Abstract
Background
Recurrent Clostridium difficile infection (CDI) remains problematic, with up to 30 % of individuals diagnosed with primary CDI experiencing at least one episode of recurrence. The success of microbial-based therapeutics, such as fecal microbiota transplantation, for the treatment of recurrent CDI underscores the importance of restoring the microbiota. However, few studies have looked at the microbial factors that contribute to the development of recurrent disease. Here we compare microbial changes over time in patients with or without recurrence to identify microbial signatures associated with the development of recurrence.
Methods
We used 16S rRNA-encoding gene sequence analysis to compare the fecal microbiota of 93 patients with recurrent and nonrecurrent CDI, sampled longitudinally. Cross-group and intra-individual differences in microbial community diversity and similarity were compared prior to the development of recurrent disease and over time.
Results
Samples from these patient groups exhibited variable community profiles, clustering into four distinct community groups. Cross-group comparison of the index sample collected from patients that did or did not develop recurrence revealed differences in diversity and community structure (analysis of molecular variance, p < 0.05). Intra-individual comparisons of the microbiota were more informative and samples from recurrent patients were less likely to recover in diversity (Chi-square test, p < 0.005), exhibiting less community similarity overall (Kruskal–Wallis test, p < 0.05). Interestingly, patients with severe disease harbored a significantly less diverse community, a trend that was observed across both nonrecurrent and recurrent patient groups (Wilcoxon test, p < 0.05).
Conclusions
To date, this study represents one of the largest studies focused on the relationship between predictive signals from the gut microbiota and the development of recurrent CDI. Our data demonstrate that specific microbiota-derived characteristics associate with disease severity and recurrence and that future studies could incorporate these characteristics into predictive models.
Similar content being viewed by others
Background
Clostridium difficile infection (CDI) has become one of the most prevalent hospital-acquired infections in recent years [1]. Adding to the impact of CDI, recurrent disease affects 20–30 % of patients after an initial diagnosis [2]. Although multiple factors are associated with recurrence, the exact contributions of these factors to the development of recurrence in certain patients remain undetermined. Strains of C. difficile belonging to the 027 ribotype have been associated with higher rates of recurrence at some institutions [3]. Similarly, certain antibiotic treatment options have also been associated with more recurrence [4]. Failure to develop an adaptive immune response against the C. difficile toxins has also been associated with increased risk for recurrence [5]. Since the relationship between the gastrointestinal microbiota, i.e., the indigenous microbes of the gastrointestinal tract, and the development of CDI has been well-established, specific differences in the gastrointestinal microbiota may contribute to susceptibility to recurrence.
The importance of the gut microbiota in recovering from a recurrent CDI cycle has been previously demonstrated. Fecal microbiota transplantation (FMT) is one of the most effective therapies for recurrent CDI, with an over 90 % success rate [6–8]. Several studies have observed a significant recovery in the diversity of the microbial community following FMT, although the specific microbes that contribute to recovery are variable between patients [9–11]. However, studies have not followed CDI patients over time to compare those that do or do not develop recurrent disease.
The objective of this study was to compare the gastrointestinal microbiota of patients diagnosed with CDI, with or without recurrence. We examined the microbiota at initial diagnosis (index) time points, as well as the longitudinal changes in the microbiota of patients over time. We observed marked differences in the recovery of recurrent patients compared with nonrecurrent cases. This study represents the most comprehensive exploration of the microbiota during development of recurrent CDI.
Methods
Study design, patient population, and sample collection
Fecal samples for this study were selected retrospectively from a biorepository created as part of the NIH Enterics Research Investigational Network (ERIN) study, and encompassed patients who received care at the University of Michigan Health System (UMHS) from October 2010 to June 2014. The ERIN study obtained the index fecal specimens through collection of discarded fecal material after C. difficile testing by the clinical microbiology laboratory and follow-up specimens through informed consent of patients who were aged over 18 years and not pregnant. Patients included in this study were selected based on the availability of multiple fecal samples following an initial CDI diagnosis (Fig. 1). The number of longitudinal samples varied between patients, as did the interval between individual samples (Table 1; Additional file 1: Table S1). Clinical data were extracted from the electronic medical record through both automated query and manual chart review by infectious disease clinicians (KR and DM) (Additional file 1: Table S1).
Study design and sample collection timeline. Relative timeline (days) of samples collected from patients diagnosed with initial Clostridium difficile infection (CDI) (index sample = 0 days) categorized into three patient groups (nonrecurrent, recurrent, and reinfected). Patients who did not develop recurrence (n = 42) remained free of a subsequent CDI diagnosis. Patients with recurrent disease (n = 32) were diagnosed with CDI (positive clinical lab result) 14–56 days following index sample collection. Patients diagnosed with another CDI index outside of the recurrence window (>56 days) were considered reinfected (n = 19) NA = test not available
Samples were collected in Cary-Blair transport media as per hospital protocol during the study period. Clinical testing for CDI was performed at the discretion of the patients’ inpatient care team, following institutional and national guidelines that recommend testing of only symptomatic patients [12]. Testing for CDI in the clinical lab followed a two-stage algorithm employing the C. diff Quik Chek Complete (TechLab, Blacksburg, Virginia, USA), which detects glutamate dehydrogenase (GDH) antigen and toxins A and B via enzyme immunoassay, with confirmation by polymerase chain reaction (PCR) for the C. difficile toxin gene, tcdB, if the toxin and GDH results from the Quik Chek test were discordant. The C. difficile clinical status (“positive” or “negative”) used in this study is based on the presence of a positive test and symptoms and is listed under “clinical lab result” in Additional file 1: Table S1. Index, recurrent, and reinfected cases were defined in conjunction with a positive Quik Chek result with a chart review confirming symptoms of CDI and the patient’s medical history. The “sample status” definition used in Additional file 1: Table S1 and Fig. 6 is based on the Infectious Diseases Society of America (IDSA) surveillance definitions [13] applied to each patient, resulting in categorization of the patient into nonrecurrent, recurrent, or reinfected clinical groups as follows: index (first positive sample collected in study), recurrence (subsequent positive sample 15–56 days from a previous positive sample), reinfection (subsequent positive sample >56 days from a previous positive sample), treatment (sample collected within 14 days of a positive sample, during antibiotic treatment), and recovery (nonrecurrent, non-reinfected sample collected >14 days from a positive sample). Disease severity (based on the IDSA criteria of a circulating white blood cell count >15,000 cells/mm3 and/or a serum creatinine >1.5 times the baseline value) [12] was recorded when available (Table 1; Additional file 1: Table S1). Following routine testing in the clinical laboratory, excess sample was transported to the research laboratory and stored at −80 °C before further processing.
We isolated C. difficile from each sample as described previously [14, 15]. Fecal samples were grown in taurocholate cycloserine cefoxitin fructose agar (TCCFA) media anaerobically overnight to enrich for C. difficile spores, then plated on TCCFA media to isolate single C. difficile colonies. The ribotypes of single C. difficile isolates were obtained using a high-throughput ribotyping protocol, previously validated at multiple centers [14]. Results from these analyses are listed in Additional file 1: Table S1 under “plating results” and “ribotype”.
DNA extraction and 16S rRNA sequencing
Total fecal DNA was extracted from 200–300 μl fecal content using the MoBio PowerMag soil isolation kit optimized for the epMotion 5075 TMX (MoBio Laboratories, #271004EP; Eppendorf) using the manufacturer’s instructions, which includes a mechanical bead-beating step. The University of Michigan Host Microbiome core prepped DNA libraries as previously described [16]. In brief, amplification of the 16S V4 region was accomplished using specific barcoded dual index primers as described in Kozich et al. [17]. The PCR reaction included the following: 5 μl of 4 μM stock combined primer set, 0.15 μl of Accuprime High-Fidelity Taq with 2 μl of 10× Accuprime PCR II buffer (Life Technologies, #12346094), 11.85 μl of PCR-grade water, and 1 μl of template. The PCR cycling conditions were as follows: 95 °C for 2 minutes, 30 cycles of 95 °C for 20 s, 55 °C for 15 s, and 72 °C for 5 minutes, and 10 minutes at 72 °C. The DNA library plates were normalized with a SequelPrep normalization kit (Life Technologies, #A10510-01) and pooled. The pooled concentration was quantified using the Kapa Biosystems Library Quantification kit for Illumina platforms (KapaBiosystems, #KK4854) and amplicon size was determined using the Agilent Bioanalyzer high-sensitivity DNA analysis kit (#5067-4626). The MiSeq Reagent 222 kit V2 (#MS-102-2003) was used to sequence the amplicons (500 total cycles) with modifications for the primer set. Illumina’s protocol for library preparation was used for 2 nM libraries, with a final loading concentration of 4 pM spiked with 10 % PhiX for diversity. Paired-end reads of FASTQ files for all samples are available in the Sequence Read Archive under BioProject PRJNA307992 (SRP068473).
Data processing and analysis
Detailed commands for data processing, presentation, and statistical analysis are available at https://github.com/aseekatz/ERIN.recurrence. Raw sequence files were processed using mothur v1.34.4 [18]. Sequences were trimmed, aligned, and binned, using UCHIME to remove chimeric sequences [19]. A mothur-adapted version of the SILVA rRNA database project (release v119) was used to align the V4 region [20]. Samples with less than 1400 reads were discarded. Sequences were taxonomically classified at 80 % bootstrap minimum using the Wang method to the mothur-adapted RDP database (v10) [21]. Standard and loadable R packages (R Foundation for Statistical Computing, Vienna, Austria, v3.1.0) were used to process the data following processing in mothur. The Partitioning Around Medoids (PAM) clustering algorithm was used to cluster samples into community clusters based on the Jensen–Shannon divergence from phylotype relative abundance in R as conducted previously [22] using the silhouette score to determine the optimal number of clusters (S(i) = 0.26, four clusters). A 97 % cutoff was used to bin sequences into operational taxonomic units (OTUs) in mothur for downstream analyses. The inverse Simpson index (λ), the Yue and Clayton dissimilarity index (θ YC ) [23], and principal coordinates analysis (PCoA) of θ YC distance were calculated in mothur using OTU abundance. Results were plotted using R. A heatmap of the relative abundance of dominant OTUs was visualized using the R package gplots [24]. Standard R commands were used to visualize the results from linear discriminant analysis (LDA) effect size (LEfSe) in nonrecurrent/recurrent patients or clinically negative/positive samples [25].
Statistical analysis
Wilcoxon rank sum tests were used to determine significance of binary group comparisons for diversity λ and community dissimilarity θ YC . The Kruskal–Wallis was used for comparison of three or more groups. Analysis of molecular variance (AMOVA) was used to compare group communities, as directed in mothur [26]. To compare diversity λ between nonrecurrent and recurrent patients, a generalized estimating equation (GEE) model was applied [27]. The R package “geepack” was used to calculate the model, using a first order autoregressive correlation structure and a binomial link-logit family specification [28]. A Chi-squared test was performed to determine distribution of sample categories in the four identified microbial community type clusters and for inference about the variable coefficients’ point estimates from GEE.
Results
Distinct microbial community features are present in the feces of patients with severe C. difficile infection but are not with recurrent infection
Following an initial diagnosis of CDI (index sample), longitudinal fecal samples were collected from patients with nonrecurrent and recurrent disease and patients who were reinfected with C. difficile past the 56-day window of the recurrence definition, as described in “Methods” (Fig. 1, Table 1). The fecal microbiota from each sample was examined by 16S rRNA-encoding gene sequence analysis.
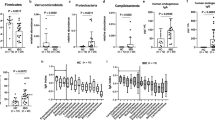
To determine the microbial community membership and investigate shared similarities or differences within the fecal microbiota of patients with or without recurrence or reinfection, we examined the phylotypes, or genus-level taxonomic classification, of the microbial community. We employed a previously used method, Partitioning Around Medoids (PAM), clustering on the taxonomic classification of phylotypes to identify distinct community clusters [22, 29]. Investigation of the community membership in samples from all patient groups revealed variable community profiles and dominance by specific organisms as represented by OTUs in patient samples (Fig. 2). Clustering (mean S(i) = 0.26) of all samples resulted in four major community clusters (Fig. 2): a high-diversity cluster defined by a high relative abundance of one of two Proteobacteria members (cluster 1); a cluster of samples rich in Bacteroidetes (cluster 3); and two low-diversity clusters characterized by Enterococcus (cluster 4) or an Enterobacteriaceae OTU (cluster 2).
Samples clustered into four major community profiles. The relative abundance of the 40 most abundant operational taxonomic units (OTUs), with classification to the genus level and organized by bacterial phylum, is shown in columns. Samples were binned into four major clusters using the Partitioning Around Medoids (PAM) algorithm based on the Jensen–Shannon divergence. The mean inverse Simpson index (λ) per cluster is shown on the left axis (samples). Sample categorization on the left axis is based on the following classifications: patient group category (nonrecurrent, recurrent, or reinfected); clinical lab results (Quik Chek, positive or negative); C. difficile cultivation results (positive or negative); and disease severity (severe or non-severe) at sample collection during a CDI diagnosis NA = text result not available
Cluster 2 contained a disproportionate amount of samples from nonrecurrent, recurrent, and reinfected samples compared with clusters 3 and 4 (Chi-square, p < 0.05); however, comparison of the proportion of index samples from each patient within the patient groups was not significant. When comparing the proportion of samples that were negative or positive for C. difficile using clinical lab results, none of the clusters was significantly disproportionate. However, when using cultivation to determine C. difficile status, cluster 4 contained a disproportionate amount of negative samples compared with clusters 2 and 3 (Chi-square, p < 0.05). Interestingly, samples during a severe diagnosis were also significantly overrepresented in cluster 4 compared with cluster 3 (Chi-square, p < 0.05).
In addition to clustering samples by overall community membership, we identified differentially abundant OTUs using linear discriminant analysis (LDA) effect size (LEfSe) [25]. LEfSe revealed five differentially represented OTUs in samples that were positive or negative for C. difficile based on University of Michigan Health System clinical lab results (n = 204; Fig. 3a). One of these OTUs, OTU12, classified to Clostridium XI, which includes C. difficile. While Clostridium XI can include other clostridial species in addition to C. difficile, this was the only differentially abundant OTU identified when the index (initial) samples from recurrent and nonrecurrent patients was compared using LEfSe (n = 93), suggesting a higher burden of Clostridium XI, potentially C. difficile, is detectable in recurrent patients at initial diagnosis (Additional file 2: Figure S1). LEfSe comparison of samples from patients with severe or non-severe disease at initial disease (using only index samples for which a severity score was available, n = 86) revealed seven differentially abundant OTUs with little overlap between differentially abundant OTUs between positive and negative samples (Fig. 3b). This suggests that within samples positive for C. difficile, patient metadata such as severity may be correlated to several different community structures.
Differentially abundant members of the microbiota in patients with C. difficile infection. The mean relative abundance plus standard error (se) of differentially abundant operational taxonomic units (OTUs) identified by linear discriminant analysis (LDA) effect size (LEfSe) in (a) samples that tested positive or negative for C. difficile by the clinical laboratory (Quik Chek) or (b) severe or non-severe samples. OTUs that were overrepresented in the specified groups are color-coded by the respective group in each panel
Microbial diversity at initial CDI diagnosis is decreased in patients with severe or recurrent disease
The diversity of the fecal microbiota community in the initial sample at diagnosis collected from nonrecurrent, recurrent, and reinfected patients was compared by calculating the inverse Simpson index (λ; n = 42, n = 32, n = 19, respectively). There was no difference in diversity when patients were classified based on clinical lab results (negative or positive for C. difficile), antibiotic exposure prior to initial diagnosis, or prior CDI history (Additional file 3: Figure S2). However, the fecal microbiota at initial diagnosis (index sample) in patients with recurrence trended towards a lower diversity compared with patients with nonrecurrent disease (Fig. 4a; Kruskal–Wallis test, p = 0.10). Furthermore, samples collected from patients with severe disease at diagnosis had lower fecal microbiota diversity compared with those without severe disease (Fig. 4b; Wilcoxon test, p = 0.022). Comparison of samples collected during severe or non-severe disease within the nonrecurrent, recurrent, and reinfected patient groups followed a similar trend; patients with severe CDI at the time of sample collection within each group exhibited lower diversity (Additional file 3: Figure S2).
Fecal microbial diversity during initial C. difficile infection. The inverse Simpson index (λ) of the microbiota in (a) index samples collected at initial C. difficile infection (CDI) diagnosis in nonrecurrent (n = 42), recurrent (n = 32), and reinfected (n = 19) patients (Kruskal–Wallis, not significant (ns)) and (b) index samples from patients diagnosed with severe (n = 36) or non-severe (n = 50) CDI (Wilcoxon test, p = 0.022)
Investigation of the microbiota diversity over time within each of the patient groups revealed time-dependent differences between patients with or without recurrence. To account for the inherent correlation present in repeated measures data, we utilized a generalized estimating equation (GEE) model to examine whether diversity (λ) was increased in nonrecurrent patients over time compared with recurrent patients. In this model, both time and repeated sampling are accounted for. We found that diversity and sampling across time were correlated in nonrecurrent patients, suggesting that as diversity increases across sampling time, recurrence is less likely to occur (p < 0.0013). In patients with recurrent disease and patients reinfected with C. difficile, no such increase in diversity across time was observed, suggesting that individual recovery of diversity is distinct in nonrecurrent patients compared with recurrent patients.
The fecal microbiota community is more dynamic within patients without recurrence
To investigate the inter- and intra-individual similarities of the fecal community within the patients and their groups, we calculated the beta diversity using the Yue and Clayton distance (θ YC ), a measure of similarity that accounts for abundance [23]. Principal coordinates analysis (PCoA) based on the θ YC revealed significant differences between samples from recurrent, nonrecurrent, and reinfected patients using analysis of molecular variance (AMOVA) (Fig. 5a; p < 0.016) [26]. Comparison of the index samples (initial sample) from each patient category also trended towards being significantly different (Additional file 4: Figure S3; p < 0.068). We also observed significant differences between samples that tested positive or negative for C. difficile based on clinical results (Fig. 5b; p < 0.015) and using cultivation (Additional file 4: Figure S3; p < 0.001). A biplot of the correlating OTUs towards PCoA axes 1 and 2 revealed four OTUs responsible for opposing directions of the PCoA-determined communities: OTU4 (classified as Escherichia), OTU2 (classified as Enterococcus), and OTU3/OTU4 (both classified as Bacteroides) (Fig. 5).
Community structure of patients with or without recurrent C. difficile infection. Principal coordinates analysis (PCoA) was used to plot the Yue and Clayton dissimilarity index (θ YC ). a The community structure of the microbiota in samples from nonrecurrent, recurrent, and reinfected patients (analysis of molecular variance (AMOVA), p = 0.016). b The community structure of samples positive or negative for C. difficile as determined by the clinical lab using Quik Chek (AMOVA, p = 0.015)
We next investigated how intra-individual similarity changed over time. We observed that all of the longitudinal samples collected from a given recurrent patient were more similar (θ YC , comparing only intra-individual samples) compared with the longitudinal samples from a nonrecurrent or reinfected patient (Fig. 6a; Kruskal–Wallis, p < 0.025). We compared the community dissimilarity θ YC within each patient throughout consecutive sampling. Sequential comparison of changes throughout time did not reveal major differences between recurrent and nonrecurrent patients (Additional file 5: Figure S4). However, consecutive sampling does not necessarily take into account the variability in each patient’s clinical history, such as a change from index sampling (initial diagnosis) to subsequent recovery (negative for C. difficile) or recurrence (a second positive). To account for the variability in the clinical status of the patient throughout sampling, we annotated each sample status to reflect the patient medical history as follows: index, recurrence, reinfection, treatment, recovery (see “Methods” section for detailed definitions) (Additional file 1: Table S1). We observed greater intra-individual similarity within the recurrent group when a patient’s index sample was compared with another recurrence or reinfection, as well as recovery or treatment. Nonrecurrent patients were more likely to exhibit more dissimilarity, suggesting changes in the microbial community, during recovery and treatment phases.
Intra-individual similarity of the microbiota in patients with or without recurrent C. difficile infection. The microbial community similarity within patients was compared using the Yue and Clayton dissimilarity index (θ YC ). a Intra-individual similarity was lower in patients with recurrence compared with patients without recurrence or reinfected with C. difficile (Kruskal–Wallis test, p = 0.025). b Microbial community similarity of the index sample from a patient was compared with different stages of clinical diagnosis in nonrecurrent, recurrent, and reinfected patients: to recovery (nonrecurrent, non-reinfected samples >14 days of a positive sample), to recurrence (subsequent positive sample within 14–56 days), reinfection (subsequent positive sample >56 days), and during treatment (sample collected within 14 days of a positive sample) (Kruskal–Wallis, not significant). ns not significant
Discussion
This study represents one of the first longitudinal studies focused on the role of the microbiota in the development of recurrent CDI. Cross-sectional studies comparing the fecal microbiota of diarrheal patients with or without CDI at diagnosis with that of healthy controls have observed a range of community types, with variable community members associated with CDI [30, 31]. It is possible that incorporation of other CDI considerations, such as severity or recurrence, can impact the identification of community members that may exacerbate disease. Combined with the inherent variability already present in the human microbiota [22, 32], generalizing all patients with CDI in one group is not ideal. Indeed, studies in murine models suggest that multiple community types from different antibiotic treatments are susceptible to C. difficile, suggesting that disease development, and the development of recurrence, is variable and complex [33–35]. As illustrated by our data, both severity and the development of recurrence were associated with decreased diversity and community resilience. Similarly, the fecal microbiota in patients with recurrent CDI prior to fecal microbiota transplantation (FMT) has been observed to be variable, albeit severely decreased in diversity [9, 10]. The study presented here attempted to dissect some of these variables within a patient population that already does not possess a healthy microbiota.
Previous studies have used results from the clinical laboratory to determine the C. difficile status of a patient [30, 36, 37]. Our current study identified differentially represented OTUs as well as differential community structure between samples positive or negative for C. difficile based on the clinical laboratory test used at our hospital (C. diff Quik Chek), and cultivation of C. difficile from these samples generally agreed with testing results. We were unable to isolate C. difficile from samples during the standard antibiotic treatment window (14 days following a positive sample), potentially due to residual antibiotics in the sample. Overall, the abundance of Clostridium XI identified via 16S rRNA-based sequencing did not correlate with a positive test from the clinical lab or cultivation efforts. However, OTU12, belonging to Clostridium XI, was the only overrepresented OTU in the index sample of recurrent patients compared with nonrecurrent patients, suggesting that abundance of C. difficile is potentially important in the development of recurrence.
Differences in the fecal microbiota at index sampling within recurrent, nonrecurrent, and reinfected patients were less pronounced compared with differences between C. difficile negative or positive samples. A previous small study demonstrated that patients with recurrent disease have a less diverse community than patients with nonrecurrent CDI [38]. We did observe a slightly lower diversity in the index samples from patients with recurrence and reinfection patients compared with the index sample from patients without recurrence. However, we did find that intra-individual changes over time were more informative. Both diversity over time and overall community dissimilarity increased in patients without recurrence, suggesting that the microbial community in these patients is more dynamic. The similarity in the microbial community of samples collected during “index” and “treatment” time points in nonrecurrent patients was variable, suggesting dynamic changes in the community regardless of the test results. In contrast, there was greater similarity between samples collected during “recurrence”, “treatment”, “recovery”, or “reinfection” in patients who developed recurrence. Community types that are less susceptible to recurrence might be very individual and rely on the ability of the microbiota to change rather than the microbiota sharing a feature with that of other recurrent patients.
Surprisingly, we also observed significant differences in microbial diversity between samples collected during severe disease or not. Although LEfSe analysis revealed seven differentially present OTUs between severe and non-severe samples, few OTUs overlapped with LEfSe comparisons of C. difficile negative and positive samples. Comparison of patient samples categorized by severity within each of the patient groups (recurrent, nonrecurrent, or reinfected) each followed similar trends, suggesting that severity and recurrence were not associated. The severity score used in our study [12] does not reflect serious complications such as pseudomembranous colitis, ileus/toxic megacolon, or sepsis. However, it does suggest that other physiological parameters may be associated with changes in the gut microbiota and this severity score is frequently positive early in the disease process, when our index samples were collected. Given that increased gut microbiota diversity is associated with recovery from recurrent CDI following FMT, a simple severity score may be of value when deciding on treatment. Recent application of FMT for the treatment of severe disease has been effective in preventing later recurrence [39, 40]. If overall diversity of a community is in part predictive of susceptibility to recurrence, preemptive measures to promote recovery of diversity may be especially important in this patient population.
We were unable to identify a single microbiota-based metric that would predict the development of recurrent CDI. However, longitudinal analysis that considers the individual’s potential for recovery implied that patients with a more dynamic fecal microbiota were less likely to develop recurrence. This warrants analysis in a larger, more structured study to understand how recovery can be managed to decrease the likelihood of a recurrent episode and to better characterize the role of microbiota-derived variables in predictive models of severity/recurrence. As we gain a better understanding of the microbiota and their functions, which may include activities such as bile acid metabolism [35, 41], we may be in a position to identify patients at increased risk of recurrent disease and intervene through therapies that are designed to restore necessary microbiome functions.
Conclusions
We observed distinct differences in microbiota diversity of patients with CDI that did or did not develop recurrent disease. Both static and longitudinal analysis indicated that recovery of the microbiota community is different in recurrent patients, suggesting that the overall microbiota structure may be important in susceptibility to recurrence. Additionally, disease severity at the time of diagnosis may be associated with the status of a patient’s fecal microbiota diversity. Validation of our observations in a larger cohort of patients that do or do not develop recurrence could aid in identification of microbial determinants that are associated with developing recurrent CDI.
Ethics approval and consent to participate
All subjects signed written consent to participate in this study. This study was approved by the University of Michigan Institutional Review Board (Study HUM33286; originally approved 8/26/2009).
Availability of data and materials
The raw sequence files supporting the conclusions of this article are available in the Sequence Read Archive (SRA) under the BioProject ID PRJNA307992, BioSamples SAMN04407535-SAMN04407764. Detailed description of data processing and generation of all figures and statistics are available at https://github.com/aseekatz/ERIN.recurrence.
Abbreviations
- θ YC :
-
Yue and Clayton distance
- λ :
-
inverse Simpson index
- AMOVA:
-
analysis of molecular variance
- CDI:
-
Clostridium difficile infection
- ERIN:
-
Enterics Research Investigational Network
- FMT:
-
fecal microbiota transplantation
- GDH:
-
glutamate dehydrogenase
- GEE:
-
generalized estimating equation
- IDSA:
-
Infectious Diseases Society of America
- LDA:
-
linear discriminant analysis
- LEfSe:
-
linear discriminant analysis effect size
- OTU:
-
operational taxonomic unit
- PAM:
-
Partitioning Around Medoids
- PCoA:
-
Principal coordinates analysis
- PCR:
-
polymerase chain reaction
- TCCFA:
-
taurocholate cycloserine cefoxitin fructose agar
References
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–34. doi:10.1056/NEJMoa1408913.
Dubberke E. Clostridium difficile infection: the scope of the problem. J Hosp Med. 2012;7 Suppl 3:S1–4. doi:10.1002/jhm.1916.
Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–9. doi:10.1056/NEJMoa051639.
Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400. doi:10.1371/journal.pone.0098400.
Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–93. doi:10.1016/s0140-6736(00)03592-3.
Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002. doi:10.1093/cid/cir632.
Rao K, Young VB. Fecal microbiota transplantation for the management of Clostridium difficile infection. Infect Dis Clin North Am. 2015;29(1):109–22. doi:10.1016/j.idc.2014.11.009.
Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, et al. Fecal microbiota transplantation for Clostridium difficile infection: a systematic review. Ann Intern Med. 2015;162(9):630–8. doi:10.7326/m14-2693.
Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5(3):e00893–14. doi:10.1128/mBio.00893-14.
Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–35. doi:10.4161/gmic.23571.
van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. doi:10.1056/NEJMoa1205037.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55. doi:10.1086/651706.
McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–5. doi:10.1086/511798.
Martinson JN, Broadaway S, Lohman E, Johnson C, Alam MJ, Khaleduzzaman M, et al. Evaluation of portability and cost of a fluorescent PCR ribotyping protocol for Clostridium difficile epidemiology. J Clin Microbiol. 2015;53(4):1192–7. doi:10.1128/jcm.03591-14.
Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2(3):145–58.
Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, Young VB. Fecal microbiota transplant eliminates Clostridium difficile in a murine model of relapsing disease. Infect Immun. 2015;83(10):3838–46. doi:10.1128/IAI.00459-15.
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79(17):5112–20. doi:10.1128/aem.01043-13.
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–41. doi:10.1128/AEM.01541-09.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200. doi:10.1093/bioinformatics/btr381.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–6. doi:10.1093/nar/gks1219.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. doi:10.1128/AEM.00062-07.
Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. doi:10.1038/nature09944.
Yue JC, Clayton MK. A similarity measure based on species proportions. Commun Stat Theor M. 2005;34:2123–31.
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T et al. gplots: various R programming tools for plotting data (v2.12.1). 2013. http://cran.r-project.org/web/packages/gplots/gplots.pdf. Accessed Dec 2015.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi:10.1186/gb-2011-12-6-r60.
Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131(2):479–91.
Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22.
Hojsgaard S, Halekoh U, Yan J. Package ‘geepack’: Generalized Estimating Equation Package. CRAN. 2014. https://cran.r-project.org/web/packages/geepack/geepack.pdf. Accessed Jan 2016.
Seekatz AM, Panda A, Rasko DA, Toapanta FR, Eloe-Fadrosh EA, Khan AQ, et al. Differential response of the cynomolgus macaque gut microbiota to Shigella infection. PLoS One. 2013;8(6):e64212. doi:10.1371/journal.pone.0064212.
Schubert AM, Rogers MA, Ring C, Mogle J, Petrosino JP, Young VB, et al. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. MBio. 2014;5(3):e01021–14. doi:10.1128/mBio.01021-14.
Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51(9):2884–92. doi:10.1128/JCM.00845-13.
David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(7):R89. doi:10.1186/gb-2014-15-7-r89.
Schubert AM, Sinani H, Schloss PD. Antibiotic-induced alterations of the murine gut microbiota and subsequent effects on colonization resistance against Clostridium difficile. MBio. 2015;6(4):e00974. doi:10.1128/mBio.00974-15.
Theriot CM, Bowman AA, Young VB, Ellermeier CD. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere. 2016;1(1):e00045–15. doi:10.1128/mSphere.00045-15.
Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(7533):205–8. doi:10.1038/nature13828.
Rao K, Erb-Downward JR, Walk ST, Micic D, Falkowski N, Santhosh K, et al. The systemic inflammatory response to Clostridium difficile infection. PLoS One. 2014;9(3):e92578. doi:10.1371/journal.pone.0092578.
LaSala PR, Ekhmimi T, Hill AK, Farooqi I, Perrotta PL. Quantitative fecal lactoferrin in toxin-positive and toxin-negative Clostridium difficile specimens. J Clin Microbiol. 2013;51(1):311–3. doi:10.1128/jcm.02735-12.
Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–8. doi:10.1086/525047.
Lagier JC, Delord M, Million M, Parola P, Stein A, Brouqui P, et al. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur J Clin Microbiol Infect Dis. 2015;34(8):1597–601. doi:10.1007/s10096-015-2394-x.
Aroniadis OC, Brandt LJ, Greenberg A, Borody T, Kelly CR, Mellow M, et al. Long-term follow-up study of fecal microbiota transplantation for severe and/or complicated Clostridium difficile infection: a multicenter experience. J Clin Gastroenterol. 2015. doi:10.1097/mcg.0000000000000374.
Theriot CM, Koenigsknecht MJ, Carlson Jr PE, Hatton GE, Nelson AM, Li B, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi:10.1038/ncomms4114.
Acknowledgements
The authors would like to thank Judy Opp, April Cockburn, and Harriette Carrington in the University of Michigan Host Microbiome Initiative Sequencing Core. We would also like to thank Dr. Daniel McClung for assisting with data collection and curation.
Funding
This study was supported by the National Institute of Allergy and Infectious Diseases Enteric Research Investigative Network (ERIN; U19AI09087), awarded to VBY. ASM has been supported by the National Center for Advancing Translational Sciences (UL1TR000433). KR has been supported by the National Institute of Allergy and Infectious Diseases (AI120599) and the Claude D. Pepper Older Americans Independence Center (AG-024824). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCATS, the Pepper Center, or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
VY has served as a consultant for Vedanta, Merck and has received a research grant from MedImmune. All other authors declare that they have no competing interests.
Authors’ contributions
AMS conceived the study, participated in sample processing, analyzed the data, and drafted the manuscript. KR participated in sample selection, interpretation of results, and inclusion of metadata in analysis. KS participated in sample collection, conducted sample processing, and facilitated inclusion of metadata. VBY participated in study design, aided interpretation of results, and helped draft the manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1: Table S1.
Sample description and metadata. The relative abundance of the top 100 operational taxonomic units (OTUs), with taxonomic classification, for all samples is listed in columns. Metadata for each sample is described across each row. (XLSX 236 kb)
Additional file 2: Figure S1.
Relative abundance of distinct community members in patient groups. The mean relative abundance plus standard error (se) of operational taxonomic units (OTUs) identified by linear discriminant analysis (LDA) effect size (LEfSe) in C. difficile-positive and -negative samples was plotted in nonrecurrent, recurrent, and reinfected patients. LEfSe analysis identified OTU12 (Clostridium XI) was also overrepresented in index samples of recurrent patients compared with nonrecurrent patients. (PDF 118 kb)
Additional file 3: Figure S2.
Comparison of diversity in the fecal microbiota in patients with C. difficile infection (CDI). Comparison of the microbial diversity (inverse Simpson index λ) in a severe and non-severe samples in nonrecurrent, recurrent, and reinfected patients (Kruskal–Wallis test, p = 0.040), b C. difficile positive versus negative samples (not significant), c patients with or without prior antibiotic exposure (not significant) and d with or without a history of prior CDI (not significant). (PDF 173 kb)
Additional file 4: Figure S3.
Community structure of patients with or without recurrent C. difficile infection. Principal coordinates analysis (PCoA) was used to plot the Yue and Clayton dissimilarity index (θ YC ). a The community structure of the microbiota in index (initial) samples from nonrecurrent, recurrent, and reinfected patients (analysis of molecular variance (AMOVA), p = 0.068). b The community structure of samples positive or negative for C. difficile as determined by cultivation (AMOVA, p = 0.001). (PDF 166 kb)
Additional file 5: Figure S4.
Intra-individual similarity of the microbiota in patients with or without recurrent C. difficile infection over sequential sampling. The microbial community similarity over sequential time within nonrecurrent and recurrent patients was compared using the Yue and Clayton dissimilarity index (θ YC ; Wilcoxon test, not significant). (PDF 102 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Seekatz, A.M., Rao, K., Santhosh, K. et al. Dynamics of the fecal microbiome in patients with recurrent and nonrecurrent Clostridium difficile infection. Genome Med 8, 47 (2016). https://doi.org/10.1186/s13073-016-0298-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13073-016-0298-8