Abstract
Introduction
Tocilizumab (TCZ), an anti-interleukin-6 receptor antibody, is clinically effective against rheumatoid arthritis (RA), and several reports have indicated how TCZ influences a number of mechanisms underlying RA pathogenesis. However, it is still unclear whether TCZ affects inflammatory cells in peripheral blood and whether any such changes are associated with clinical response. We evaluated associations between proportions of subsets of peripheral immune cells and clinical response in patients with RA treated with TCZ.
Methods
Thirty-nine consecutive patients with RA who started to receive TCZ as their first biologic between March 2010 and April 2012 were enrolled. The proportions of several subsets of peripheral cells with their levels of expression of differentiation markers, activation markers and costimulatory molecules were measured sequentially from baseline to week 52 by flow cytometry analysis.
Results
Clinical Disease Activity Index (CDAI) remission was achieved in 53.8% of patients at week 52 of TCZ therapy. The proportions of CD4+CD25+CD127low regulatory T cells (Treg) and HLA-DR+ activated Treg cells significantly increased with TCZ therapy (P < 0.001 and P < 0.001, respectively), whereas proportions of CD3+CD4+CXCR3−CCR6+CD161+ T helper 17 cells did not change over the 52 weeks. The proportions of CD20+CD27+ memory B cells, HLA-DR+CD14+ and CD69+CD14+ activated monocytes, and CD16+CD14+ monocytes significantly decreased (P < 0.001, P < 0.001, P < 0.001 and P < 0.001, respectively). Among them, only the change in Treg cells was inversely correlated with the change in CDAI score (ρ = −0.40, P = 0.011). The most dynamic increase in Treg cells was observed in the CDAI remission group (P < 0.001).
Conclusion
This study demonstrates that TCZ affected proportions of circulating immune cells in patients with RA. The proportion of Treg cells among CD4+ cells correlated well with clinical response.
Similar content being viewed by others
Introduction
T cells (especially CD4+ T cells), monocytes and B cells are considered to be involved in the pathogenesis of rheumatoid arthritis (RA) [1]. It is frequently considered that decreasing the number and/or activity of lymphocytes and other immune cells by RA treatment can reduce disease activity. The first evidence of this was seen in preliminary clinical trials in which targeting CD4+ T cells with anti-CD4 monoclonal antibodies (mAbs) resulted in clinical improvement of RA, albeit only modestly [2]. Abatacept, a cytotoxic T lymphocyte antigen 4 immunoglobulin recombinant fusion protein that inhibits CD4+ T cell activation by blocking costimulation with antigen-presenting cells such as B cells and monocytes, showed clinical efficacy against RA and has been approved worldwide for the treatment of RA [3]. Depletion of peripheral B cells by the anti-CD20 antibody rituximab also improves disease activity [4].
The anti-interleukin-6 receptor (IL-6R) antibody tocilizumab (TCZ) is also clinically effective against RA [5]. Several studies have shown that blocking the IL-6 signaling with TCZ can affect proportions of peripheral blood cells. Given that IL-6 was originally identified as a B cell differentiation factor [6,7], it is not surprising that TCZ affects proportions of B cell populations in patients with RA. IL-6 also influences differentiation of T cells into effector T cells (TH1, TH2 and TH17 cells) or regulatory T cells (Treg) [8-10]. In recent studies, researchers have shown that IL-6 blockade could favorably affect the TH17/Treg cell imbalance in patients with RA [11,12]. Moreover, IL-6 seems to affect the proliferation and activation of monocytes that express IL-6R [13,14]. However, because in previous studies the number of patients, the period of study and the examined cell populations were limited, it is not clear whether there is a key population of peripheral immune cells that attenuates RA clinical symptoms through anti-IL-6R therapies [11,12,15]. If these relationships could be clarified, it would enable medical researchers to comprehend the pathogenesis of RA from the view of lymphocyte populations and to find surrogate markers in order to choose an optimal therapeutic strategy for RA.
The primary objective of this study was to evaluate multiple different types of peripheral blood cells by using flow cytometry analysis to identify populations modulated by anti-IL-6R therapy. The secondary objective was to determine whether any of these populations is strongly associated with various clinical measures in response to anti-IL-6R therapy.
Methods
Patients
Eligible patients were those who met the 1987 revised criteria of the American College of Rheumatology (ACR) for the classification of RA or the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria [16,17]. Consecutive patients at our institute who commenced TCZ as their first biologic agent between March 2010 and April 2012 were enrolled. They all showed insufficient response to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD). The enrolled patients were administered 8 mg/kg TCZ every 4 weeks, either with or without other csDMARDs, including methotrexate (MTX). The study protocol was approved by the ethics committee at Keio University School of Medicine and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all patients.
Clinical assessments and evaluation of effectiveness
Demographic and clinical characteristics including age, sex, disease duration, tender joint count (TJC), swollen joint count (SJC), patient global assessment (patient visual analogue scale (Pt-VAS)), physician global assessment (doctor’s visual analogue scale (D-VAS)), Health Assessment Questionnaire Disability Index (HAQ-DI) score, C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), matrix metalloproteinase-3 (MMP-3) level, rheumatoid factor (RF) value and anticyclic citrullinated peptide (CCP) antibody value were obtained from the patients’ medical records.
Disease activity was assessed using the Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI). The cutoff values for remission, low disease activity (LDA), moderate disease activity (MDA) and high disease activity (HDA) were as follows: for remission, CDAI ≤2.8, SDAI ≤3.3; for LDA, 2.8 < CDAI ≤ 10, 3.3 < SDAI ≤ 11; for MDA, 10 < CDAI ≤ 22, 11 < SDAI ≤ 26; and for HDA, CDAI > 22, SDAI > 26 [18].
Cell surface staining and flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were obtained at baseline and at weeks 24 and 52 of TCZ treatment. PBMCs were separated by density gradient with Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) and cryopreserved in CELLBANKER 1 (Nippon Zenyaku Kogyo, Fukushima, Japan) until use. Thawed cells were stained for 30 minutes at room temperature under darkened conditions with the following fluorophore-labeled mAbs: anti-CD4-VioGreen (Miltenyi Biotec, Bergisch Gladbach, Germany); anti-CD3-Pacific Blue/fluorescein isothiocyanate (FITC), anti-CD8-Pacific Blue, anti-CD14-(APC)-Cy7, anti-CD20 allophycocyanin-cyanine 7 (APC-Cy7), anti-CD25 phycoerythrin (PE)-Cy5, anti-CD27-PE-Cy7, anti-CD38-PE-Cy5, anti-CD45RO-PE-Cy7, anti-CD56-PE/PE-Cy7, anti-CD69-APC/PE-Cy7, anti-CD80-FITC, anti-CD86-PE-Cy5, anti-CD127-FITC, anti-CD161-APC, anti-chemokine (C-X-C motif) receptor 3 (CXCR3)-PE and anti-HLA-DR-APC/APC-Cy7 (all from BD Biosciences, Franklin Lakes, NJ, USA); anti-CD16-Brilliant Violet 510 and anti-CCR6-Brilliant Violet 421 (both from BioLegend, San Diego, CA, USA); and anti-mouse immunoglobulin G isotype-matched controls (VioGreen from Miltenyi Biotec, the others from BD Biosciences).
Stained cells were washed twice with 2 ml of phosphate-buffered saline and analyzed on a MACSQuant analyzer (Miltenyi Biotec). Dead cells were confirmed with a propidium iodide fluorescence solution (Miltenyi Biotec) and excluded on the basis of scatter signals. The subsets analyzed were CD4 and CD8 T cells (including memory, effector and activation markers) and TH1, TH2, TH17, Treg, B cells, natural killer cells, and monocytes, including their subpopulations and activation markers. The peripheral cell subsets identified in this study were defined by using cell surface markers on the basis of peripheral cell subsets described in a previous report (Additional file 1: Table S1) [19].
Statistical analysis
Continuous data are presented as median and interquartile range (IQR) or as a number with percentage value, as appropriate. The Wilcoxon test and Kruskal-Wallis test were used to examine the differences between continuous variables. Correlation of two continuous variables was analyzed using the Spearman rank correlation coefficient. Fisher’s exact test was used to compare the proportion of categorical data between groups. A P-value <0.05 was considered statistically significant. All statistical analyses were performed with JMP 10 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of patients and associations between peripheral cell populations and disease activity at baseline
Table 1 shows the baseline demographics and clinical characteristics of the enrolled patients (N = 39). In this population, there was no difference between TCZ monotherapy and TCZ in combination with MTX in terms of baseline characteristics.
At baseline, a higher proportion of HLA-DR+CD8+ T cells among the CD8+ T cells was significantly associated with higher CRP, Pt-VAS, SDAI and HAQ-DI, as well as a higher proportion of naïve and memory CD8+ T cells among the CD8+ T cells, was significantly associated with RF, ACPA, SJC, CDAI and SDAI (Additional file 1: Table S2). A higher proportion of TH2 cells among the CD4+ T cells was also significantly associated with TJC, D-VAS, Pt-VAS, CDAI and SDAI scores. No other baseline subsets or surface markers correlated with CDAI or SDAI score (Additional file 1: Table S3).
Changes from baseline in clinical response
All patients in this study received TCZ for the entire 52 weeks. The CDAI and SDAI scores (mean ± standard deviation) significantly decreased from 19.6 ± 9.3 and 21.1 ± 9.9, respectively, at baseline to 5.5 ± 5.2 and 5.5 ± 5.2 at week 24 and to 5.2 ± 6.0 and 5.6 ± 6.8 at week 52 (P < 0.0001). The number and percentage of patients categorized as having attained remission or as having LDA, MDA and HDA were as follows for CDAI and SDAI: at baseline, 0 (0%) and 0 (0%) for remission, 3 (7.7%) and 4 (10.3%) for LDA, 24 (61.5%) and 25 (64.1%) for MDA, and 12 (30.8%) and 10 (25.6%) for HDA, respectively; and at week 52, 21 (53.8%) and 22 (56.4%) for remission, 11 (28.2%) and 10 (25.6%) for LDA, 7 (17.9%) and 7 (17.9%) for MDA, and 0 (0%) and 0 (0%) for HDA, respectively (Additional file 1: Figure S2).
Changes from baseline in peripheral cell subsets
The proportions of memory CD4+ T cells among all CD4+ T cells, HLA-DR+CD8+ T cells among CD8+ T cells, Treg cells among CD4+ T cells, HLA-DR+ Treg cells among Treg cells, naïve B cells among all B cells, and CD16−CD14+ monocytes among CD14+ monocytes increased after TCZ treatment (Tables 2 and 3). On the other hand, the proportions of naïve CD4+ T cells among all CD4+ T cells, HLA-DR+CD4+ T cells among CD4+ T cells, CD86+ B cells among all B cells, memory B cells among all B cells, HLA-DR+CD14+ monocytes among CD14+ monocytes, CD69+CD14+ monocytes among CD14+ monocytes, and CD16+CD14+ monocytes among CD14+ monocytes decreased after TCZ treatment (Tables 2 and 3). The other subsets and their activation markers were not significantly changed during TCZ therapy.
Evaluation of the relationships between peripheral cell subsets and clinical response after tocilizumab therapy
Associations between changes in peripheral cell subsets and changes in clinical endpoints (∆CDAI and ∆SDAI) after TCZ treatment are summarized in Table 4. A significant correlation was observed between the change in the proportion of Treg cells among CD4+ T cells and the changes in CDAI score from baseline to week 52: the greater the increase in proportion of Treg cells among CD4+ T cells from baseline to week 52, the greater the improvement in CDAI score during the same period (ρ = −0.346, P = 0.031). Also, the change in CD38 expression on CD8+ T cells over 52 weeks was negatively correlated with change in CDAI score (ρ = −0.355, P = 0.026). The other peripheral cell subsets, including naïve and memory CD4+ T cells, HLA-DR+CD4+ T cells, CD69+CD4+ T cells, HLA-DR+CD8+ T cells, HLA-DR+ Treg naïve and memory B cells, CD86+ B cells, HLA-DR+CD14+ monocytes, CD69+CD14+ monocytes, CD16+CD14+ monocytes and CD16−CD14+ monocytes, the proportions of which significantly changed over 52 weeks (as shown above), did not demonstrate any correlation with the change in CDAI score. The same results were found when ΔSDAI was used as the clinical endpoint.
Association between the change in Treg cells and the effectiveness of tocilizumab therapy
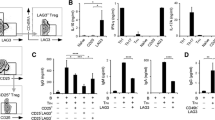
The time course of changes in Treg cells as a proportion of CD4+ T cells is shown in Figure 1A. The median proportion of Treg cells significantly increased over 52 weeks from 3.6 (IQR, 2.2 to 4.3) to 5.6 (IQR 4.5 to 7.1) (P < 0.001). The change in CDAI and SDAI scores at week 52 was significantly associated with the change in Treg cells at week 52 (Table 4, Figure 1B). Of the 39 patients examined in this study, 21 (53.8%) met the CDAI criteria for remission and 22 (56.4%) attained SDAI remission at week 52. When we divided the patients into two groups according to their remission status at week 52, the change in peripheral blood Treg cells was significantly higher in the remission group than in the nonremission group at week 52 (Figure 1C). Moreover, the proportion of peripheral blood Treg cells was significantly higher in the remission group than in the nonremission group at week 52 (Figure 1D).
Relations between effects of tocilizumab on the proportion of regulatory T cells and clinical responses. (A) Chronological change in the proportion of regulatory T (Treg) cells among CD4+ T cells from baseline to week 52 of tocilizumab (TCZ) treatment. (B) The relationships between the change in clinical activities (Clinical Disease Activity Index (ΔCDAI) and Simplified Disease Activity Index (ΔSDAI)) and the change in proportion of Treg cells among CD4+ T cells (ΔTreg/CD4) at week 52. (C) The relationships between CDAI and SDAI scores at week 52 and the change in proportion of Treg cells among CD4+ T cells (ΔTreg/CD4) at week 52. (D) The relationships between CDAI and SDAI scores at week 52 and the proportion of Treg cells among CD4+ T cells at week 52. The squares signify medians and interquartile ranges, and the diamonds signify means and 95% confidence intervals. Data were analyzed by using the Kruskal-Wallis test (A), Spearman’s rank correlation coefficient (B) and the Wilcoxon rank-sum test (C,D). *Significant differences.
As for CD38 expression on CD8+ T cells, the overall expression did not change during 52 weeks (P = 0.913), and there was no difference in its proportion between the remission and nonremission groups at Week 52 (P = 0.453 for CDAI, P = 0.345 for SDAI).
Discussion
In this article, we report the results of the first comprehensive study to show the effect of TCZ on various peripheral cell subsets. In this study, we have shown that the proportion of CD4+CD25+CD127low Treg cells among CD4+ cells and the proportion of HLA-DR+-activated Treg cells among Treg cells significantly increased from baseline over the course of treatment with TCZ, and also that the proportions of CD20+CD27+ memory B cells, HLA-DR+CD14+ activated monocytes, CD69+CD14+ activated monocytes, and CD16+CD14+ nonclassical monocytes significantly decreased from baseline. Among them, only the increase in Treg cells was significantly associated with achieving remission by TCZ treatment.
Related studies have reported the increase in Treg cell levels 1 year after TCZ administration in a small population [20], for 3 months [15] and for 6 months [12]. Though definitions of Treg cells in our study, using only cell surface markers without stimulatory modification for cells in vitro, were different from those in these previous studies, our results showed the same trends as previously reported. The mechanism is a question that is not yet fully understood. However, this is still an important biomarker, as no suitable biomarker that can help predict outcome of RA has not been identified.
Several mechanisms may contribute to the potentiation of Treg cells by TCZ therapy. It is conceivable that IL-6 has an effect on Treg differentiation via inhibiting the expression of a specific transcriptional factor, FoxP3 [21,22]. This may suggest a mechanism by which neutralizing of IL-6 signaling in patients with RA can induce an increase in the number and function of Treg cells. The quantitative increase in Treg cells expressing elevated levels of human leukocyte antigen (HLA)-DR in patients with RA treated with TCZ could also be a result of activation and proliferation of preexisting Treg cells or of their differentiation by conversion from Foxp3− precursors.
One question arising from the result is whether the increase of Treg proportion after TCZ therapy is a result of disease remission or is attributable to use of TCZ. To answer the question, we also analyzed peripheral Treg cells in 12 patients with RA longitudinally treated with MTX alone (Additional file 1: Table S4). All the patients achieved LDA or remission at week 52 after starting MTX therapy (Additional file 1: Figure S3). The proportion of Treg cells did not show a certain tendency or change over 52 weeks after administration of MTX (P = 0.729) (Additional file 1: Figure S4A). In addition, the change in Treg cells at week 52 was not associated with the change in CDAI and SDAI scores at week 52 (Additional file 1: Figure S4B). These results suggest that, albeit the comparison in a small number of patients with MTX, the increase in Treg proportion after TCZ therapy is not a result of disease remission but caused by TCZ therapy itself.
Another question is whether other biologic agents for RA treatment also induce Treg cells. It has been reported that neither adalimumab, an anti-tumor necrosis factor (anti-TNF) mAb, nor etanercept, a soluble TNF receptor, modified percentages or absolute numbers of circulating CD4+CD25high Treg cells or other Treg phenotypes after being administered for 6 and 12 weeks to patients with RA, regardless of their response [23]. However, there is one report that high concentrations of TNFα can block the immunosuppressive functions of Treg cells in vitro and that the treatment of patients with RA with infliximab, an anti-TNF mAb, bolsters Treg suppression of the proliferation of effector cells [24]. It is assumed that these conflicting results might be ascribed to a small number of subjects (N = 10 to 30) and a short observational period (12 to 24 weeks). Regarding abatacept, the therapy diminishes the absolute numbers of Treg cells but enhances their function in patients with RA [25]. It is conceivable that the different targets of these therapies influence the different performance of Treg cells and that the results of the present study indicate a part of the unique mechanism of TCZ.
Although much still remains to be clarified about how Treg defects might contribute to the pathogenesis of RA, approaches that specifically boost Treg activity could be useful in the treatment of RA. In this study, the change in Treg was correlated with disease activities after TCZ therapy. This is the first time that the effect of increasing Treg has been shown to be strictly associated with clinical efficacy.
The effects of IL-6 on the late stages of B cell differentiation in vitro are well documented [26]. In vivo IL-6 overexpression is associated with B cell hyperactivity, autoantibody production and immunopathology [27,28]. In patients with RA, chronic activation of B cells and an accumulation of memory B cells in the peripheral blood and synovial membranes have been described [29,30]. Within this context, B cell–targeted therapies utilizing rituximab have been widely explored in RA. Because IL-6 has been described as an important B cell–stimulating factor with effects on memory B cell survival and on plasma cell differentiation and survival in the bone marrow, it is easy to comprehend the effect of TCZ on peripheral B cells, especially the ratio of naïve B cells to memory B cells [31]. Although the proportion of memory B cells significantly decreased over 52 weeks of TCZ therapy, it did not correlate with any component of activity status, SJC, TJC, Pt-VAS, D-VAS, CRP and ESR. Therefore, the decrease can be attributed to the effect of TCZ therapy rather than to disease activity. When we compared the proportion of B cell subsets in the same 12 patients that were effectively treated with MTX alone during 52 weeks as mentioned above (Additional file 1: Table S5), we observed that the proportion of memory B cells tended to decrease in patients with MTX therapy, as in the case with TCZ, suggesting that the trend was not specific to TCZ therapy. However, the proportions of CD80+ and CD86+ B cells among all B cells did not change in patients who received MTX therapy. Therefore, the decrease in the proportion after TCZ therapy may be characteristic of TCZ.
In peripheral blood, two monocyte subpopulations with distinct functional properties have been defined by their expression of CD14 and CD16 molecules. Compared with classical CD14+CD16− monocytes, CD16+ nonclassical monocytes have been shown to possess several features of inflammatory tissue macrophages, notably, higher expression of major histocompatibility complex class II antigens and several adhesion molecules and lower expression of IL-10, transforming growth factor β, macrophage colony-stimulating factor, IL-1β and TNFα [32]. The pathophysiologic significance of the CD16+ nonclassical monocyte subset has been demonstrated by its expansion under various inflammatory conditions, such as RA, sepsis, asthma and solid tumors. We demonstrated that TCZ reduced the peripheral level of CD16+ nonclassical monocytes. Although it remains to be clarified how monocytes differentiate into CD16+ nonclassical monocytes, this study revealed that IL-6 appears to be involved in the proliferation of CD16+ nonclassical monocytes or in shifting the balance of monocytes to CD16+ nonclassical monocytes. In the same 12 patients with RA described above who were treated with MTX alone, the proportions of HLA-DR+CD14+ monocytes and CD69+CD14+ monocytes among all monocytes did not change (Additional file 1: Table S5), which was different from the results for TCZ. The decrease of those activated monocytes after TCZ therapy did not seem to be the cause of disease remission, but rather the effect of TCZ. The proportion of CD16+CD14+ nonclassical monocytes tended to decrease during 52 weeks of MTX therapy compared with that of TCZ therapy. This may have been a result of the improvement of RA disease activity, or there may some actions on immune cells in common between TCZ and MTX.
Conclusions
Our findings suggest that TCZ affected proportions of circulating Treg cells, B cells and monocytes in patients with RA. Especially, the increase in the proportion of Treg cells among CD4+ T cells correlated well with clinical response. Then the possible mode of action of TCZ against RA could increase the proportion of Treg cells.
Abbreviations
- ACPA:
-
Anticitrullinated protein antibody
- ACR:
-
American College of Rheumatology
- CCP:
-
Cyclic citrullinated peptide
- CDAI:
-
Clinical Disease Activity Index
- CRP:
-
C-reactive protein
- csDMARD:
-
Conventional synthetic disease-modifying antirheumatic drug
- D-VAS:
-
Doctor’s visual analogue scale
- ESR:
-
Erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- HAQ-DI:
-
Health Assessment Questionnaire Disability Index
- HDA:
-
High disease activity
- IL-6R:
-
Interleukin-6 receptor
- IQR:
-
Interquartile range
- LDA:
-
Low disease activity
- mAb:
-
Monoclonal antibody
- MDA:
-
Moderate disease activity
- MMP-3:
-
Matrix metalloproteinase-3
- MTX:
-
Methotrexate
- PBMC:
-
Peripheral blood mononuclear cell
- Pt-VAS:
-
Patient’s visual analogue scale
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- SDAI:
-
Simplified Disease Activity Index
- SJC:
-
Swollen joint count
- TCZ:
-
Tocilizumab
- TJC:
-
Tender joint count
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
References
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Choy EH, Panayi GS, Emery P, Madden S, Breedveld FC, Kraan MC, et al. Repeat-cycle study of high-dose intravenous 4162 W94 anti-CD4 humanized monoclonal antibody in rheumatoid arthritis. A randomized placebo-controlled trial. Rheumatology (Oxford). 2002;41:1142–8.
Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15.
Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, et al. for the IMAGE Investigators. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis. 2011;70:39–46.
Nam JL, Ramiro S, Gaujoux-Viala C, Takase K, Leon-Garcia M, Emery P, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014;73:516–28.
Kishimoto T. Factors affecting B-cell growth and differentiation. Annu Rev Immunol. 1985;3:133–57.
Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6.
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8.
Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60.
Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, et al. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J Exp Med. 2002;196:39–49.
Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, et al. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–503.
Pesce B, Soto L, Sabugo F, Wurmann P, Cuchacovich M, López MN, et al. Effect of interleukin-6 receptor blockade on the balance between regulatory T cells and T helper type 17 cells in rheumatoid arthritis patients. Clin Exp Immunol. 2013;171:237–42.
Jansen JH, Kluin-Nelemans JC, Van Damme J, Wientjens GJ, Willemze R, Fibbe WE. Interleukin 6 is a permissive factor for monocytic colony formation by human hematopoietic progenitor cells. J Exp Med. 1992;175:1151–4.
Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4.
Thiolat A, Semerano L, Pers YM, Biton J, Lemeiter D, Portales P, et al. Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014;66:273–83.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham 3rd CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8.
Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8.
Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200.
Sarantopoulos A, Tselios K, Gkougkourelas I, Pantoura M, Georgiadou AM, Boura P. Tocilizumab treatment leads to a rapid and sustained increase in Treg cell levels in rheumatoid arthritis patients: comment on the article by Thiolat et al. Arthritis Rheumatol. 2014;66:2638.
Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 2011;186:32–40.
Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25− T cells. J Immunol. 2007;179:2041–5.
Blache C, Lequerré T, Roucheux A, Beutheu S, Dedreux I, Jacquot S, et al. Number and phenotype of rheumatoid arthritis patients’ CD4+CD25hi regulatory T cells are not affected by adalimumab or etanercept. Rheumatology (Oxford). 2011;50:1814–22.
Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–61.
Álvarez-Quiroga C, Abud-Mendoza C, Doníz-Padilla L, Juárez-Reyes A, Monsiváis-Urenda A, Baranda L, et al. CTLA-4-Ig therapy diminishes the frequency but enhances the function of Treg cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:588–95.
Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167:332–44.
Hirano T, Taga T, Yasukawa K, Nakajima K, Nakano N, Takatsuki F, et al. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A. 1987;84:228–31.
Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989;86:7547–51.
Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–8.
Dörner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol. 2003;15:246–52.
Roll P, Muhammad K, Schumann M, Kleinert S, Einsele H, Dörner T, et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63:1255–64.
Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92.
Acknowledgements
This study was supported by Chugai Pharmaceutical Co, Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
JK, KY, NN and YK declare that they have no competing interest. MH is an employee of Chugai Pharmaceutical Co, Ltd. TT has received grants from Astellas Pharma Inc, Bristol-Myers KK, Chugai Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Eisai Co, Ltd, Mitsubishi Tanabe Pharma Corp, Pfizer Japan, Santen Pharmaceutical Co, Ltd, Takeda Pharmaceutical Co, Ltd, Teijin Pharma Ltd, AbbVie GK, Asahi Kasei Pharma Corp, Taisho Toyama Pharmaceutical Co, Ltd, and SymBio Pharmaceuticals Ltd; speaking fees from AbbVie GK, Bristol-Myers KK, Chugai Pharmaceutical Co, Ltd, Eisai Co, Ltd, Janssen Pharmaceutical KK, Mitsubishi Tanabe Pharma Corp, Pfizer Japan Inc, Takeda Pharmaceutical Co, Ltd, Astellas Pharma Inc, and Daiichi Sankyo Co, Ltd; and consultant fees from Astra Zeneca KK, Eli Lilly Japan KK, Novartis Pharma KK, Mitsubishi Tanabe Pharma Corp, Asahi Kasei Medical Co, Ltd, AbbVie GK, Daiichi Sankyo Co, Ltd, and Bristol-Myers KK.
Authors’ contributions
JK carried out flow cytometry, participated in the design of the study, analyzed the data, performed the statistical analysis, evaluated the results and drafted the manuscript. MH carried out flow cytometry, participated in the design of the study, analyzed the data, evaluated the results and drafted the manuscript. YK participated in the design of the study, evaluated the results and helped to draft the manuscript. KY participated in flow cytometry, evaluated the results and helped to draft the manuscript. NN analyzed the data, evaluated the results and helped to draft the manuscript. TT conceived of the study, participated in its design and coordination, evaluated the results and helped to draft the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1: Table S1.
Identified subsets of peripheral blood mononuclear cells defined using cell surface markers. Table S2. Correlations between peripheral T cell subsets and serum markers, composite measures of disease activity, and Health Assessment Questionnaire Disability Index score at baseline. Spearman’s rank correlation coefficient was used for analysis. Table S3. Correlations between peripheral B cell subsets, natural killer (NK) cells and monocyte subsets and serum markers, composite measures of disease activity, and Health Assessment Questionnaire Disability Index score at baseline. Spearman’s rank correlation coefficient was used for analysis. Table S4. Baseline demographics and clinical characteristics of the patients treated with MTX. Table S5. Changes in proportions (%) of subsets and surface markers of B cells and monocytes during MTX treatment. Subsets and surface markers were selected as the changed ones in TCZ therapy. Figure S1. Gating strategy for flow cytometry analysis of Treg cells and subsets of monocytes. Figure S2. Chronological changes in and proportions of clinical activity from baseline until week 52 of TCZ treatment. Figure S3. Chronological changes in clinical activity from baseline until week 52 of MTX treatment. Figure S4. Relationships between effects of MTX on the proportion of Treg cells and clinical responses. (A) Chronological change in the proportion of Treg cells among CD4+ T cells from baseline to week 52 of MTX treatment. (B) The relationships between the change in clinical activity (ΔCDAI and ΔSDAI scores) and the change in proportion of Treg cells among CD4+ T cells (ΔTreg/CD4) at week 52. The squares signify medians and interquartile ranges.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kikuchi, J., Hashizume, M., Kaneko, Y. et al. Peripheral blood CD4+CD25+CD127low regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther 17, 10 (2015). https://doi.org/10.1186/s13075-015-0526-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-015-0526-4