Abstract
Neuroimaging genomics is a relatively new field focused on integrating genomic and imaging data in order to investigate the mechanisms underlying brain phenotypes and neuropsychiatric disorders. While early work in neuroimaging genomics focused on mapping the associations of candidate gene variants with neuroimaging measures in small cohorts, the lack of reproducible results inspired better-powered and unbiased large-scale approaches. Notably, genome-wide association studies (GWAS) of brain imaging in thousands of individuals around the world have led to a range of promising findings. Extensions of such approaches are now addressing epigenetics, gene–gene epistasis, and gene–environment interactions, not only in brain structure, but also in brain function. Complementary developments in systems biology might facilitate the translation of findings from basic neuroscience and neuroimaging genomics to clinical practice. Here, we review recent approaches in neuroimaging genomics—we highlight the latest discoveries, discuss advantages and limitations of current approaches, and consider directions by which the field can move forward to shed light on brain disorders.
Similar content being viewed by others
Background
Neuroimaging genomics is a relatively new and rapidly evolving field that integrates brain imaging and individual-level genetic data to investigate the genetic risk factors shaping variations in brain phenotypes. Although this covers a broad range of research, one of the most important aims of the field is to improve understanding of the genetic and neurobiological mechanisms underlying various aspects of neuropsychiatric disorders—from symptoms and etiology, to prognosis and treatment. The goal is to identify key components in biological pathways that can be evaluated or monitored to improve diagnostic and prognostic assessments, and that can ultimately be targeted by novel therapies.
Broadly speaking, existing brain imaging methods can be divided into those that provide data on structure—for example, computed tomography (CT), structural magnetic resonance imaging (MRI), and diffusion–tensor imaging (DTI); function—for example, functional MRI (fMRI), arterial spin labeling (ASL); and molecular imaging—for example, single-photon emission computed tomography (SPECT) and positron-emission tomography (PET) using receptor-binding ligands and magnetic resonance spectroscopy (MRS) [1]. A range of additional new methods have become available for animal and/or human brain imaging, including optical imaging, cranial ultrasound, and magnetoencephalography (MEG), but to date these have been less widely studied in relation to genomics. Future work in imaging genomics will rely on further advances in neuroimaging technology, as well as on multi-modal approaches.
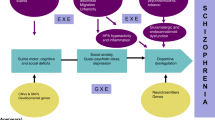
Progress in both neuroimaging and genomic methods has contributed to important advances—from candidate-gene (or more precisely, single-variant) approaches initiated almost two decades ago [2, 3], to recent breakthroughs made by global collaborations focused on GWAS [4], gene–gene effects [5], epigenetic findings [6], and gene–environment interactions [7] (Fig. 1). Developments in the field of neuroimaging genomics have only recently begun to provide biological insights through replicated findings and overlapping links to disease—we now know the field holds much promise, but further work and developments are needed to translate findings from neuroimaging genomics into clinical practice. In this review, we discuss the most recent work in neuroimaging genomics, highlighting progress and pitfalls, and discussing the advantages and limitations of the different approaches and methods now used in this field.
Timeline of methodological approaches common in neuroimaging-genomics studies of neuropsychological disorders. The field of neuroimaging genomics was initiated in the early 2000s using a hypothesis-driven candidate-gene approach to investigate brain and behavior phenotypes [2, 3]. Towards the end of the decade, other candidate-gene approaches, investigating alternative genetic models, began to emerge. These included gene–gene interactions [172], gene–environment interactions [7], and epigenetic effects [6]. Simultaneously, hypothesis-free approaches such as genome-wide association studies (GWAS) were initiated [173] and the need for increased statistical power to detect variants of small individual effects soon led to the formation of large-scale consortia and collaborations [36, 37]. The emergence of the “big data” era presented many statistical challenges and drove the development of multivariate approaches to account for these [174]. GWAS of neuropsychological disorders soon identified significant associations with genetic variants with unknown biological roles, resulting in candidate neuroimaging genomics studies to investigate and validate the genetic effects on brain phenotypes [175]. The emergent polygenic nature of these traits encouraged the development of polygenic models and strategies to leverage this for increased power in genetic-overlap studies between clinical and brain phenotypes [114]. Most recently, hypothesis-free approaches are starting to extend to alternative genetic models, such as gene–gene interactions [70]
Heritability estimates and candidate gene associations with imaging-derived traits
Approximately two decades ago, neuroimaging genomics had its inception—twin and family designs from population genetics were used to calculate heritability estimates for neuroimaging-derived measures, such as brain volume [8], shape [9, 10], activity [11], connectivity [12], and white-matter microstructure [13]. For almost all these imaging-derived brain measures, monozygotic twin pairs showed greater correlations than dizygotic twins, who in turn showed greater correlations than more-distant relatives and unrelated individuals. These studies confirm that brain measures derived from non-invasive scans have a moderate to strong genetic underpinning [14, 15] and open the doors for more-targeted investigations. These brain features might now be considered useful endophenotypes (using only certain symptoms—for example, altered brain volume—of a trait such as schizophrenia, which might have a more-robust genetic underpinning) for psychiatric disorders [16]. A focus on the underlying mechanisms is central to the now highly regarded Research Domain Criteria (RDoC) research framework [17]. In contrast to classifications that focus on diagnoses or categories of disorders [18, 19], RDoC emphasizes transdiagnostic mechanisms (investigating overlapping symptoms across diagnoses) that emerge from translational neuroscience [20].
Early imaging genomics work (from approximately 2000 to 2010; Fig. 1) focused predominantly on candidate-gene approaches—in the absence of large GWAS datasets, investigators relied on biological knowledge to develop hypotheses. Genetic variants or single-nucleotide polymorphisms (SNPs) identified through linkage studies or located near or within genes with putative biological roles, particularly those involved in neurotransmission, were investigated in brain imaging studies. Early candidate genes studied in relation to brain phenotypes included the sodium-dependent serotonin transporter gene (SLC6A4) in individuals with anxiety and depression [21,22,23] and the catechol-O-methyltransferase gene (COMT) in individuals with schizophrenia [24,25,26,27,28].
A key criticism of this early work was that candidate-gene studies were insufficiently powered, with the possibility that small false-positive studies were being published, whereas larger negative analyses were being “filed away” [29, 30]. In support of this view, several meta-analyses have emphasized the inconsistency of small candidate-gene studies [31,32,33]. These studies noted that, given relatively small effect sizes, larger studies were needed and that a clear focus on harmonization of methods across studies was needed for meaningful meta-analyses. For example, a meta-analysis of candidate studies of the rs25532 polymorphism of SLC6A4 (commonly referred to as the “short variation”) and amygdala activation, which incorporated unpublished data, was unable to identify a significant association [31]. This finding cast doubt on the representativeness of effect sizes reported in early studies with positive findings, highlighting a potential “winner’s curse” and emphasized the importance of publication bias in the field.
However, borrowing strategic approaches from studies of anthropometric traits (GIANT consortium), psychiatric disorders (PGC, psychiatric genomics consortium [34]), cancer (CGC, cancer genomics consortium [35]), and cardiovascular health and aging (CHARGE [36]), the imaging-genomics community has built large-scale collaborations and consortia in order to obtain the statistical power necessary to disentangle the genetic architecture of brain phenotypes [37].
Genome-wide association studies in imaging genomics
Imaging genomics has increasingly moved towards a GWAS approach, using large-scale collaborations to improve power for the detection of variants with small independent effects [29]. Examples of such consortia include the Enhancing Neuro-imaging through Meta-analysis (ENIGMA) consortium [37], Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium [36], Alzheimer's Disease Neuroimaging Initiative (ADNI), IMAGEN, which is focused on adolescents [38], and the Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement (UNIVRSE) consortium [39]. The growing number of GWAS of brain phenotypes and of neuropsychiatric disorders has, on occasion, lent support to previously reported candidate variants [40], but importantly has identified many new variants of interest [41].
An early study by the ENIGMA consortium consisted of approximately 8000 participants, including healthy controls and cases with psychiatric disorders [42]. This study identified significant associations between intracranial volume and a high-mobility group AT-hook 2 (HMGA2) polymorphism (rs10784502), and between hippocampal volume and an intergenic variant (rs7294919). A subsequent collaboration with the CHARGE consortium, including over 9000 participants, replicated the association between hippocampal volume and rs7294919, as well as identifying another significant association with rs17178006 [43]. In addition, this collaboration has further validated and identified other variants associated with hippocampal volume [44] and intracranial volume [45], with cohorts of over 35,000 and 37,000 participants, respectively. Another analysis of several subcortical volumes (ENIGMA2), with approximately 30,000 participants, identified a significant association with a novel intergenic variant (rs945270) and the volume of the putamen, a subcortical structure of the basal ganglia [4]. More recently, a meta-analysis of GWAS of subcortical brain structures from ENIGMA, CHARGE, and the United Kingdom Biobank was conducted [46]. This study claims to identify 25 variants (20 novel) significantly associated with the volumes of the nucleus accumbens, amygdala, brainstem, caudate nucleus, globus pallidus, putamen, and thalamus amongst 40,000 participants (see the “Emerging pathways” section later for a more detailed discussion). Moreover, many large-scale analyses [15, 46] are now first being distributed through preprint servers and social media. In another example, in over 9000 participants from the UK Biobank, Elliot and colleagues [15] used six different imaging modalities to perform a GWAS of more than 3000 imaging-derived phenotypes, and identified statistically significant heritability estimates for most of these traits and implicated numerous associated single-nucleotide polymorphisms (SNPs) [15]. Such works still need to undergo rigorous peer-review and maintain strict replication standards for a full understanding of findings, yet this work highlights the fact that the depth of possibilities now available within the field of neuroimaging genomics appears to be outpacing the current rate of publications. As of November 2017, ENIGMA is currently undertaking GWAS of the change in regional brain volumes over time (ENIGMA-Plasticity), cortical thickness and surface area (ENIGMA-3), white-matter microstructure (ENIGMA-DTI), and brain function as measured by EEG (ENIGMA-EEG).
Although neuroimaging measurements only indirectly reflect the underlying biology of the brain, they remain useful for in vivo validation of genes implicated in GWAS and lend insight into their biological significance. For example, the rs1006737 polymorphism in the gene encoding voltage-dependent L-type calcium channel subunit alpha-1C (CACNA1C) was identified in early GWAS of bipolar disorder [47, 48] and schizophrenia [49, 50], but its biology was unknown. Imaging-genomics studies of healthy controls and individuals with schizophrenia attempted to explain the underlying biological mechanisms. Studies reported associations of this variant with increased expression in the human brain, altered hippocampal activity during emotional processing, increased prefrontal activity during executive cognition, and impaired working memory during the n-back task [51,52,53], a series of task-based assessments relying on recognition memory capacity. As the psychiatric genomics field advances and more reliable and reproducible genetic risk factors are identified, imaging genomics will continue to help understand the underlying biology.
The limitations of GWAS of complex traits and neuropsychiatric disorders deserve acknowledgment. In particular, although GWAS can identify statistically significant associations, these have particularly small individual effect sizes and, even cumulatively, do not account for a substantial fraction of the heritability of the relevant phenotype estimated from family models [54]. Furthermore, many associated variants are currently not functionally annotated and most often are found in non-coding regions of the genome, which are not always well understood [55, 56]. Increasing power, through increasing sample sizes, will likely implicate additional variants, but these might not necessarily play a directly causal role [57]. This could be because of the small effect sizes of causative variants, linkage disequilibrium with other variants, and the indirect effects of other variants in highly interconnected pathways [57]. Currently, most studies utilize participants of European ancestry, and replication studies using alternative ethnic groups are required for further discovery and validation of significant associations, which might be influenced by the populations under investigation [58]. Thus, additional strategies are needed to understand fully the genetic architecture of brain phenotypes and neuropsychiatric disorders. These methods can be summarized into three categories: first, delving deeper into rarer genetic variations; second, incorporating models of interactions; and, third, investigating more than a single locus and instead expanding to incorporate aggregate or multivariate effects; these methods and more are discussed below [57].
Copy-number variation and brain variability
Growing recognition of the neuropsychiatric and developmental abnormalities that arise from rare genetic conditions, such as 22q11 deletion syndrome [59], has led imaging-genomic studies to further explore the relationships between copy-number variations (CNVs) and neural phenotypes [60,61,62,63]. For example, in a recent large-scale study of over 700 individuals, 71 individuals with a deletion at 15q11.2 were studied to examine the effects of the genetic deletion on cognitive variables [60]. These individuals also underwent brain MRI scans to determine the patterns of altered brain structure and function in those with the genetic deletion. This study identified significant associations between this CNV and combined dyslexia and dyscalculia, and with a smaller left fusiform gyrus and altered activation in the left fusiform and angular gyri (regions in the temporal and parietal lobes of the brain, respectively). Another study investigating the 16p11.2 CNV, with established associations with schizophrenia and autism, found that the CNVs modulated brain networks associated with established patterns of brain differences seen in patients with clinical diagnoses of schizophrenia or autism [61]. These studies indicate that CNVs might play an important role in neural phenotypes, and initiatives such as ENIGMA-CNV [63] aim to explore this further.
Gene–gene interactions
Gene–gene interactions (epistasis), where the phenotypic effect of one locus is affected by the genotype(s) of another, can also play significant roles in the biology of psychiatric disorders [64]; such interactions might help account for the missing heritability observed with genetic association testing [54]. Singe-locus tests and GWAS might not detect these interactions as they use additive genetic models [64]. The inclusion of interaction tests has also, for example, been shown to improve the power for detection of the main effects in type 1 diabetes [65]. Recently, this has emerged as a focus of imaging-genomic studies, predominantly using a candidate-gene approach [66,67,68,69].
Studies of epistasis are, however, at an early stage and currently have relatively small sample sizes and lack replication attempts, limiting the validity of these findings [70]. Selecting candidate genes for investigation, usually based on significance in previous association studies, may miss important interactions with large effects [71]. Genome-wide interaction approaches may provide for a more unbiased approach towards understanding epistatic effects. As a proof of concept, one such study investigated genome wide SNP–SNP interactions using participants from the ADNI cohort, and the Queensland Twin Imaging study for replication [70]. While larger scale studies are needed to confirm specific findings, this study identified a significant association between a single SNP–SNP interaction and temporal lobe volume, which accounted for an additional 2% of the variance in temporal lobe volume (additional to the main effects of SNPs) [70]. As the power for GWAS in imaging genomics increases through growing consortia and biobanks, large-scale epistatic studies may become possible and explain more of the genetic variance underlying brain structure and function.
Gene–environment interactions
Most neuropsychiatric disorders have a multifactorial etiology [72, 73], with varying heritability estimates under different conditions [74]. Imaging-genomics studies have begun to investigate how genes and the environment interact (GxE) to influence brain structure and function in relation to neuropsychiatric disorders [75]. These interactions are of further interest as emerging evidence indicates that some individuals exposed to certain environmental factors have altered treatment responses [75]. For example, GxE studies of the rs25532 polymorphism within the SLC6A4 gene indicate that carriers with depression, and who are exposed to recent life stressors, respond poorly to treatment with certain antidepressants [76,77,78,79], but have better responses to psychotherapy compared to those with the alternative genotype [80]. Therefore, imaging genomics is ideally suited to identify possible interactions that may affect treatment responses, lend insight into these mechanisms potentially leading to altered or new therapeutic regimens, and identify at-risk individuals who may benefit from early interventions [81, 82].
Small exploratory studies have suggested that potentially interesting gene–gene interactions might exist [7, 83,84,85,86,87,88,89]; however, the statistical power of published analyses is low, and replication is key [90, 91]. Candidate-gene approaches to GxE studies have been commonplace, but these might oversimplify genetic models, as each of these variants contributes minimally to disease risk [90, 91]. To ensure the effect is indeed an interaction and not due to one component of the interaction, all terms (G, E, GxE) will need to be included in a regression model. Naturally, this implies genome-wide interaction studies would require even larger sample sizes than GWAS if they are to be appropriately powered [90, 91]. Concerns about the measures of both phenotype and the exposome (lifetime environmental exposures) have also been raised, as studies using different measures and at different stages of life can produce conflicting results [91,92,93]. Large-scale collaborations using carefully harmonized protocols will likely be able to mitigate these limitations.
Epigenetics
Approaches investigating the associations between epigenetic alterations and brain measures once again began with candidate genes [94, 95]. However, disparities between the methylation states of blood, saliva, and brain tissue remain important limitations for untangling the discrepancies found with epigenetic studies [96]. To illustrate this, several projects, such as the Human Roadmap Epigenomics project [97], the International Human Epigenome Consortium [98], and Braincloud [99], have begun developing reference epigenomes, which could pave the way for harmonizing and pooling data across independent datasets. These projects might also provide new biologically based candidates for research—it has been suggested that genes most similarly methylated between blood and brain tissue be investigated first in neuroimaging studies [100, 101]. Recently, imaging consortia such as ENIGMA have begun epigenome-wide association studies for key brain measures such as hippocampal volume, revealing promising associations [102]. Longitudinal and trans-generational studies of both healthy and at-risk individuals might also prove useful for understanding the impact of the environment on the epigenome [101].
Mapping the genetic structure of psychiatric disease onto brain circuitry
Recent large-scale GWAS of psychiatric disorders have begun to identify significantly associated variants [41, 103]—however, the effect sizes of these variants are small (usually less than 1%) and do not account for the predicted heritability of these traits (as high as 64–80% in schizophrenia [104, 105]). It is hypothesized that many psychiatric disorders have a polygenic (effected by multiple genetic variants) and heterogeneous (disease-causing variants can differ between affected individuals) genetic architecture, resulting in a failure to reach statistical significance and contributing to the phenomenon of missing heritability [106]. GWAS of subcortical brain structure and cortical surface area have also started to reveal significant genetic associations and a polygenic etiology [44,45,46, 107], although the extent of polygenicity appears to be less than that predicted for psychiatric disorders [107]. Recent studies have begun to disentangle whether the genetics of brain phenotypes overlap with that of psychiatric disorders by making use of their polygenic nature [108, 109].
Polygenic risk scoring (PRS) is one such analytical technique that exploits the polygenic nature of complex traits by generating a weighted sum of associated variants [106, 110, 111]. PRS uses variants of small effect (with p values below a given threshold), identified in a GWAS from a discovery dataset to predict disease status for each participant in an independent replication dataset [111]. In large-scale GWAS of schizophrenia, for example, the PRS now accounts for 18% of the variance observed [41]. PRS in imaging genomics has the potential advantage of addressing many confounders, such as the effects of medication and the disease itself through investigation of unaffected and at-risk individuals [112, 113]. For example, PRS for major depressive disorder (MDD; n = 18,749) has been associated with reduced cortical thickness in the left amygdala-medial prefrontal circuitry among healthy individuals (n = 438) of European descent [114].
However, as with other approaches, PRS is not without limitations. For example, an additive model of variant effects is assumed, disregarding potentially more-complex genetic interactions [115]. The predictive capacity of PRS is also largely dependent on the size of the discovery dataset (ideally greater than 2000 individuals), which is likely still underpowered in many instances [106]. Furthermore, PRS does not provide proportionate weight to biologically relevant genes for neural phenotypes as it is also subject to the confounding elements of GWAS emphasized earlier [57, 113, 116]. Thus, other approaches such as linkage disequilibrium score regression for genetic correlation (a technique that uses GWAS summary statistics to estimate the degree of genetic overlap between traits) [117], Bayesian-type analyses [118], and biologically informed multilocus profile scoring [119, 120] might be alternatives worth exploring, perhaps in conjunction with PRS [121]. More recently, an omnigenic model has been proposed—which takes into account the interconnected nature of cellular regulatory networks that can confound other polygenic models [57].
Linkage-disequilibrium score regression [117] did not identify genetic overlap between schizophrenia (33,636 cases, 43,008 controls) and subcortical volumes (n = 11,840 healthy controls), but provided a useful proof-of-principle of this approach [108]. A partitioning-based heritability analysis [122], which estimates the variance explained by all the SNPs on a chromosome or the whole genome rather than testing the association of particular SNPs with the trait, indicated that variants associated with schizophrenia (n = 1750) overlapped with eight brain structural phenotypes, including intracranial volume and superior frontal gyrus thickness [109]. Publicly available GWAS data for several other psychiatric disorders were also investigated and indicated that intracranial volume was enriched for variants associated with autism spectrum disorder (ASD), and right temporal pole surface area was enriched for variants associated with MDD, and left entorhinal cortex thickness showed enrichment for bipolar disorder risk variants [109]. These types of analyses confirm a common genetic basis between risk for altered brain structure and neuropsychiatric disorders [16].
Multivariate approaches
To explain more of the variance in gene-imaging findings, techniques for data-driven discovery using multivariate approaches have begun to emerge in this field. These techniques include methods such as independent component analysis (ICA) [123], canonical correlation analysis [124], sparse partial least squares [125], and sparse reduced-rank regression [126]. To date, the increased explanatory power provided by these approaches has mainly been shown in single datasets or relatively small studies—these often claim to identify significant associations at a genome-wide level [127,128,129]. Owing to the large number of input variables and parameters (many dimensions), often paired with limited data-points and split-sample training and testing from the same cohort, there can be concerns about overfitting and models that do not generalize. Thus, dimensionality reduction, in the imaging or genetic domain, is often necessary. Dimensionality-reduction techniques can group or cluster these large sets of variables (dimensions) in either domain; approaches guided by a priori knowledge might prove useful as the field advances [130]. Each multivariate approach has particular advantages and limitations. Data-driven multivariate techniques, such as ICA, in particular, can lead to sample-specific solutions that are difficult to replicate in independent datasets. The large datasets now available through collaborative efforts provide the opportunity to assess and compare the utility of these approaches [37]; on the other hand, larger datasets can also overcome the need for dimensionality-reduction methods if the sample sizes prove sufficient for mass univariate testing.
Emerging pathways
Understanding the pathways involved in brain development, structure, function, and plasticity will ultimately lead to an improved ability to navigate neuropsychiatric disease pathophysiology. Investigation of the signatures of selection affecting neuropsychiatric, behavioral, and brain phenotypes have indicated both recent and evolutionarily conserved polygenic adaptation, with enrichment in genes affecting neurodevelopment or immune pathways [131] (Table 1). Annotation of the loci associated with subcortical brain volumes has already identified an enrichment of genes related to neurodevelopment, synaptic signaling, ion transport and storage, axonal transport, neuronal apoptosis, and neural growth and differentiation [4, 15, 46] (Table 1). Studies have also implicated pleiotropy (a single locus that affects multiple phenotypes) amongst these loci [46]. Furthermore, many of the associated neurodevelopmental genes are conserved across species, providing a foundation for translational research in imaging genomics [46].
Advances in our concepts of brain connectivity can provide a useful framework for further integration of imaging and genomics data. Recent work has emphasized that hubs of neural connectivity are associated with transcriptional differences in genes affecting ATP synthesis and metabolism in mice [132], consistent with their high energy demands [132]. Analogous findings have been found in humans [133, 134]. Studies of the transcriptome and the metabolome, now curated by efforts such as the Allen Brain atlas [135], increasingly allow study of issues such as the relationship between resting-state functional connectivity and gene-expression profiles, with early work indicating enrichment in hubs of genes related to ion channels, synaptic activity, and ATP metabolism [136, 137].
Key considerations in imaging-genomic analyses
While imaging genomics has great potential, the limitations associated with both genetic [57, 138] and imaging [139] studies, as well as some unique concerns, deserve consideration. Here we discuss three important issues, namely (i) possible confounders of heritability estimates in imaging measures, (ii) the necessity of methodological harmonization for cross-site collaborations, and (iii) accounting for the multiple testing burden.
Environmental, physiological, and demographic influences can affect heritability estimates and measurements of brain-related features [72, 73, 140]. Most psychiatric disorders produce subtle changes in brain phenotypes and multiple potential confounding factors might obscure disease-related effects, limiting their utility as endophenotypes. Examples of such potential factors include motion [141, 142] and dehydration [143, 144], to name a few. Differences in data acquisition and analysis types might also contribute to variation between studies [145], particularly for small structures and grey-matter volumes [146,147,148]. These potential confounding factors can, however, be included as covariates and adjusted. This approach was used, for example, to control for the effects of height in the largest imaging-genetics meta-analysis of intracranial volume [45]. The distribution of these covariates can also be balanced between cases and controls. Furthermore, potential confounders can be mitigated by investigating healthy individuals only or a single ethnic group, sex, or age group, for example [149]. However, healthy individuals with certain genotypes might be more susceptible to certain confounding factors, such as smoking, which could lead to spurious associations [139].
Furthermore, caution should be taken when interpreting results from fMRI studies, owing to the dependence on quality of both the control and task of interest [150]. These tasks should improve sensitivity and power of genetic effects, adequately stimulate regions of interest, be appropriate for the disorder of interest, reliably evoke reactions amongst individuals, and highlight variability between them [150,151,152]. Resting-state fMRI studies also require consideration as these might be experienced differently between patients and controls [153]. Studies of unaffected siblings could be beneficial to minimize the potential confounders of disease on brain measures [154]. Meta-analytical approaches need to take the comparability of tasks into account, as apparently slight differences can considerably confound associations [155]. ENIGMA, for example, attempts to reduce these effects through predetermined protocols and criteria for study inclusion [37].
There is often a need to account for multiple testing in imaging genomics beyond that which is done in genetics alone. This is an important issue to emphasize [149, 156]. Studies performing a greater number of tests, especially genome-wide analyses [157] and multimodal and multivariate approaches [130], might require more-stringent corrections. Approaches to reduce the dimensions of these datasets are being developed and include the use of imaging or genetic clusters [66, 158,159,160,161,162] and machine learning methods [163]. However, replication studies and meta-analyses of highly harmonized studies remain the most reliable method for reducing false-positive associations [164].
Conclusions and future directions
The field of imaging genomics is moving forward in several research directions to overcome the initial lack of reproducible findings and to identify true findings that can be used in clinical practice. First, well-powered hypothesis-free genome-wide approaches remain key. Research groups are now routinely collaborating to ensure adequate power to investigate CNVs and epigenetic, gene–gene, and gene–environment interactions. Second, advances in both imaging and genetic technologies are being used to refine the brain–gene associations; next-generation sequencing (NGS) approaches now allow for more-in-depth investigation of the genome and deeper sequencing (whole-exome and genome); and more-refined brain mapping will ideally allow the field to localize genetic effects to specific tissue layers and subfields as opposed to global structural volumes. Third, replication attempts are crucial, and investigations in various population groups might validate associations and discover new targets that lend further insights into the biological pathways involved in these traits. Finally, specific initiatives to integrate neurogenetics and neuroimaging data for translation into clinical practice are being routinely advocated. These might include efforts in translational neuroscience [165], a systems-biology perspective [16, 166,167,168], and longitudinal data collection in community and clinical contexts [169].
Current psychiatric treatments have important limitations. First, many patients are refractory to treatment. For example, only approximately 60% of patients with depression achieve remission after either, or a combination of, psychotherapy and pharmacotherapy [170]. Second, clinical guidelines often focus on the “typical” patient, with relatively little ability to tailor individual treatments to the specific individual. Such limitations speak to the complex nature of the brain and of psychiatric disorders, and the multiple mechanisms that underlie the relevant phenotypes and dysfunctions. [20]. In order to progress into an era of personalized medicine, addressing the unique environmental exposures and genetic makeup of individuals [171], further efforts to improve statistical power and analyses are needed.
Ultimately, understanding the mechanisms involved in associated and interconnected pathways could lead to identification of biological markers for more-refined diagnostic assessment and new, more effective, and precise pharmacological targets [20, 171]. These goals can be fostered through continued efforts to strengthen collaboration and data sharing. Indeed, such efforts have led to a growing hope that findings in imaging genomics might well be translated into clinical practice [166,167,168]. The studies reviewed here provide important initial insights into the complex architecture of brain phenotypes; ongoing efforts in imaging genetics are well positioned to advance our understanding of the brain and of the underlying neurobiology of complex mental disorders, but, at the same time, continued and expanded efforts in neuroimaging genomics are required to ensure that this work has clinical impact.
Abbreviations
- ADNI:
-
Alzheimer's Disease Neuroimaging Initiative
- ATP:
-
Adenosine triphosphate
- CHARGE:
-
Cohorts for Heart and Aging Research in Genomic Epidemiology
- CNV:
-
Copy number variation
- DTI:
-
Diffusion-tensor imaging
- ENIGMA:
-
Enhancing Neuro Imaging Genetics through Meta-analysis
- fMRI:
-
Functional magnetic resonance imaging
- GWAS:
-
Genome-wide association study
- GxE:
-
Gene–environment interaction
- ICA:
-
Independent component analysis
- MDD:
-
Major depressive disorder
- MRI:
-
Magnetic resonance imaging
- PRS:
-
Polygenic risk scoring
- RDoC:
-
Research Domain Criteria project
References
Kovelman I. Neuroimaging methods. In: Hoff E, editor. Research methods in child language: a practical guide. Oxford, UK: Wiley-Blackwell; 2011. p. 43–59.
Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–6.
Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–9.
Hibar DP, Stein JL, Renteria ME. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–9.
Nicodemus KK, Callicott JH, Higier RG, Luna A, Nixon DC, Lipska BK, et al. Evidence of statistical epistasis between DISC1, CIT and NDEL1 impacting risk for schizophrenia: Biological validation with functional neuroimaging. Hum Genet. 2010;127:441–52.
Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A, et al. Stress-related methylation of the catechol-o-methyltransferase val158 allele predicts human prefrontal cognition and activity. J Neurosci. 2011;31:6692–8.
Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–95.
Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–8.
Roshchupkin GV, Gutman BA, Vernooij MW, Jahanshad N, Martin NG, Hofman A, et al. Heritability of the shape of subcortical brain structures in the general population. Nat Commun. 2016;7:13738.
Ge T, Reuter M, Winkler AM, Holmes AJ, Lee PH, Tirrell LS, et al. Multidimensional heritability analysis of neuroanatomical shape. Nat Commun. 2016;7:13291.
Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, et al. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010;107:1223–8.
Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–69.
Patel V, Chiang MC, Thompson PM, McMahon KL, De Zubicaray GI, Martin NG, et al. Scalar connectivity measures from fast-marching tractography reveal heritability of white matter architecture. 2010 7th IEEE International Symposium: Biomedical Imaging: From Nano to Macro. IEEE. 2010. p. 1109–12.
Jansen AG, Mous SE, White T, Posthuma D, Polderman TJC. What twin studies tell us about the heritability of brain development, morphology, and function: a review. Neuropsychol Rev. 2015;25:27–46.
Elliott L, Sharp K, Alfaro-Almagro F, Douaud G, Miller K, Marchini J, et al. The genetic basis of human brain structure and function: 1,262 genome-wide associations found from 3,144 GWAS of multimodal brain imaging phenotypes from 9,707 UK Biobank participants. bioRxiv. 2017. doi: https://doi.org/10.1101/178806.
Rose EJ, Donohoe G. Brain vs behavior: An effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull. 2013;39:518–26.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51.
World Health Organization. International Statistical Classification of Diseases and Related Health Problems (International Classification of Diseases); ICD-10, version:2010. 2010. http://apps.who.int/classifications/icd10/browse/2016/en. Accessed 15 Oct 2017.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126.
Lesch KP, Bengel D, Heils a, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31.
Lesch KP, Mössner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998;44:179–92.
Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3.
Kunugi H, Vallada HP, Sham PC, Hoda F, Arranz MJ, Li T, et al. Catechol-O-methyltransferase polymorphisms and schizophrenia: a transmission disequilibrium study in multiply affected families. Psychiatr Genet. 1997;7:97–101.
Li T, Ball D, Zhao J, Murray RM, Liu X, Sham PC, et al. Family-based linkage disequilibrium mapping using SNP marker haplotypes: application to a potential locus for schizophrenia at chromosome 22q11. Mol Psychiatry. 2000;5:77–84.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22.
Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, et al. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45:44–51.
Mechelli A, Tognin S, McGuire PK, Prata D, Sartori G, Fusar-Poli P, et al. Genetic vulnerability to affective psychopathology in childhood: a combined voxel-based morphometry and functional magnetic resonance imaging study. Biol Psychiatry. 2009;66:231–7.
Button KS, Ioannidis JP, Mokrysz C, Nosek B, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76.
de Vries YA, Roest AM, Franzen M, Munafò MR, Bastiaansen JA. Citation bias and selective focus on positive findings in the literature on the serotonin transporter gene (5-HTTLPR), life stress and depression. Psychol Med. 2016;46:2971–9.
Bastiaansen JA, Servaas MN, Marsman JBC, Ormel J, Nolte IM, Riese H, et al. Filling the gap: relationship between the serotonin-transporter-linked polymorphic region and amygdala activation. Psychol Sci. 2014;25:2058–66.
González-Castro TB, Hernández-Díaz Y, Juárez-Rojop IE, López-Narváez ML, Tovilla-Zárate CA, Fresan A. The role of a catechol-o-methyltransferase (COMT) Val158Met genetic polymorphism in schizophrenia: a systematic review and updated meta-analysis on 32,816 subjects. Neuromolecular Med. 2016;18:216–31.
Jahanshad N, Ganjgahi H, Bralten J, den Braber A, Faskowitz J, Knodt A, et al. Do candidate genes affect the brain’s white matter microstructure? Large-scale evaluation of 6,165 diffusion MRI scans. bioRxiv. 2017. https://www.biorxiv.org/content/early/2017/02/20/107987.
What is the PGC? Psychiatric Genomics Consortium. http://www.med.unc.edu/pgc. Accessed Sep 27 2017.
Cancer Genomics Consortium. https://www.cancergenomics.org/. Accessed Sep 27 2017.
Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium design of prospective meta-analyses of genome-wide association studies from 5 Cohorts. Circ Cardiovasc Genet. 2009;2:73–80.
Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. The ENIGMA Consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–82.
Schumann G, Loth E, Banaschewski T, Barbot a, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–39.
Adams HHH, Hilal S, Schwingenschuh P, Wittfeld K, van der Lee SJ, DeCarli C, et al. A priori collaboration in population imaging: The Uniform Neuro-Imaging of Virchow-Robin Spaces Enlargement consortium. Alzheimer’s Dement (Amst). 2015;1:513–20.
Cai N, Bigdeli TB, Kretzschmar W, Li Y, Liang J, Song L, et al. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–91.
Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Stein JL, Medland SE, Vasquez AA, Derrek P, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44:552–61.
Bis JC, DeCarli C, Smith AV, van der Lijn F, Crivello F, Fornage M, et al. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat Genet. 2012;44:545–51.
Hibar DP, Adams HHH, Jahanshad N, Chauhan G, Stein JL, Hofer E, et al. Novel genetic loci associated with hippocampal volume. Nat Commun. 2017;8:13624.
Adams HHH, Hibar DP, Chouraki V, Stein JL, Nyquist PA, Rentería ME, et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat Neurosci. 2016;19:1569–82.
Satizabal CL, Adams HHH, Hibar DP, White CC, Stein JL, Scholz M, et al. Genetic architecture of subcortical brain structures in over 40,000 individuals worldwide. bioRxiv. 2017. doi: https://doi.org/10.1101/173831.
Sklar P, Smoller JW, Fan J, Ferreira MAR, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–69.
Ferreira MAR, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–8.
Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2009;15:1–7.
Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sørensen KM, et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry. 2010;15:119–21.
Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–45.
Zhang Q, Shen Q, Xu Z, Chen M, Cheng L, Zhai J, et al. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology. 2012;37:677–84.
Paulus FM, Bedenbender J, Krach S, Pyka M, Krug A, Sommer J, et al. Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp. 2014;35:1190–200.
Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53.
Pickrell JK. Joint analysis of functional genomic data and genome-wide association studies of 18 human traits. Am J Hum Genet. 2014;94:559–73.
Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–6.
Boyle EA, Li YI, Pritchard JK. An expanded view of complex traits: from polygenic to omnigenic. Cell. 2017;169:1177–86.
Dalvie S, Koen N, Duncan L, Abbo C, Akena D, Atwoli L, et al. Large scale genetic research on neuropsychiatric disorders in african populations is needed. EBioMedicine. 2015;2:1259–61.
Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–52.
Ulfarsson MO, Walters GB, Gustafsson O, Steinberg S, Silva A, Doyle OM, et al. 15q11.2 CNV affects cognitive, structural and functional correlates of dyslexia and dyscalculia. Transl. Psychiatry. 2017;7:e1109.
Maillard AM, Ruef A, Pizzagalli F, Migliavacca E, Hippolyte L, Adaszewski S, et al. The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry. 2015;20:140–7.
Liu J, Ulloa A, Perrone-Bizzozero N, Yeo R, Chen J, Calhoun VD. A pilot study on collective effects of 22q13.31 deletions on gray matter concentration in schizophrenia. PLoS One. 2012;77(12):e52865.
Sonderby I, Doan NT, Gustafsson O, Hibar D, Djurovic S, Westlye LT, et al. Association of subcortical brain volumes with CNVS: a mega-analysis from The Enigma-CNV Working Group. Eur Neuropsychopharmacol. 2017;27:S422–3.
Carlborg O, Haley CS. Epistasis: too often neglected in complex trait studies? Nat Rev Genet. 2004;5:618–25.
Cordell HJ, Todd JA, Hill NJ, Lord CJ, Lyons PA, Peterson LB, et al. Statistical modeling of interlocus interactions in a complex disease: rejection of the multiplicative model of epistasis in type 1 diabetes. Genetics. 2001;158:357–67.
Chiang MC, Barysheva M, McMahon KL, de Zubicaray GI, Johnson K, Montgomery GW, et al. Gene network effects on brain microstructure and intellectual performance identified in 472 twins. J Neurosci. 2012;32:8732–45.
Schott BH, Assmann A, Schmierer P, Soch J, Erk S, Garbusow M, et al. Epistatic interaction of genetic depression risk variants in the human subgenual cingulate cortex during memory encoding. Transl Psychiatry. 2014;4, e372.
Papaleo F, Burdick MC, Callicott JH, Weinberger DR. Epistatic interaction between COMT and DTNBP1 modulates prefrontal function in mice and in humans. Mol Psychiatry. 2014;19:311–6.
Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R, et al. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67:991–1001.
Hibar DP, Stein JL, Jahanshad N, Kohannim O, Hua X, Toga AW, et al. Genome-wide interaction analysis reveals replicated epistatic effects on brain structure. Neurobiol Aging. 2015;36:S151–8.
Cordell HJ. Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009;10:392–404.
Rijsdijk FV, van Haren NEM, Picchioni MM, McDonald C, Toulopoulou T, Hulshoff Pol HE, et al. Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med. 2005;35:1399–409.
Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, et al. Genetic and environmental contributions to neonatal brain structure: a twin study. Hum Brain Mapp. 2010;31:1174–82.
Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47(3-4):226–61.
Halldorsdottir T, Binder EB. Gene × environment interactions: from molecular mechanisms to behavior. Annu Rev Psychol. 2017;68:215–41.
Mandelli L, Marino E, Pirovano A, Calati R, Zanardi R, Colombo C, et al. Interaction between SERTPR and stressful life events on response to antidepressant treatment. Eur Neuropsychopharmacol. 2009;19:64–7.
Keers R, Uher R, Huezo-Diaz P, Smith R, Jaffee S, Rietschel M, et al. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenomics J. 2011;11:138–45.
Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22:239–58.
Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuro-Psychopharmacology Biol Psychiatry. 2013;45:183–94.
Eley TC, Hudson JL, Creswell C, Tropeano M, Lester KJ, Cooper P, et al. Therapygenetics: the 5HTTLPR and response to psychological therapy. Mol Psychiatry. 2012;17:236–7.
Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, et al. Real-time fMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9:e88785.
Hamilton JP, Glover GH, Bagarinao E, Chang C, Mackey S, Sacchet MD, et al. Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Res. 2016;249:91–6.
Aas M, Haukvik UK, Djurovic S, Bergmann Ø, Athanasiu L, Tesli MS, et al. BDNF val66met modulates the association between childhood trauma, cognitive and brain abnormalities in psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:181–8.
Carballedo A, Morris D, Zill P, Fahey C, Reinhold E, Meisenzahl E, et al. Brain-derived neurotrophic factor Val66Met polymorphism and early life adversity affect hippocampal volume. Am J Med Genet B NeuroPsychiatr Genet. 2013;162:183–90.
Gerritsen L, Tendolkar I, Franke B, Vasquez a a, Kooijman S, Buitelaar J, et al. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol Psychiatry. 2012;17:597–603.
Ho B-C, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr Res. 2011;128:66–75.
Onwuameze OE, Nam KW, Epping EA, Wassink TH, Ziebell S, Andreasen NC, et al. MAPK14 and CNR1 gene variant interactions: effects on brain volume deficits in schizophrenia patients with marijuana misuse. Psychol Med. 2013;43:619–31.
Tozzi L, Carballedo A, Wetterling F, McCarthy H, O’Keane V, Gill M, et al. Single-nucleotide polymorphism of the FKBP5 gene and childhood maltreatment as predictors of structural changes in brain areas involved in emotional processing in depression. Neuropsychopharmacology. 2016;41:487–97.
Grabe HJ, Wittfeld K, van der Auwera S, Janowitz D, Hegenscheid K, Habes M, et al. Effect of the interaction between childhood abuse and rs1360780 of the FKBP5 gene on gray matter volume in a general population sample. Hum Brain Mapp. 2016;37:1602–13.
Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–9.
Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, et al. Candidate gene-environment interaction research: reflections and recommendations. Perspect Psychol Sci. 2015;10:37–59.
Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–46.
Caspi A, Hariri AR, Andrew H, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27.
Swartz JR, Hariri AR, Williamson DE. An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Mol Psychiatry. 2016;22:1–6.
Frodl T, Tozzi L, Farrell C, Doolin K, O’Keane V, Pomares F, et al. Association of stress hormone system, epigenetics and imaging. Eur Psychiatry. 2017;41:S19–20.
Walton E, Hass J, Liu J, Roffman JL, Bernardoni F, Roessner V, et al. Correspondence of DNA methylation between blood and brain tissue and its application to schizophrenia research. Schizophr Bull. 2016;42:406–14.
Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–30.
Bujold D, Morais DA de L, Gauthier C, Côté C, Caron M, Kwan T, et al. The International Human Epigenome Consortium Data Portal. Cell Syst. 2016;3:496–9. e2.
Bigos KL, Trangle J, Weinberger DR. Brain cloud and clinical research. Schizophr Bull. 2013;39:S97.
Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43.
Nikolova YS, Hariri AR. Can we observe epigenetic effects on human brain function? Trends Cogn Sci. 2015;19:366–73.
Desrivières S, Jia T, Ruggeri B, Liu Y, Sakristan D, Syvänen A-C, et al. Identifying epigenetic markers affecting the brain. 22nd Annual Meeting of the Organization for Human Brain Mapp. Geneva; 2016. http://www.humanbrainmapping.org/files/2016/OHBM_2016_Geneva_Abstracts.pdf. Accessed 28 Sep 2017.
Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, et al. Largest GWAS of PTSD (N = 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. 2017. doi:10.1038/mp.2017.77.
Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–7.
Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9.
Dudbridge F. Power and predictive accuracy of polygenic scores. PLoS Genet. 2013;9:e1003348.
Chen C-H, Peng Q, Schork AJ, Lo M-T, Fan C-C, Wang Y, et al. Large-scale genomics unveil polygenic architecture of human cortical surface area. Nat Commun. 2015;6:7549.
Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJE, et al. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19:420–31.
Lee PH, Baker JT, Holmes AJ, Jahanshad N, Ge T, Jung J-Y, et al. Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Mol Psychiatry. 2016;21:1680–9.
Evans DM, Visscher PM, Wray NR. Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum Mol Genet. 2009;18:3525–31.
Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;10:8192.
Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–20.
Bogdan R, Salmeron BJ, Carey CE, Agrawal A, Calhoun VD, Garavan H, et al. Imaging genetics and genomics in psychiatry: a critical review of progress and potential. Biol Psychiatry. 2017;82:165–75.
Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, et al. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci. 2012;32:18087–100.
Hill WG, Goddard ME, Visscher PM. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 2008;4:e1000008.
Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, et al. DSM-5 field trials in the United States and Canada, part II: Test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59–70.
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Vilhjalmsson BJ, Yang J, Finucane HK, Gusev A, Lindstrom S, Ripke S, et al. Modeling linkage disequilibrium increases accuracy of polygenic risk scores. Am J Hum Genet. 2015;97:576–92.
Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–7.
Bogdan R, Pagliaccio D, Baranger DA, Hariri AR. Genetic moderation of stress effects on corticolimbic circuitry. Neuropsychopharmacology. 2015;41:275–96.
Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, et al. Genetic differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. Neuron. 2015;86:1189–202.
Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82.
Chen J, Calhoun VD, Pearlson GD, Perrone-Bizzozero N, Sui J, Turner JA, et al. Guided exploration of genomic risk for gray matter abnormalities in schizophrenia using parallel independent component analysis with reference. Neuroimage. 2013;83:384–96.
Li F, Huang X, Tang W, Yang Y, Li B, Kemp GJ, et al. Multivariate pattern analysis of DTI reveals differential white matter in individuals with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2643–51.
Le Floch E, Guillemot V, Frouin V, Pinel P, Lalanne C, Trinchera L, et al. Significant correlation between a set of genetic polymorphisms and a functional brain network revealed by feature selection and sparse Partial Least Squares. Neuroimage. 2012;63:11–24.
Vounou M, Nichols TE, Montana G. Discovering genetic associations with high-dimensional neuroimaging phenotypes: a sparse reduced-rank regression approach. Neuroimage. 2010;53:1147–59.
Ge T, Feng J, Hibar DP, Thompson PM, Nichols TE. Increasing power for voxel-wise genome-wide association studies: The random field theory, least square kernel machines and fast permutation procedures. Neuroimage. 2012;63:858–73.
Chen J, Calhoun VD, Pearlson GD, Ehrlich S, Turner JA, Ho BC, et al. Multifaceted genomic risk for brain function in schizophrenia. Neuroimage. 2012;61:866–75.
Jahanshad N, Rajagopalan P, Hua X, Hibar DP, Nir TM, Toga AW, et al. Genome-wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity. Proc Natl Acad Sci U S A. 2013;110:4768–73.
Liu J, Calhoun VD. A review of multivariate analyses in imaging genetics. Front Neuroinform. 2014;8:29.
Beiter ER, Khramtsova EA, Merwe C van der, Chimusa ER, Simonti C, Stein J, et al. Polygenic selection underlies evolution of human brain structure and behavioral traits. bioRxiv. 2017. doi: https://doi.org/10.1101/164707.
Fulcher BD, Fornito A. A transcriptional signature of hub connectivity in the mouse connectome. Proc Natl Acad Sci U S A. 2016;113:1435–40.
Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–72.
Vértes PE, Rittman T, Whitaker KJ, Romero-Garcia R, Váša F, Kitzbichler MG, et al. Gene transcription profiles associated with inter-modular hubs and connection distance in human functional magnetic resonance imaging networks. Philos Trans R Soc Lond B Biol Sci. 2016;371:735–69.
Shen EH, Overly CC, Jones AR. The Allen Human Brain Atlas. Comprehensive gene expression mapping of the human brain. Trends Neurosci. 2012;35:711–4.
Wang GZ, Belgard TG, Mao D, Chen L, Berto S, Preuss TM, et al. Correspondence between resting-state activity and brain gene expression. Neuron. 2015;88:659–66.
Richiardi J, Altmann A, Jonas R. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:11–4.
Korte A, Farlow A. The advantages and limitations of trait analysis with GWAS: a review. Plant Methods. 2013;9:29.
Weinberger DR, Radulescu E. Finding the elusive psychiatric ‘lesion’ with 21st-century neuroanatomy: a note of caution. Am J Psychiatry. 2016;173:27–33.
Turkheimer E. Weak genetic explanation 20 years later. Perspect Psychol Sci. 2016;11:24–8.
Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–48.
Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015;107:107–15.
Hajnal JV, Saeed N, Oatridge A, Williams EJ, Young IR, Bydder GM. Detection of subtle brain changes using subvoxel registration and subtraction of serial MR images. J Comput Assist Tomogr. 1995;19:677–91.
Streitbürger DP, Möller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7:e44195.
Brent BK, Thermenos HW, Keshavan MS, Seidman LJ. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia. A review of structural MRI findings. Child Adolesc Psychiatr Clin N Am. 2013;22:689–714.
Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–92.
Schnack HG, Van Haren NEM, Brouwer RM, Van Baal GCM, Picchioni M, Weisbrod M, et al. Mapping reliability in multicenter MRI: voxel-based morphometry and cortical thickness. Hum Brain Mapp. 2010;31:1967–82.
Shokouhi M, Barnes A, Suckling J, Moorhead TW, Brennan D, Job D, et al. Assessment of the impact of the scanner-related factors on brain morphometry analysis with Brainvisa. BMC Med Imaging. 2011;11:23.
Bigos KL, Weinberger DR. Imaging genetics—days of future past. Neuroimage. 2010;53:804–9.
Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78.
Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–32.
Buckner RL, Hrienen FM, Yeo TBT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Rev Neurosci. 2013;16:832–7.
Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, et al. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci U S A. 2012;109:3131–6.
Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Curr Opin Genet Dev. 2011;21:340–8.
Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: Meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6(10):e25322.
Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–61.
Hoggart CJ, Clark TG, De Iorio M, Whittaker JC, Balding DJ. Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol. 2008;32:179–85.
Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk to disease from genome-wide association studies. Genome Res. 2007;17:1520–8.
Chiang MC, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, et al. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage. 2011;54:2308–17.
Chen CH, Panizzon MS, Eyler LT, Jernigan TL, Thompson W, Fennema-Notestine C, et al. Genetic influences on cortical regionalization in the human brain. Neuron. 2011;72:537–44.
Chen C-H, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, et al. Hierarchical genetic organization of human cortical surface area. Science. 2012;335:1634–6.
Wu MC, Kraft P, Epstein MP, Taylor DM, Chanock SJ, Hunter DJ, et al. Powerful SNP-set analysis for case-control genome-wide association studies. Am J Hum Genet. 2010;86:929–42.
Yang H, Liu J, Sui J, Pearlson G, Calhoun VD. A hybrid machine learning method for fusing fMRI and genetic data: combining both improves classification of schizophrenia. Front Hum Neurosci. 2010;4:192.
Carter CS, Bearden CE, Bullmore ET, Geschwind DH, Glahn DC, Gur RE, et al. Enhancing the informativeness and replicability of imaging genomics studies. Biol Psychiatry. 2017;82(3):157–64.
Woods RP, Fears SC, Jorgensen MJ, Fairbanks LA, Toga AW, Freimer NB. A web-based brain atlas of the vervet monkey, Chlorocebus aethiops. Neuroimage. 2011;54:1872–80.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83.
Chang H, Hoshina N, Zhang C, Ma Y, Cao H, Wang Y, et al. The protocadherin 17 gene affects cognition, personality, amygdala structure and function, synapse development and risk of major mood disorders. Mol Psychiatry. 2017;231:1–13.
Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2:150031.
Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–51.
Holtzheimer PE, Mayberg HS. Stuck in a rut: Rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9.
Ozomaro U, Wahlestedt C, Nemeroff CB. Personalized medicine in psychiatry: problems and promises. BMC Med. 2013;11:132.
Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:709–16.
Potkin SG, Turner JA, Fallon JA, Lakatos A, Keator DB, Guffanti G, et al. Gene discovery through imaging genetics: identification of two novel genes associated with schizophrenia. Mol Psychiatry. 2008;14:416–28.
Liu J, Pearlson G, Windemuth A, Ruano G, Perrone-Bizzozero NI, Calhoun V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp. 2009;30:241–55.
Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605.
Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806–12.
Hibar DP, Westlye LT, van Erp TGM, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;21:1710–6.
van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53.
Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2017. doi:10.1038/mp.2017.170.
Ramaker RC, Bowling KM, Lasseigne BN, Hagenauer MH, Hardigan AA, Davis NS, et al. Post-mortem molecular profiling of three psychiatric disorders. Genome Med. 2017;9:72.
Funding
DJS is supported by the SA Medical Research Council. NAG is supported by the Claude Leon Foundation. PMT and NJ are supported in part by the National Institutes of Health Big Data to Knowledge program U54 EB020403 and the Kavli Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mufford, M.S., Stein, D.J., Dalvie, S. et al. Neuroimaging genomics in psychiatry—a translational approach. Genome Med 9, 102 (2017). https://doi.org/10.1186/s13073-017-0496-z
Published:
DOI: https://doi.org/10.1186/s13073-017-0496-z