekly or biweekly. Both serum BUN and CRE and urine protein increased more dramatically in vehicle-treated NZB/W F1 mice than in vehicle-treated MRL/lpr mice. Serum BUN was significantly ameliorated in both mouse models with HM71224 treatment, whereas serum CRE was significantly reduced in NZB/W F1 mice only (Fig. 5). The percentage of mice with severe proteinuria (urine protein ≥ 300 mg/dl or urine protein score ≥ 3) sequentially increased in both mouse models, and vehicle-treated NZB/W F1 mice especially had dramatically increased high-grade proteinuria, whereas HM71224 decreased the severe proteinuria in both mouse models (Fig. 6a and c). Although there was some fluctuation in the proteinuria score, the area under the curve (AUC) for proteinuria during the treatment periods was significantly decreased in HM71224-treated MRL/lpr and NZB/W F1 mice (Fig. 6b and d).
HM71224 limits renal impairment. Serum blood urea nitrogen (BUN) and creatinine (CRE) were measured at the end of the study. Treatment of MRL/lpr mice with HM71224 reduced BUN (a) but increased CRE was not observed at 28 weeks of age, even in vehicle-treated mice (b). Treatment of NZB/W F1 mice with HM71224 resulted in a significant decrease in BUN (c) and CRE (d) at 40 weeks of age. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle control determined by analysis of variance (BUN and CRE in c and d) and the Kruskal–Wallis test (BUN in a)
HM71224 reduced glomerular dysfunction in animal models of lupus. The severity of urine protein concentration was graded using a urine protein scoring system. The percentage of mice with severe proteinuria (urine protein ≥ 300 mg/dl or urine protein score ≥ 3) sequentially increased in both mouse models (a, c). Especially, severe proteinuria in NZB/W F1 mice dramatically increased from 30 weeks of age in vehicle-treated mice, whereas few HM71224-treated mice had severe proteinuria c. The area under the curve (AUC) for the urine protein score in all treatment periods was significantly reduced in HM71224-treated groups in both mouse models (b, d). Data are shown as the mean ± SEM. **P < 0.01 and ****P < 0.0001 versus vehicle control determined by analysis of variance
HM71224 ameliorates renal injury and inflammation in animal models of lupus
To assess whether HM71224 can limit renal damage and inflammation, histopathological analysis was conducted in both animal models. Among MRL/lpr mice, the vehicle-treated mice had moderate renal damage, as indicated by the membranous glomerulonephritis (GN) score (2.9 ± 0.7), renal interstitial inflammation (IN) score (2.9 ± 1.0), and vasculitis (VI) score (3.6 ± 0.2), whereas HM71224-treated mice had a dose-dependent decrease in the GN score (reduced by 43%, P < 0.01), IN score (reduced by 40%, P not significant), and VI score (reduced by 31%, P < 0.01) (Fig. 7a). Among NZB/W F1 mice, the vehicle-treated mice had moderate to severe renal damage, as indicated by the GN score (3.0 ± 0.3), IN score (3.4 ± 0.2), and VI score (3.5 ± 0.2), whereas HM71224-treated mice had significantly less renal damage and inflammation, as shown by a 39% reduction in GN score (P < 0.05), 49% reduction in IN score (P < 0.01), and 55% reduction in VI score (P < 0.001) (Fig. 7b). Moreover, HM71224 ameliorated the glomerular basement membrane thickening and tubule distention containing proteinaceous casts in the kidney in NZB/W F1 mice (Fig. 7c).
HM71224 ameliorates renal damage and inflammation in animal models of lupus. Renal histopathological analysis was performed using the membranous glomerulonephritis score (GN) score, the renal interstitial inflammation/fibrosis (IN), and the vasculitis (VI) score. a Treatment of MRL/lpr mice with HM71224 for 20 weeks produced a dose-dependent reduction in renal destruction and lymphocyte infiltration and a statistically significant reduction in GN and VI scores. b Treatment of NZB/W F1 mice with HM71224 for 22 weeks resulted in statistically significant amelioration of the GN, IN, and VI scores. Scores are shown as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle control determined by the Kruskal–Wallis test. Representative histopathological Periodic acid Schiff stained sections of the kidneys of MRL/lpr mice at 28 weeks of age (c) and NZB/W F1 mice at 40 weeks of age (d). Thickening of the basement membrane (arrow), tubule distention with proteinaceous fluid (asterisk), and inflammation were ameliorated in the HM71224-treated groups. All images were captured at × 200 magnification
HM71224 improves the survival rate in animal models of lupus
Because the development of murine SLE-like disease features ultimately lead to premature death, the cumulative survival rate was estimated during treatment with HM71224. None of the MRL/lpr mice treated with HM71224 died during the course of treatment, whereas one of the vehicle-treated mice died at 16 weeks (Fig. 8a). Furthermore, in NZB/W F1 mice, the survival rate at 22 weeks in vehicle-treated mice was 67%, while all mice treated with 10 or 30 mg/kg of HM71224 were alive at 22 weeks (P < 0.01) (Fig. 8b).
HM71224 increases the survival rate in animal models of lupus. a None of mice treated with HM71224 died through all by 20 weeks after administration compared to the survival rate of 92% (11/12) in vehicle treated MRL/lpr mice. b All mice treated with 10 and 30 mg/kg of HM71224 survived through all by 22 weeks following administration and had a statistically significantly better survival rate, whereas vehicle-treated mice and mice treated with 3 mg/kg of HM71224 had survival rates of 67% (8/12) and 75% (9/12), respectively, among NZB/W F1 mice (n = 12 per group). **P < 0.01 versus vehicle control determined by the log-rank test
Discussion
B cell hyperactivation and immune complex-mediated FcR activation are known to play a major role in the pathogenesis of immune-mediated LN [5,6,7,8,9]. A previous report demonstrated the benefits of BTK inhibition in mouse models of TLR7/IFN-driven lupus by affecting both BCR and FcR signaling [23]. We also have previously demonstrated that BTK inhibition by HM71224 effectively blocks not only the phosphorylation of both BTK and PLCγ2 through downstream BCR signaling in human B cells, but also FcR-stimulated cytokine production in human monocytes [27].
Our present study demonstrates that HM71224 effectively reduced the numbers of several splenic B cell types. Germinal centers are central to development of long-lived plasma B cells, their clonal expansion, and somatic hypermutation affinity maturation. In SLE a large proportion of these autoreactive plasma cells, which are generated in the spleen, preferentially migrate to the inflamed kidneys. Therefore, the spontaneous formation of these germinal centers, which are an important source of autoreactive plasma cells, is considered a hallmark of SLE, and disease progression of LN is associated with an increase in the number and size of germinal centers [33, 34]. In this study, HM71224 dose-dependently and considerably decreased the number of splenic B220+GL7+ germinal center B cells, and this inhibition of B cell differentiation for germinal center formation may exert a therapeutic effect on LN. Inflamed tissue and secondary lymphoid organs can contribute to the number of autoreactive plasma cells in SLE [35]. In this study, HM71224 dose-dependently and significantly decreased the number of splenic B220+CD138+ plasma cells, which are considered the major producers of autoantibodies. Moreover, HM71224 strikingly inhibited splenic B220+CD69+ B cell numbers, which are early markers of B cell activation and antigen presentation. This result is consistent with previously reported data showing that B cells with defective BCR signaling fail to mature and are non-responsive to BCR cross-linking in terms of proliferation and upregulation of the activation marker CD69 [36, 37]. These results suggest that HM71224 may effectively inhibit autoantibody production and cytokine enhancement by B cells, which are caused by presenting autoantigens to CD4+ T cells as antigen-presenting cells and stimulating dendritic cells.
These numerous inhibitory effects of splenic B cells are consistent with the prevention of splenomegaly and enlargement of cervical lymph nodes observed following treatment with HM71224. Approximately 50% of patients with SLE have enlarged lymph nodes and these are usually detected in the cervical, axillary, and inguinal areas. In particular, lymphadenopathy is more frequently noted at the onset of disease or during disease flares [38]. Splenomegaly also occurs in 10–45% of patients with SLE owing to disordered immunoregulation and is particularly observed during active disease [39]. Thus, inhibition of both lymph node enlargement and splenomegaly are considered to be positive signals of the suppression of SLE-like disease progression by HM71224 in an animal model of lupus.
The first major therapeutic finding was that treatment with HM71224 resulted in no skin lesions in MRL/lpr mice. Skin rash is the second most common clinical manifestation in patients with SLE and in MRL/lpr mice, which represent a unique mice strain, with development of a skin rash [40]. The pathogenesis of skin lesions, hair loss, and scab formation in MRL/lpr mice is characterized by immunoglobulin and/or complement depositions and accumulation of various other components of the immune system including macrophage/monocytes and dendritic cells [41, 42]. The B cell deficient and spleen tyrosine kinase (SYK) inhibitor-treated MRL/lpr mice were found not to develop spontaneous dermatitis [8, 43] and rituximab has been shown to have a therapeutic effect against cutaneous lesions in patients with SLE [44]. Here, we demonstrated that the typical skin lesions around the nose or eyes were observed in vehicle-treated mice but not in HM71224-treated mice, and these effects in skin lesions may be associated with B cell modulation by HM71224.
The second major therapeutic finding was that HM71224 had therapeutic effects related to the amelioration of kidney damage caused by lupus-like renal inflammation. Intrarenal B cells can contribute to renal damage and inflammation by enhancing the immunological response as antigen-presenting cells, inducing cytokine-promoting T cell proliferation and lymphatic angiogenesis, and enhancing the local immune response to persisting autoantigens in the tubulointerstitium [45, 46]. In both MRL/lpr and NZB/W F1 mice, activation of TLR9 caused accelerated LN [47]. On the other hand, LN develops via unique mechanisms in each strain like the highly heterogenous nature of SLE. NZB/W F1 mice promote the renal damages B cell dependent manner including secretion of autoantibody, whereas MRL/lpr mice develop the renal damages via antigen presentation and cytokine production by B cells, not secretion of autoantibodies [5].
As discussed previously, HM71224 markedly reduced splenic B cell numbers, including those of germinal center B cells, plasma B cells, and activated B cells. We also reported the inhibitory effects of HM71224 on BCR and FcR signaling [27]. These mechanisms of action of HM71224 might lead to significant amelioration of renal injury and lymphocyte infiltration in both animal models, although differences in strains in the pathogenesis of LN can lead to different degrees of therapeutic drug efficacy. Therefore, we suggest that the therapeutic effects of HM71224 in glomerulonephritis, interstitial nephritis, and vessel inflammation in murine LN were mediated by B cell inhibition.
Finally, one of the major therapeutic findings was that there was no mortality during the period of HM71224 administration in both mice models (Fig. 8). Prevention of renal damage by HM71224 might have led to the improvement in survival rate, because LN leads to proteinuria and renal failure and is associated with significant mortality [48]. Although cyclophosphamide treatment has markedly reduced mortality in LN from more than 70% in the 1960s to less than 10% in recent years, mortality remains relatively high [49]. Therefore, despite continuous improvements in SLE therapeutics, mortality remains a serious problem in LN therapy [3, 4]. Thus, the most notable result of this study is that HM71224 can markedly increase the survival rate of animals with SLE by preventing renal damage and inflammation.
Conclusions
This study indicated that HM71224 causes B cell modulation and has therapeutic effects via BTK inhibition of disease progression in SLE and LN in murine models of lupus.
Abbreviations
- ACK:
-
Ammonium-chloride-potassium
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- BCR:
-
B cell antigen receptor
- BMX:
-
Bone-marrow tyrosine kinase
- BTK:
-
Bruton’s tyrosine kinase
- BUN:
-
Blood urea nitrogen
- CO2 :
-
Carbon dioxide
- CRE:
-
Creatinine
- dsDNA:
-
Double strand deoxyribonucleic acid
- ECL:
-
Enhanced chemiluminescence
- ELISA:
-
Enzyme-linked immunosorbent assay
- Fc:
-
Crystallizable fragment
- FcR:
-
Crystallizable fragment receptor
- FCS:
-
Forward scatter
- FITC:
-
Fluorescein isothiocyanate
- GAPH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GN:
-
Glomerulonephritis
- IC50 :
-
Half maximal inhibitory concentration
- IFN-γ:
-
Interferon gamma
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IL-6:
-
Interleukin 6
- IN:
-
Interstitial nephritis
- LN:
-
Lupus nephritis
- MNC:
-
Mononuclear cell
- NZB/WF1:
-
New Zealand Black/White F1
- PE:
-
phycoerythrin
- PLCγ:
-
Phosphoinositide phospholipase C gamma
- RIPA:
-
Radioimmunoprecipitation assay
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM:
-
Standard error of mean
- SLE:
-
Systemic lupus erythematosus
- SSC:
-
Side scatter
- SYK:
-
Spleen tyrosine kinase
- TEC:
-
Tec protein tyrosine kinase
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- VI:
-
Vasculitis
- xid :
-
X-linked immunodeficiency
References
Liu Z, Davidson A. Taming lupus – a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18:871–82.
Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus-an update. Curr Opin Immunol. 2012;24:651–7.
Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10(2):413–24.
Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken). 2010;62:873–80.
Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–48.
Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24:1357–66.
Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–4.
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33.
Otten MA, Groeneveld TW, Flierman R, Rastaldi MP, Trouw LA, Faber-Krol MC, et al. Both complement and IgG fc receptors are required for development of attenuated antiglomerular basement membrane nephritis in mice. J Immunol. 2009;183:3980–8.
Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately to severely active systemic lupus erythematosus: the randomized, double-blind, phaseII/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33.
Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 2012;64:1215–26.
Reddy V, Jayne D, Close D, Isenberg D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res Ther. 2013;15(Suppl1):S2.
Carter LM, Isenberg DA, Ehrenstein MR. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 2013;65(10):2672–9.
Wallace DJ, Gordon C, Strand V. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow up. Rheumatology. 2013;52:1313–22.
Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. 2014;73:183–90.
Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–30.
Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomized, placebo-control, phase 3 trial. Lancet. 2011;377:721–31.
Smith CI, Baskin B, Humire-Greiff P, Zhou JN, Olsson PG, Maniar HS, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994;152(2):557–65.
Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton’s tyrosine kinase separately regulates NFκB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR-9 stimulated B cells. J Biol Chem. 2008;283:11189–98.
Xu D, Kim Y, Postelnek J, Vu MD, Hu DQ, Liao C, et al. RN486, a selective Bruton’s tyrosine kinas inhibitor, abrogates immune hypersensitivity responses and arthritis in rodents. J Pharmacol Exp Ther. 2012;341:90–103.
Mangla A, Khare A, Vineeth V, Panday NN, Mukhopadhyay A, Ravindran B, et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood. 2004;104:1191–7.
Halcomb KE, Musuka S, Gutierrez T, Wright HL, Satterthwaite AB. Btk regulates localization, in vivo activation, and class switching of anti-DNA B cells. Mol Immunol. 2008;46:233–41.
Mina-Osorio P, LaStant J, Keirstead N, Whittard T, Ayala J, Stefanova S, et al. Suppression of glomerulonephritis in lupus-prone NZB x NZW mice by RN486, a selective inhibitor of Bruton’s tyrosine kinase. Arthritis Rheum. 2013;65:2380–91.
Hutcheson J, Vanarsa K, Bashmakov A, Grewal S, Sajitharan D, Chang BY, et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res Ther. 2012;14:R243.
Rankin AL, Seth N, Keegan S, Andreyeva T, Cook TA, Edmonds J, et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J Immunol. 2013;191:4540–50.
Shao WH, Cohen PL. The role of tyrosine kinase in systemic lupus erythematosus and their potential as therapeutic targets. Expert Rev Clin Immunol. 2014;10:573–82.
Park JK, Byun JY, Park JA, Kim YY, Lee YJ, Oh JI, et al. HM71224, a novel Bruton’s tyrosine kinase inhibitor, suppresses B cell and monocyte activation and ameliorates arthritis in a mouse model: a potential drug for rheumatoid arthritis. Arthritis Res Ther. 2016;18:91.
Weintraub JP, Godfrey Y, Wolthusen PA, Cheek RL, Eisenberg RA, Cohen PL. Immunological and pathological consequences of mutations in both Fas and Fas ligand. Cell Immunol. 1998;186:8–17.
Rigby RJ, Vyse TJ. Models of systemic lupus erythematosus. Drug Discov Today Disease Models. 2004;1:445–9.
Rigby RJ, Rozzo SJ, Boyle JJ, Lewis M, Kotzin BL, Vyse TJ. New loci from New Zealand Black and New Zealand White mice on chromosomes 4 and 12 contribute to lupus like disease in context of BALB/c. J Immunol. 2004;172:4609–17.
Seavey MM, Lu LD, Stump KL. Animals of systemic lupus erythematosus (SLE) and ex vivo assay design for drug discovery. Curr Protoc Pharmacol. 2011;Chapter 5:Unit 5.60.
Annegret K. Percy L, Thomas R. Cutaneous lupus erythematosus. In: Fukumi F. Experimental models of lupus erythematosus. Springer; 2005. p. 221–238
Cecile K. T follicular helper cells and B cells. Immunity. 2011;34:827–8.
Wang W, Rangel-Moreno J, Owen T, Barnard J, Nevarez S, Ichikawa HT, et al. Long-Term B cell depletion in murine lupus eliminates autoantibody-secreting cells and is associated with alterations in the kidney plasma cell niche. J Immunol. 2014;192:3011–20.
Mumtaz IM, Hoyer BF, Panne D, Moser K, Winter O, Cheng QY, et al. Bone marrow of NZB/W mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: implications for the treatment of autoimmunity. J Autoimmun. 2012;39:180–8.
Xu S, Tan JE, Wong EP, Manickam A, Ponniah S, Lam KP. B cell development and activation defects resulting in xid like immunodeficiency in BLNK/SLP-65-deficient mice. Int Immunol. 2000;12:397–404.
Schram BR, Tze LE, Ramsey LB, Liu J, Najera L, Vegoe AL, et al. B cell receptor basal signaling regulates antigen induced Ig light chain rearrangements. J Immunol. 2008;180:4728–41.
Fozya B. Hematological disorders in patients with systemic lupus erythematosus. Open Rheumatol J. 2013;7:87–95.
Eular intranet. In: Bertsias G, Cervera R, Boumps DT. Systemic lupus erythematosus: pathogenesis and clinical features. Eular Fpp Indd. 2012;20:476-505. https://www.eular.org/myuploaddata/files/sample%20chapter20_mod%2017.pdf. Accessed 12 Sept 2017.
Rottman JB, Willis CR. Mouse models of systemic lupus erythematosus reveal a complex pathogenesis. Vet Pathol. 2010;47:664–76.
Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathological studies on skin lesions of MRL mice. Arch Dermatol Res. 1984;276:186–94.
Deng GM, Tsokos GC. Pathogenesis and targeted treatment of skin injury in SLE. Nat Rev Rheumatol. 2015;11:663–9.
Deng GM, Liu L, Bahjat FR, Pine PR, Tsokos GC. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 2010;62(7):2086–92.
Tim YTL, Kristine PNG, Geraldine C, Maria JL, Jonathan CWE, Michael E, et al. A respective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum. 2009;61:482–7.
Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, et al. The contribution of B cells to renal interstitial inflammation. Am J Path. 2007;170:457–68.
Segerer S, Schlondorff D. B cells and tertiary lymphoid organs in renal inflammation. Kidney Int. 2008;73:533–7.
Hasegawa K, Hayashi T. Synthetic CpG oligodeoxynucleotides accelerate the development of lupus nephritis during preactive phase in NZB × NZWF1 mice. Lupus. 2003;12:838–45.
Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2009;21:489–94.
Bevra HH, Maureen M, Alan W, Wallace WD, David ID, John F, et al. American college of rheumatology guidelines for screening, case definition, treatment and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64:797–808.
Acknowledgements
This study was supported by funding from Hanmi Pharmaceutical Co., Ltd. in the Republic of Korea and copyreading from Chung-Ang University in Republic of Korea. HM71224 (Hanmi Pharmaceutical Co., Ltd.) is also referred to as LY3337641 (Eli Lilly and Company).
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors were engaged in the experiment design. SYJ provided HM71224. JYB performed cell-based assay. YYK and KTP performed all animal studies. YYK wrote the manuscript and received significant input from all other authors, and KWH edited the manuscript. All authors listed have read the manuscript for content and have approved the final version.
Corresponding authors
Ethics declarations
Authors’ information
Not applicable.
Ethics approval
All experiments were conducted in compliance with the guidelines for the care and use of laboratory animals. In all animal experiments, protocols were approved by the Institutional Animal Care and Use Committee of the Hanmi Research Center, and the procedures were performed according to the approved guideline.
Consent for publication
Only group data are presented in this manuscript, and they are not attributable to any individual person. Consent to use and publish the data collected in this way was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, YY., Park, K.T., Jang, S.Y. et al. HM71224, a selective Bruton’s tyrosine kinase inhibitor, attenuates the development of murine lupus. Arthritis Res Ther 19, 211 (2017). https://doi.org/10.1186/s13075-017-1402-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-017-1402-1