Abstract
Background
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). The aim of this study was to explore the safety and efficacy of open-label tofacitinib following blinded treatment with adalimumab or tofacitinib for moderate to severe RA.
Methods
Analyses included patients treated with adalimumab 40 mg once every 2 weeks or tofacitinib 10 mg twice daily (BID) with background methotrexate (MTX) in a 12-month randomized study (NCT00853385), who subsequently received tofacitinib 10 mg BID (with/without background MTX) in an open-label extension (NCT00413699). Patients with treatment-related serious adverse events (AEs) and serious or recurrent infections in the index study were excluded from the extension study. Exposure-adjusted incidence rates of safety-related events were assessed in 3-month and 12-month periods in the year before and in the year after switching. Efficacy was assessed 3 months before, at the time of, and 3 months after switching.
Results
There were 233 (107 adalimumab to tofacitinib 10 mg BID, 126 blinded to open-label tofacitinib 10 mg BID) patients included in these analyses. Patients in both treatment sequences had similar incidence rates (per 100 patient-years) of discontinuation due to AEs, serious AEs, and serious infections in the year before and in the year after switching. Incidence rates of AEs were increased in the first 3 months after switching compared with the last 3 months before switching in both treatment groups. Switching from either blinded adalimumab or tofacitinib to open-label tofacitinib resulted in numerically higher incidence of responders for signs and symptoms of disease and improved physical function.
Conclusions
Treatment can be directly switched from adalimumab to tofacitinib. A similar safety and efficacy profile was seen when patients received open-label tofacitinib after receiving either blinded adalimumab or tofacitinib.
Trial registration
ClinicalTrials.gov Identifiers: NCT00853385, registered 27 February 2009; NCT00413699, registered 18 December 2006.
Similar content being viewed by others
Background
The long-term treatment of rheumatoid arthritis (RA) often involves a sequence of different therapies. Patients who have not responded to conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs) have been routinely treated with a tumor necrosis factor inhibitor (TNFi); however, approximately 20 % of patients discontinue TNFi therapy within 12 months due to inefficacy or adverse events (AEs) [1, 2]. Management of patients who have not responded to treatment with one TNFi may involve switching to another TNFi or an agent with an alternative mechanism of action. Several randomized controlled trials have demonstrated the efficacy of agents with a different mechanism of action versus placebo in patients with an inadequate response to a TNFi [3–5]. In clinical practice, patients receiving a TNFi, such as adalimumab (ADA), may need or choose to switch to an agent with an alternative mechanism of action; an understanding of the impact of switching without an extended washout period is needed.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA [6]. The tofacitinib phase 3 program included six randomized, controlled studies in which tofacitinib was assessed in adult patients with active moderate to severe RA [7–12] as monotherapy or combined with conventional synthetic DMARDs. A 12-month, double-blind, randomized, controlled trial, conducted in 115 centers worldwide, compared the safety and efficacy of tofacitinib 5 or 10 mg twice daily (BID) or ADA 40 mg every 2 weeks (Q2W) with placebo in patients with active RA taking background methotrexate (MTX; 7.5 to 25 mg weekly), who had an inadequate response to MTX (defined as sufficient residual disease activity to meet entry criteria). The primary safety and efficacy data have been reported previously [10]. Eligible patients who had participated in this blinded study could be enrolled in an open-label extension where they received tofacitinib 10 mg BID (with or without background MTX), regardless of their treatment in the blinded study [13].
The objective of the current analyses was to describe the safety and efficacy of open-label tofacitinib in an extension study, following a direct switch from blinded treatment with either ADA or tofacitinib for the treatment of moderate to severe RA. The group of patients who received blinded tofacitinib followed by open-label tofacitinib in the extension study could act as a control for such an assessment by representing potential changes to the clinical profile introduced by transition from the double-blind to the open-label study with minimal changes in treatment (dose adjustments permitted for concomitant RA medications and tofacitinib in the extension study).
Methods
Study design and treatment
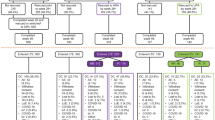
ORAL Standard (A3921064; NCT00853385) was a double-blind, phase 3 trial [10] in which patients were randomly assigned to one of five regimens, all with stable background MTX: tofacitinib 5 mg BID, tofacitinib 10 mg BID, ADA 40 mg Q2W (subcutaneous injection), placebo for 3 or 6 months followed by tofacitinib 5 mg BID, and placebo for 3 or 6 months followed by tofacitinib 10 mg BID for a total of 12 months.
ORAL Sequel (A3921024; NCT00413699) is an open-label long-term extension study [13] in which all patients from ORAL Standard were eligible to receive tofacitinib 10 mg BID with or without conventional synthetic DMARDs. Exclusion criteria included treatment-related serious AEs and serious or recurrent infections (including serious or recurrent herpes zoster) in the index study. Patients with latent tuberculosis were allowed to continue in the extension study if they had received an adequate course of therapy. Patients from certain countries (e.g., Korea, Croatia, Denmark, Czech Republic, Germany, Spain, Sweden, Ireland and the United Kingdom) who enrolled in the extension study ≥14 days after their final index study visit were excluded if their absolute lymphocyte count (ALC) values were <750 cells/mm3. Patients from the remaining countries were excluded if their ALC values were <500 cells/mm3.
The current post-hoc analyses included patients who were randomized at baseline to treatment with blinded ADA 40 mg Q2W + MTX or blinded tofacitinib 10 mg BID + MTX in ORAL Standard and subsequently received open-label tofacitinib 10 mg BID (with or without MTX) in the extension study. Although the patients initiated treatment in the long-term extension with tofacitinib 10 mg BID, this could be reduced to 5 mg BID for safety reasons. Additionally, dose adjustments, including tapering and discontinuation (DC) of permitted concomitant RA medications (such as MTX, prednisone and non-steroidal anti-inflammatory drugs) were allowed in response to inadequate efficacy, disease improvement, or for safety reasons.
Each study was performed in compliance with the Declaration of Helsinki and Good Clinical Practice Guidelines established by the International Conference on Harmonization. The final protocols, amendments, and informed consent documentation were reviewed and approved by the institutional review board and the independent ethics committee of each investigational center (Additional file 1: Table S1).
Patients
Patients in this analysis included those who were initially enrolled in the phase 3, ORAL Standard study [10] and subsequently elected to enroll in the extension study [13]. Eligible patients in the phase 3 study were ≥18 years of age and had received a diagnosis of active RA, as classified according to the American College of Rheumatology (ACR) 1987 Revised Criteria [14] and had not failed or had an AE to a TNFi in the past. Additional inclusion and exclusion criteria have been described previously [10]. Patients who completed participation in the study or discontinued for reasons other than treatment-related serious AEs and who met other inclusion/exclusion criteria were invited to enroll in the long-term extension [13]. For patients enrolling in the long-term extension study within 2 weeks of their last dose in the blinded study, baseline values were obtained from the blinded study.
Statistical analyses
Post-hoc descriptive statistics are reported (data cutoff date 19 April 2012). As the extension study is ongoing, the study database has not yet been locked (i.e., some values may change for the final, locked study database).
Safety and tolerability assessments
The incidence of treatment-emergent AEs (TEAEs) was calculated at scheduled visits of the blinded study (baseline, and months 1, 3, 6, 9, and 12) and extension study (baseline, months 1, 2, 3, and every 3 months thereafter).
In the current analyses, the incidence of TEAEs was compared during the last 3 months of blinded therapy and the first 3 months of open-label treatment.
Exposure-adjusted incidence rates (and corresponding 95 % confidence intervals) of DCs due to AEs, serious AEs and serious infections per 100 patient-years from baseline were calculated for patients who completed treatment with ADA 40 mg Q2W + MTX or tofacitinib 10 mg BID + MTX in the blinded study for patients, (1) regardless of whether they entered the extension study, and (2) who then enrolled in the extension study. These analyses were performed for 3-month (months 0 − 3, 3 − 6, 6 − 9, and 9 − 12) and 12-month (months 0 − 12) periods for both the blinded study and the first 12 months of the extension study. Of note, there were two study visits in the last 3 months of the blinded study (visits 5 and 6 at months 9 and 12, respectively), and four study visits in the first 3 months of the extension study (visits 1 − 4, for baseline and months 1, 2, and 3).
Efficacy assessments
The following efficacy parameters were measured in patients who completed treatment with ADA + MTX or tofacitinib 10 mg + MTX in the blinded study (or discontinued for reasons other than a treatment-related serious AE) and then switched to the tofacitinib 10 mg group in the extension study: ACR response rates (20 %, 50 %, and 70 % improvement), mean change in Health Assessment Questionnaire-Disability Index (HAQ-DI) scores from the baseline of the blinded study, and Disease Activity Score for 28-joint counts based on the erythrocyte sedimentation rate (DAS28-4(ESR)) <2.6 response rates at: (1) month 9 (visit 5) of the blinded study (“-3 months” relative to the switch); (2) the time of switching from the blinded to the extension study (“switch”), and (3) month 3 (visit 4) of the extensions study (“+3 months”) allowing visit windows of ±1.5 months to capture all efficacy and safety data.
Results
Patient disposition and demographics
Of 204 patients randomized to ADA 40 mg Q2W + MTX in the blinded study, 107 switched to open-label tofacitinib 10 mg BID (105 (98 %) with MTX on day 1) within 2 weeks after their last dose of ADA. Of 201 patients randomized to blinded tofacitinib 10 mg BID + MTX in the blinded study, 126 switched to open-label tofacitinib 10 mg BID (123 (98 %) with MTX on day 1) within 2 weeks. Patient demographic and baseline characteristics were similar across sequences and between those patients who were randomized to ADA 40 mg Q2W + MTX or tofacitinib 10 mg BID + MTX in the blinded study and those who then received tofacitinib 10 mg BID (with or without MTX) in the extension study (Table 1).
Safety and tolerability
On analysis of the proportions of patients with TEAEs occurring 3 months prior to and 3 months after the switch, there was no clear pattern of changes in either the ADA to tofacitinib group or the blinded to open-label tofacitinib group (Table 2). Incidence rates per 100 patient-years of DCs due to AEs, serious AEs, and serious infections over 3-month and 12-month periods were similar in the year before and in the year after switching in both groups (Table 3). Incidence rates were increased in the first 3 months of the extension study (0 − 3 months) compared with the last 3 months of the blinded study (9 − 12 months) for both groups.
In the extension study, small increases from baseline in ALC were observed at month 1 and month 3 in patients in the ADA to tofacitinib group; ALC levels returned to baseline at month 6 and were maintained through month 12. Small decreases from baseline in ALC were observed at all time points through month 12 in patients in the blinded to open-label tofacitinib group (Table 3). In the extension study, patients in the ADA to tofacitinib group and patients in the blinded to open-label tofacitinib group had small increases from baseline in total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides at all time points through month 12 (except for LDL at month 3 in patients in the ADA to tofacitinib group) (Table 3). At month 3 of the extension study, 2 patients (2 %) in the ADA to tofacitinib group and 4 patients (3 %) in the blinded to open-label tofacitinib group were receiving lipid-lowering agents.
Serious AEs occurring up to the month-3 visit of the extension study - that is, following the switch to tofacitinib - are presented in Table 4 alongside the action taken and patient outcomes. Two serious AEs resulted in death (lung malignancy, n = 1 (day 132); suicide, n = 1 (day 1); it is unknown whether the patient took the study drug on this day). One patient who developed vertigo during this period was still experiencing it 20 months later. All other patients who experienced a serious AE in the first 3 months of the extensions study recovered from the AE.
Efficacy
Greater proportions of patients had improvement in the signs and symptoms of RA, as measured by increased ACR response rates (Fig. 1) and reductions in mean DAS28-4(ESR), during the first 3 months of the extension study compared with the last 3 months of the blinded study in both the ADA to tofacitinib group and the blinded to open-label tofacitinib group (Fig. 2a). Of the 74 patients on ADA and 84 patients on tofacitinib who achieved an ACR20 response 3 months prior to switching in the double-blind study, 7 % (n = 5) and 7 % (n = 6), respectively, did not maintain an ACR20 response 3 months after switching to tofacitinib in the extension study.
American College of Rheumatology (ACR) response rates 3 months before switch, at switch, and 3 months after switch*. ADA adalimumab, BID twice daily. *Includes patients who completed treatment with ADA 40 mg Q2W or tofacitinib 10 mg BID in ORAL Standard (or discontinued treatment for reasons other than a tofacitinib-related serious AE) and then enrolled in ORAL Sequel and switched treatment with minimal washout (≤2 weeks after their last dose of study drug in ORAL Standard). ACR, American College of Rheumatology; ACR20/50/70, 20/50/70% reduction in the number of tender and swollen joints, as well as 20/50/70% improvement in ≥3 of the other five ACR components; ADA, adalimumab; BID, twice daily; Q2W, every 2 weeks
Disease Activity Score for 28-joint counts based on the erythrocyte sedimentation rate (DAS28-4(ESR)) and changes from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) 3 months before switch, at switch, and 3 months after switch*. ADA adalimumab, BID twice daily. *Includes patients who completed treatment with ADA 40 mg Q2W or tofacitinib 10 mg BID in ORAL Standard (or discontinued treatment for reasons other than a tofacitinib-related serious AE) and then enrolled in ORAL Sequel and switched treatment with minimal washout (≤2 weeks after their last dose of study drug in ORAL Standard). Lower scores indicate less disease activity (DAS28–4[ESR]) or lower disability (HAQ-DI). ADA, adalimumab; BID, twice daily; DAS28–4(ESR), Disease Activity Score for 28 joint counts based on the erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire-Disability Index; Q2W, every 2 weeks; SE, standard error. (a) Changes from Baseline in Disease Activity Score for 28-joint counts based on Erythrocyte sedimentation rate (DAS28-4(ESR) 3 months before switch, at switch, and 3 months after switch.* (b) Changes from Baseline in the Health Assessment QUestionnaire-Disability Index (HAQ-DI) 3 months before switch, at switch, and 3 months after switch*
Similarly, further improvement in physical function, as measured by greater mean reductions in HAQ-DI score from baseline, was seen in both treatment groups (Fig. 2b).
Discussion
The clinical, functional, and radiographic efficacy and safety of tofacitinib 5 and 10 mg BID with and without MTX has been reported in patients with active RA in randomized phase 2 [15–19] and phase 3 studies [7–12]. The phase 3 study that included an ADA treatment arm demonstrated efficacy and safety results of tofacitinib consistent with the other phase 3 studies [10]. The current analyses describe the safety and efficacy of open-label tofacitinib (with or without MTX) following a direct switch from blinded treatment with either ADA + MTX or tofacitinib + MTX.
Both proportions and exposure-adjusted incidence rates of DCs due to AEs, serious AEs, and serious infections over time were similar when switching from blinded ADA or tofacitinib to open-label tofacitinib. The increased incidence rates for these events in the initial 3 months of open-label tofacitinib, compared with the last 3 months of either blinded ADA or tofacitinib, suggests that these differences may be due to aspects of the study design inherent in the transition period rather than as a result of any overlapping immunomodulatory effects of ADA and tofacitinib. One potential explanation for the observed changes is the possibility of a measurement/detection effect. For example, the transition from the double-blind study to the open-label extension study may have driven an increase in patient/investigator sensitivity to reporting, whereby there was increased attribution of events in the extension study. Another limitation could have been the number of visits per 3-month period. Patients in the extension study had more frequent visits over the first 3 months of treatment (four visits) than they had in the final 3 months of the blinded study (two visits); thus, there were more opportunities to report an event in the first 3 months after the switch, which could have had an impact on the apparent incidence rate. Although a mechanistic or immunologic origin for the difference in safety profile cannot be ruled out, it is thought to be unlikely, as an increase was observed in both treatment sequences. On analysis of the proportions of patients experiencing TEAEs for 3 months before and 3 months after the switch, there was no clear pattern of changes during the switch. A further limitation is the potential change in dosing of permitted concomitant RA medications, including MTX, after switching.
Although the analyses of incidence rates per 100 patient-years of AEs in the blinded study included all patients regardless of whether they entered the extension, the data for the extension study included only those patients who completed the blinded study (that is, those who had data for the last 3 months of this study and who then enrolled in the extension). This introduces a potential censoring/truncation effect that is associated with identifying and analyzing a “completer” population. This effect may also apply to the efficacy response: patients with lesser efficacy responses may have been less likely to remain in the extension study to month 3, thereby, leading to seemingly greater improvements at month 3 among patients in this study. However, the incidence of both DCs and improvements in efficacy during the first 3 months of the extension study were similar between those patients who switched to ADA and those who continued to receive tofacitinib.
With these limitations in mind, we observed that switching directly from double-blind ADA 40 mg Q2W to open-label tofacitinib 10 mg BID resulted in sustained clinical response, with numeric improvements in all ACR response rates assessed, DAS28-4(ESR) scores, and mean change from baseline in HAQ-DI scores. Efficacy assessed at the same time points was similar in the double-blind tofacitinib 10 mg BID to open-label tofacitinib 10 mg BID group and the ADA to tofacitinib 10 mg BID group, and there was a similar pattern of increases in efficacy from the double-blind to the open-label extension.
One of the questions of interest was whether there are clinical consequences of potential overlapping immunomodulatory effects in the switch from ADA to tofacitinib. Analysis of AEs do not support such a conclusion but, in contrast, the contribution that overlapping pharmacologic exposure to ADA and tofacitinib makes to efficacy may be more difficult to assess. Although the first efficacy assessment at 3 months is well beyond the terminal half-life of the last dose of ADA [20, 21], pharmacodynamic effects may persist beyond what might be predicted from half-life/clearance data, and co-administered MTX reduces ADA clearance [22]. Nevertheless, efficacy assessed at the same time points was similar in the tofacitinib to tofacitinib group and the ADA to tofacitinib group, with similar numeric patterns of improvement.
Conclusions
In summary, analysis of incidence rates of AEs showed a similar pattern over time for both ADA to tofacitinib and blinded to open-label tofacitinib treatment sequences in the year before and the year after switching, and there was no clear pattern of changes in TEAEs in the 3 months before and 3 months after the switch. Switching from both double-blind ADA and tofacitinib to open-label tofacitinib resulted in numeric improvements in signs and symptoms of disease and physical function. These results indicate that a patient’s treatment can be directly switched from ADA to tofacitinib 10 mg BID, with a safety and efficacy profile similar to switching from blinded to open-label tofacitinib 10 mg BID. This analysis did not address potential differences between the safety profiles of ADA and tofacitinib in the phase 3 study for this cohort of patients, as this comparison is more appropriate in the primary safety analysis population [10].
Abbreviations
ACR, American College of Rheumatology; ADA, adalimumab; AE, adverse event; ALC, absolute lymphocyte count; BID, twice daily; CCP, cyclic citrullinated peptide; CI, confidence interval; CRP, C-reactive protein; DAS28-4(ESR), Disease Activity Score for 28-joint counts based on the erythrocyte sedimentation rate; DC, discontinuation; DMARD, disease-modifying anti-rheumatic drug; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, incidence rate; LDL, high-density lipoprotein; MTX, methotrexate; Q2W, every 2 weeks; RA, rheumatoid arthritis; HDL, high-density lipoprotein; SD, standard deviation; TEAE, treatment-emergent adverse event; TNFi, tumor necrosis factor inhibitor
References
Fisher MD, Watson C, Fox KM, Chen YW, Gandra SR. Dosing patterns of three tumor necrosis factor blockers among patients with rheumatoid arthritis in a large United States managed care population. Curr Med Res Opin. 2013;29(5):561–8.
Marchesoni A, Zaccara E, Gorla R, Bazzani C, Sarzi-Puttini P, Atzeni F, et al. TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann NY Acad Sci. 2009;1173:837–46.
Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–806.
Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–23.
Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23.
Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond). 2010;7:41.
Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–60.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507.
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–70.
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–19.
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159(4):253–61.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86.
Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase Inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41(5):837–52.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64(3):617–29.
Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–905.
Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64(4):970–81.
Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Investigators TS. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken). 2011;63(8):1150–8.
Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25(4):514–21.
den Broeder A, van de Putte L, Rau R, Schattenkirchner M, van Riel P, Sander O, et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002;29(11):2288–98.
Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25(6):1700–21.
AbbVie Inc. Humira prescribing information. 2015. www.rxabbvie.com/pdf/humira.pdf. Accessed 1 Dec 2015.
Acknowledgements
The authors would like to thank the patients who were involved in these studies, the A3921064 and A3921024 investigators, and the study teams including Kenneth Kwok and Clifford Aspinall. Medical writing support was provided by Gary Dever, PhD, and Annette Smith, PhD, at Complete Medical Communications and was funded by Pfizer Inc.
Funding
This study and post-hoc analysis was supported by Pfizer Inc.
Authors’ contributions
MG, RvV, BW, LW, SHZ, DG, RR, and TVJ were involved in the study design, and MG, BW, LW, SHZ, RR, and TVJ were responsible for the study conduct. MG, BW, LW, and RR contributed to data collection. MG, RvV, BW, LW, SHZ, DG, PB, RR, LT, and TVJ were involved in data analysis and interpretation. All authors critically reviewed the manuscript and approved the final draft prior to submission.
Competing interests
M Genovese has received grant/research support from and has been a consultant for Pfizer. R van Vollenhoven has received grant/research support from and has been a consultant for AbbVie, BMS, GSK, MSD, Pfizer, Roche and UCB, and has been a consultant for Lilly and Vertex. B Wilkinson, L Wang, SH Zwillich, D Gruben, P Biswas, L Takiya, and TV Jones are employees of, and have stock or stock options in Pfizer. R Riese was an employee of Pfizer at the time of the study. All authors confirm that they have no non-financial competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Ethical review boards and study centers. (DOCX 34 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Genovese, M.C., van Vollenhoven, R.F., Wilkinson, B. et al. Switching from adalimumab to tofacitinib in the treatment of patients with rheumatoid arthritis. Arthritis Res Ther 18, 145 (2016). https://doi.org/10.1186/s13075-016-1049-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-016-1049-3