Abstract
Background
Over the past two decades, Cameroon has recorded one of the highest rates of urban population growth in sub-Saharan Africa. It is estimated that more than 67% of Cameroon's urban population lives in slums, and the situation is far from improving as these neighbourhoods are growing at an annual rate of 5.5%. However, it is not known how this rapid and uncontrolled urbanization affects vector populations and disease transmission in urban versus rural areas. In this study, we analyse data from studies conducted on mosquito-borne diseases in Cameroon between 2002 and 2021 to determine the distribution of mosquito species and the prevalence of diseases they transmit with regards to urban areas versus rural areas.
Methods
A search of various online databases, such as PubMed, Hinari, Google and Google Scholar, was conducted for relevant articles. A total of 85 publications/reports were identified and reviewed for entomological and epidemiological data from the ten regions of Cameroon.
Results
Analysis of the findings from the reviewed articles revealed 10 diseases transmitted by mosquitoes to humans across the study regions. Most of these diseases were recorded in the Northwest Region, followed by the North, Far North and Eastern Regions. Data were collected from 37 urban and 28 rural sites. In the urban areas, dengue prevalence increased from 14.55% (95% confidence interval [CI] 5.2–23.9%) in 2002–2011 to 29.84% (95% CI 21–38.7%) in 2012–2021. In rural areas, diseases such as Lymphatic filariasis and Rift valley fever, which were not present in 2002–2011, appeared in 2012–2021, with a prevalence of 0.4% (95% CI 0.0– 2.4%) and 10% (95% CI 0.6–19.4%), respectively. Malaria prevalence remained the same in urban areas (67%; 95% CI 55.6–78.4%) between the two periods, while it significantly decreased in rural areas from 45.87% (95% CI 31.1–60.6%) in 2002–2011 to 39% (95% CI 23.7–54.3%) in the 2012–2021 period (*P = 0.04). Seventeen species of mosquitoes were identified as involved in the transmission of these diseases, of which 11 were involved in the transmission of malaria, five in the transmission of arboviruses and one in the transmission of malaria and lymphatic filariasis. The diversity of mosquito species was greater in rural areas than in urban areas during both periods. Of the articles reviewed for the 2012–2021 period, 56% reported the presence of Anopheles gambiae sensu lato in urban areas compared to 42% reported in 2002–2011. The presence of Aedes aegypti increased in urban areas in 2012–2021 but this species was absent in rural areas. Ownership of long-lasting insecticidal nets varied greatly from one setting to another.
Conclusions
The current findings suggest that, in addition to malaria control strategies, vector-borne disease control approaches in Cameroon should include strategies against lymphatic filariasis and Rift Valley fever in rural areas, and against dengue and Zika viruses in urban areas.
Graphical Abstract

Similar content being viewed by others
Background
Mosquito-borne diseases (MBDs) such as malaria and arboviruses remain a major public health problem worldwide [1, 2]. In recent years, these diseases have become increasingly widespread, accounting for a significant proportion among the overall burden of infectious diseases. The WHO reported 2 million more cases of malaria in 2021 compared to 2020 [3]. Concerning arboviruses, recent estimates suggest that there are more than 3.5 billion people at risk of dengue virus (DENV) infection in > 100 countries and around 390 million DENV infections are reported each year, of which 100 million cause clinical symptoms [4]. The explosive 2015–2016 Zika virus (ZIKV) epidemic infected > 1 million people in 73 countries, inducing an increased incidence of microcephaly in newborns and requiring the WHO to declare ZIKV infection a public health emergency of international concern [5]. Chikungunya virus (CHIKV) epidemics also occur frequently around the world on all continents, with the number of cases reaching hundreds of thousands [6]. Overall, MBDs result in significant mortality and morbidity, causing millions of deaths, long-term disability and lifelong sequelae annually [7].
In the past, many of these diseases were largely confined to specific regions, especially in rural areas of the tropics and subtropics [8]. This situation is now being profoundly altered by many factors, including climate change, increased travel, migration and refugee movements, global trade, deforestation and unplanned urbanization [9]. Pathogens are not limited by national borders; local and international movements of people and goods can contribute to their rapid spread [10]. Increasing urbanization results in large and dense populations, which in turn increases the likelihood of transmission and outbreak of infectious diseases. In addition, climate change and human activities may expand the habitats of some vectors into new areas, exposing new populations to the diseases they transmit, with patterns and intensity fluctuating seasonally [11]. The diagnosis and prevention of and response to infectious disease threats are therefore critical to national and global health security.
In Cameroon, the situation is of concern because the country provides an ideal environment for mosquito-vector proliferation [12, 13]. One of the crucial elements in reducing the burden of vector-borne diseases is behavioural change of the affected populations, which can be achieved through education and increased public awareness, so that people know how to protect themselves and their communities from mosquitoes and other vectors, such as flies and ticks, among others. It is now well known that about 80% of "malaria" cases in Cameroon are treated by non-conventional means in many families. Indeed, there is a need to develop a health education programme in local communities and schools, with the aim to implement sanitation measures in neighbourhoods and teach people how to recognize early the febrile symptoms of common vector-borne diseases. The development of these programs will depend on the information collected at the community level regarding the MBDs and the bio-ecology of the vectors. However, little research has been conducted to improve understanding of the transmission of MBDs in municipalities. Furthermore, little information is available regarding changing epidemiological patterns and the real coverage of vector control interventions. Meanwhile, combining community knowledge on vector-borne diseases with diagnostic and surveillance strategies, may strengthen the effectiveness of prevention and control interventions.
The main objective of this review was to analyze the evolution of MBD transmission in urban versus rural areas in Cameroon over the past 20 years, in order to improve current knowledge on their differential burden and better inform, strengthen and guide community-based strategies against vector-borne diseases.
Methods
Literature search
An online search of online bibliographic databases, such as PubMed, Hinari, Google and Google Scholar, for scientific articles on MBDs in urban and rural areas of Cameroon was undertaken using different search terms. Search terms included a combination of key words, such as "urban/rural mosquito-borne pathogens", "urban/rural mosquito-borne diseases", "urban/rural mosquito-borne viruses", "urban/rural mosquito-borne bacteria", "mosquito fauna in urban or rural areas", mosquito genera (including "Aedes", "Culex", "Mansonia", "Anopheles"), "arboviruses", "malaria", "filariasis" and "Cameroon". In addition to the online bibliographic databases consulted, data were also extracted from reports and theses.
Selection of articles
Articles selected were published between 2002, which is the year Cameroon developed the very first National Strategic Plan for Malaria Control (NSPMC) and restructured the National Malaria Control Programme to make it more operational and effective (NMCP), and 2021. The selected articles meet the following criteria: (i) data on Cameroon; (ii) no records repeated; (iii) prevalence data; (iv) entomological data; (v) diseases transmitted by mosquitoes; (vi) representative sampling; (vii) studies with field data collected between 2002 and 2021; and (viii) description of geographic or behavioural risk factors and changes in distribution of MBDs as a result of interventions.
Study periods
The study was conducted between January and August 2022. It consisted of collecting data from published databases over two decades: (1) between 2002 and 2011; (ii) between 2012 and 2021.
Data analysis
Selected articles were categorized based on their geographic location and type of MBDs. The information extracted from each selected study was recorded in a Microsoft Excel (Microsoft Corp., Redmond, WA, USA) spreadsheet for data analysis. This information included authors’ names, year of publication, study site, ecological profile (urban and rural), malaria transmission indices (entomological inoculation rate [EIR], human biting rate [HBR]), mosquito species, study period, prevalence of other MBDs and pathogen species. The EIR estimates and prevalences were not always available in selected papers in an adequate format for analysis. Consequently, a number of steps were undertaken to adjust data presentation (see Table 1). The prevalence of diseases in this paper was defined as the number of recorded infected people in a study population. As with the EIR data, a number of steps, as indicated in Table 2, were taken to adjust the presentation of prevalence data for different urban and rural areas. These same steps were taken to adjust data presentation of discarded prevalence. The Kruskal–Wallis and Mann–Whitney tests were used to compare the means of EIR estimates between urban and rural settings. The EIRs and parasite prevalence were also compared between the two periods study period, namely before 2011 and after 2011, because the studies conducted from 2012 to 2021 commonly used modern molecular and serological tools for mosquito processing and diagnosis, such as PCR and ELISA, while studies conducted in the previous decade (2002–2011) commonly used dissection and microscopy techniques and methods. Indeed, modern tests have a high sensitivity and specificity that improve diagnosis. Analyses were performed using R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and Epi Info 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA)
Results
Articles included in the study
Our search identified a total of 642 records published between 2002 and 2021, among which 625 were identified from database searches and 17 were identified through other means (reports and theses). Ultimately, 53 articles were retained for analysis according to the above criteria. The selection procedure and validation of articles of interest areshown in Fig. 1. Among the 53 articles analysed (Table 3), 31.54% (47/149) focussed on the distribution of MBDs, 15.44% (23/149) reported the prevalence of MBDs, 18.12% (27/149) determined Plasmodium EIRs, 32.88% (49/149) showed the distribution of mosquito species involved in pathogen (arboviruses and parasites) transmission and 2.01% (3/149) addressed mosquito control interventions.
PRISMA chart showing the steps for the selection of articles. In total, 642 articles on mosquito-borne diseases published between 2002 and 2021 were identified from online bibliographic databases using different search terms and from other sources. After sorting the titles of publications and removing duplicates, 281 articles were assessed for eligibility and 288 were excluded. Ultimately, 53 articles were retained for analysis in this review
Geographical distribution of the studies analysed on mosquito-borne diseases in Cameroon
The 53 articles included in the review are representative of the whole country but are unevenly distributed across the ten administrative regions of Cameroon. The highest number of publications reported studies in the Centre and Southwest Regions, followed by the Littoral, Far North, North and West Regions. The lowest number of publications reported studies in the Adamaoua, East, South and Northwest Regions (Fig. 2).
Geographic distribution of studies conducted in Cameroon related to mosquito-borne diseases between 2002 and 2021. The articles cover the whole country but are unevenly distributed among the 10 administrative regions. The number of items recorded per region are indicated by different colours (1–12). The black lines represent the boundaries between regions. Scale bars: 100 km
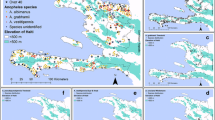
The location of the study sites and their ecological features, the pathogens described and the references of the publications are given in Table 4. The pathogens belong to three groups, namely protozoans, arboviruses and parasitic worms. The group of protozoans is represented by the genus Plasmodium, the group of arboviruses by the genera Flavivirus, Alphavirus and Phlebovirus and the group of parasitic worms by Wuchereria spp. Overall, each region of the country recorded at least one case study on malaria within the past 20 years. CHIKV, West Nile virus (WNV), Spondweni virus (SPOV), Wesselsbron (WSL) virus and Yellow fever virus (YFV) were reported in the Southwest Region. DENV was recorded in the Centre, East, Littoral, North, Far North, South and West Regions. Lymphatic filariasis (LF) was reported in the North and Far North Regions, Rift Valley Fever (RVF) virus was recorded in the East Region and ZIKV was recorded in the Centre, East, Littoral, North, Far North and Southwest Regions (Fig. 3).
Map showing the localization of mosquito-borne infections across the 10 regions of Cameroon. Overall, malaria is present in all regions of the country. Chikungunya virus, West Nile virus, Spondweni virus, Wesselsbron disease virus and yellow fever virus were reported in the Southwest Region; dengue virus was reported in the Centre, East, Littoral, North, Far North, South and West Regions; lymphatic filariasis was reported in the North and Far North Regions; Rift Vally fever virus was reported in the Eastern Region; and Zika virus was reported in the Central, Eastern, Littoral, Northern, Far North and Southwestern Regions. The black lines represent the boundaries between regions. Scale bars: 100 km
Prevalence of mosquito-borne diseases
The reported prevalences of CHIKV, SPOV, WSL, WNV and YFV ranged from 38% to 51% and from %1.3 to 40% in rural and urban areas, respectively, during the period 2002–2011. The diseases caused by these viruses completely disappeared after 2011. DENV, which had a prevalence of 37.07% (95% confidence interal [CI] 24.3–49.8%) in rural areas between 2002 and 2011, was no longer recorded in the same areas from 2012 to 2021. Conversely, in urban areas, the prevalence of this disease increased from 14.55% (95% CI 5.2–23.9%) in 2002–2011 to 29.84% (95% CI 21–38.7%) in 2012–2021. In rural areas, neglected tropical MBDs, such as LF and RVF, were not recorded in 2002–2011 while they appeared in 2012–2021, with prevalences ranging from 0.4% (95% CI 0.0–2.3%]) to 10% (95% CI 0.6–19.4%]), respectively. The prevalence of malaria remained the same in urban areas (67%) during the two periods 2002–2011 and 2012–2021; however, this prevalence may have decreased in rural areas from 45.87% (95% CI 31.1–60.6%) in 2002–2011 to 39% (95% CI 23.7–54.3%) in 2012–2021 (P = 0.91) (Fig. 4). Comparisons of the EIR estimates between urban and rural areas during the periods 2002–2011 and 2012–2021 revealed that malaria transmission has significantly decreased in rural areas (*P = 0.04), while it has significantly increased in urban areas (*P = 0.02) (Fig. 5).
Distribution of mosquito species involved in vector-borne disease transmission during the last two decades
Seventeen species of mosquitoes involved in vector-borne disease transmission have been recorded in published papers between 2002 and 2021. Of these 17 species, 11 were involved in the transmission of malaria, five in the transmission of arboviruses and one in the transmission of both malaria and LF. The diversity of mosquito species was greater in rural areas than in urban areas during both periods (Fig. 6a, b). Two major malaria vectors (Anopheles gambiae and Anopheles funestus) were more prevalent in urban areas than in rural areas (Fig. 6c, d). Of the articles included in this review that were published within the 2012–2021 period, 56% reported the presence of A. gambiae sensu lato in urban areas; in the 2002–2011 period, this was 42% of papers. The presence of Aedes aegypti increased in urban areas in 2012–2021 while this species was absent from rural areas; in the latter areas, Aedes spp. (50%) and Culex spp. (50%) were the most important arboviruses vectors for the 2002–2011 decade (Fig. 7). Of the papers published between 2012 and 2021, 50% reported A. funestus as the vector for LF in rural areas (Fig. 7b). Arbovirus vectors showed a significant decrease in species diversity in urban areas (Fig. 7c, d). Aedes aegypti (95%) and Aedes albopictus (5%) were the most prevalent species in urban areas during the 2012–2021 decade.
Distribution of malaria vector species between rural and urban areas. Seventeen species of mosquitoes involved in vector-borne disease transmission were identified in the studies published between 2002 and 2021 included in this review. a–d Proportion of studies reporting the presence of malaria vector species in rural areas in 2002–2011 (a), in rural areas in 2012–2021 (b), in urban areas in 2002–2011 (c) and in urban areas in 2012–2021 (d)
Distribution of arbovirus and lymphatic filariasis vector species between rural and urban areas. a Proportion of studies reporting the presence of arbovirus vector species in rural areas in 2002–2011, b proportion of studies reporting the presence of arbovirus and lymphatic filariasis vector species in rural areas in 2012–2021, c proportion of studies reporting the presence of arbovirus vector species in urban areas in 2002–2011, d proportion of studies reporting the presence of arbovirus vector species in urban areas in 2012–2021
Mosquito control interventions
Vector control made significant progress between 2002 and 2021, particularly for malaria control [67, 68]. Since 2000, Cameroon has benefitted from the support of various international partners to implement malaria control interventions [68]. Environmental management has been improved through major development projects (water drainage, filling of ponds) and maintenance of canals and banks, elimination of aquatic plants and management of water levels on a regular basis, with the overall aim to limit the development of mosquito larvae. This mechanical control also includes the elimination of all containers known as suitable breeding sites for mosquito larvae. This latter strategy is of considerable importance in the fight against Aedes, the vectors of arboviruses, since many of their breeding sites are created by humans. Insecticide-based mosquito control is still very important in vector control strategies, especially in areas with non-removable breeding sites and/or to target adult mosquitoes. Free distribution of insecticide-treated nets (ITNs) and long-lasting insecticidal nets (LLINs) is a major component of malaria vector control in Cameroon [69], in both rural and urban areas. Therefore, no significant difference in LLIN ownership was recorded in this study between urban and rural areas. However, an increase in LLIN ownership was observed over the time covered by this study in all regions, although it greatly varied from one region to another. The percentage of households owning at least one LLIN increased from 51.09% (Fig. 8a) to 54.49% (Fig. 8c) in urban areas and from 54.49% (Fig. 8b) to 78.43% (Fig. 8d) in rural areas between 2002–2011 and 2012–2021, respectively. During the 2002–2011 decade and for both urban and rural areas, the West (urban: 43.1%; rural: 46.5%), East (urban: 44.6%; rural: 48%) and Adamaoua (urban: 46.3%; rural: 49.7%) Regions had the lowest proportion of households owning at least one net. Net ownership was somewhat higher in the North (urban: 62.5%; rural: 65.9%) and Far North (urban: 56.5%; rural: 59.9%) Regions.
Rates of LLINs use between 2002 and 2021. The rate of LLIN use is higher in rural areas in both periods (2002–2011 and 2012–2021). a–d The rates of LLIN use by households in urban areas between 2002 and 2011 (a), by households in rural areas between 2002 and 2011 (b), by households in urban areas between 2012 and 2021 (c) and by households in rural areas between 2012 and 2021 (d). LLINs, Long-lasting insecticidal nets
However, for the 2012–2021 decade, the Southwest (urban: 51.9%; rural: 58.3%) and East (urban: 60.9%; rural: 67.6%) Regions had the lowest percentage of households owning at least one LLIN. Other vector control interventions based on larviciding and the drainage of watercourses in the framework of the YSDP (Yaoundé Sanitation and Development Programme) have been carried out in the city of Yaoundé during the 2011–2022 decade [70].
Discussion
The publications and other reports included in this review provided data on the evolution of MBD transmission in urban and rural areas over the past 20 years in Cameroon. Urbanization is increasingly blamed for influencing the epidemiology and evolution of vector-borne diseases in Cameroon [71, 73]. According to reports drawn up by the Monitoring Committee of the 4th General Census of Population and Housing in 2015, the rate of urbanization in Cameroon is increasing in an exponential manner, rising from 48.8% in 2002 to nearly 60% in 2021. Conversely, the growth rate of the rural population has dropped from 1.6% in 2002 to 1.2% in 2021 [72]. Nevertheless, the urbanization that has taken place over the last two decades has been punctuated by progress in social development, with the aim to improve hygiene and public health conditions of the populations. The decades prior to 2011 were marked by the creation and development of disease control programmes, including those against vector-borne diseases such as malaria (NMCP in 1997), onchocerciasis and LF, human and animal trypanosomiasis and schistosomiasis, all in 2003. It was also a time of increasing awareness of vector-borne diseases, with the introduction of a number of new diseases. The distribution of MBD studies included in this review, across the country, is very heterogeneous; in addition to the eco-climatic and phytogeographic characteristics which are specific to each of the 10 regions of Cameroon, the choice of the study sites in rural or urban areas depends on many other factors, including the availability of financial resources requested to collect samples in the field and to preserve and analyse the collected samples in the laboratory. Many research studies are conducted in the Central, Littoral and Southwest Regions where research institutions and universities are located.
Among the MBDs identified in this review, malaria and DENV are the most predominant, as they are distributed in almost all regions of Cameroon. The zonal distribution of other mosquito-transmitted diseases, such as YFV, LF and WNV, for example, reflect rather the lack of studies. Mosquito-borne diseases, except malaria and arboviral diseases, were reported more frequently in rural and urban areas in the 2002–2011 decade than in the 2012–2021 period. During the second period, the number of cases of urban malaria was stable while those of rural malaria decreased, but the number of DENV cases increased in urban areas. This observation reflects the country's progress over the 2012–2021 decade in the area of public health, particularly in terms of chemoprophylaxis through vaccination (e.g. the case of the yellow fever vaccine), vector control (e.g. distribution of mosquito nets) and diagnosis and case management in urban and rural areas.
Despite significant improvements in health conditions for human populations, malaria and DENV have remained endemic in rural and urban areas over this last decade. Although several strategies are deployed in the fight against malaria, this parasitic disease persists in urban and rural areas with high EIRs (approx. 600 ib/man/year) in urban areas. LLINs appear to be the most widely used preventive tool by urban and rural populations. This rate of use, although not satisfactory, is linked to the massive and free distribution of LLINs during campaigns conducted by the public authorities and their development partners with a view to providing every household in Cameroon with LLINs [74]. However, many studies have shown that this tool only has a real impact in an environment when it is used on a large scale at the community level and, therefore, the effect of LLINs depends on the habits of the resident population [75]. Unfortunately, in both urban and rural areas in most administrative regions, with the exception of the North and Far North Regions, usage rates are far from the 80% recommended by WHO for effective LLIN action [75]. Indeed, in most localities in Cameroon, a significant proportion of the population uses LLINs for other purposes (fishing and agriculture) [75], while others do not use LLINs for various reasons such as heat, allergies, the feeling of being locked up in a coffin and the preference not to use them. In addition, the poverty of the population leads some people to self-medicate and to use street medicines to treat themselves. This situation may explain the persistence of malaria cases in urban and rural areas. The government should undertake actions to assess knowledge, attitudes and practices regarding malaria treatment and prevention in order to provide sustainable solutions.
Many of the mosquito species involved in disease transmission show a preference for rural areas, but those responsible for malaria and DENV transmission, including A. gambiae and A. funestus complexes and Aedes are also prevalent in urban areas. Species belonging to the A. gambiae and A. funestus complexes are associated with human activities and are the main vectors of malaria in Cameroon and in other African countries [11]. The high frequencies of insecticide resistance mutations and genes in members of these species’ complexes coupled with their vectorial capacities and competences make them the most abundant and widespread mosquitoes in rural and urban areas. The increase in population density of these anopheline species through resistance to control products is further facilitated by the insecticide pressure acting on aquatic stages developing in agricultural settings, including coffee, sugar cane, cotton and rice fields [70], market gardening areas [76] and timber yards [59]. This insecticide pressure has progressively increased with the intensification of vector control through the distribution of ITNs to pregnant women and children aged < 5 years in the 2002–2011 decade and LLINs in the 2012–2021 decade.
Conclusion
This study provides an update on MBDs in urban versus rural areas in Cameroon over the past two decades. While the prevalence of MBDs has decreased in rural areas between the two compared periods, MBDs in urban areas have increased and are likely to increase with continued unplanned urbanization. To halt this trend in disease burden, concerted actions must be taken quickly at various levels to improve prevention and control, through case detection and management and through vector control. In addition to malaria control strategies, vector-borne disease control approaches in Cameroon should include strategies against LF and RVF in rural areas, or against dengue in urban areas. Future research efforts should prioritize a better understanding of how the different interventions can be integrated and adapted to local context either in urban or in rural areas.
Abbreviations
- CHIKV:
-
Chikungunya virus
- DENV:
-
Dengue virus
- EIRs:
-
Entomological inoculation rate
- ITNs:
-
Insecticide-treated nets
- LF:
-
Lymphatic filariasis
- LLINs:
-
Long-lasting insecticidal nets
- MBDs:
-
Mosquito-borne diseases
- RVF:
-
Rift Valley fever
- SPOV:
-
Spondweni virus
- WNV:
-
West Nile virus
- WSL:
-
Wesselsbron disease
- YFV:
-
Yellow fever virus
- ZIKV:
-
Zika virus
References
Ortiz DI, Piche-Ovares M, Romero-Vega LM, Wagman J, Troyo A. The impact of deforestation, urbanization, and changing land use patterns on the ecology of mosquito and tick-borne diseases in Central America. Insects. 2021;13:20.
Ligsay A, Telle O, Paul R. Challenges to mitigating the urban health burden of mosquito-borne diseases in the face of climate change. Int J Environ Res Public Health. 2021;18:5035.
WHO. World malaria report 2022. 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 10 Nov 2022.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7.
WHO. Zika virus disease. 2016. https://www.who.int/news-room/fact-sheets/detail/zika-virus?gclid=CjwKCAjwoIqhBhAGEiwArXT7K6VyaHNhsdpl-NSGBc0CPmk_a3h70T2MzanvsGGKr0OTmHnZgyoosxoCebYQAvD_BwE. Accessed 10 Nov 2022.
WHO. Chikungunya. 2019.https://www.who.int/news-room/fact-sheets/detail/chikungunya. Accessed 10 Nov 2022.
Salazar V, Jagger BW, Mongkolsapaya J, Burgomaster KE, Dejnirattisai W, Winkler ES, et al. Dengue and Zika virus cross-reactive human monoclonal antibodies protect against spondweni virus infection and pathogenesis in mice. Cell Rep. 2019;26:1585-1597.e4.
WHO. Evaluation of genetically modified mosquitoes for the control of vector-borne diseases. 2020. https://www.who.int/publications/i/item/9789240013155. Accessed 10 Nov 2022.
Laurent J, Stéphane A, Leïla B. Impacts des changements climatiques sur les arboviroses dans une île tropicale en développement (Mayotte). VertigO. 2011;10:3.
Braack L, de Almeida APG, Cornel AJ, Swanepoel R, de Jager C. Mosquito-borne arboviruses of african origin: review of key viruses and vectors. Parasit Vectors. 2018;11:29.
Bamou R, Mayi MPA, Djiappi-Tchamen B, Nana-Ndjangwo SM, Nchoutpouen E, Cornel AJ, et al. An update on the mosquito fauna and mosquito-borne diseases distribution in Cameroon. Parasit Vectors. 2021;14:527.
Pascal Nyam. La lutte contre le paludisme au Cameroun: autopsie d’un phénomène pluriel dans un contexte d’hyper endémicité. 2020. hal-03078921. https://hal.science/hal-03078921v1/document. Accessed 31 Mar 2023.
Antonio-Nkondjio C, Sonhafouo-Chiana N, Ngadjeu CS, Doumbe-Belisse P, Talipouo A, Djamouko-Djonkam L, et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit Vectors. 2017;10:472.
Akono PN, Tonga C, Hondt ON, Nsangou MP, Ngaha R, Magne GT, et al. Aggressive mosquito fauna and malaria transmission in a forest area targeted for the creation of an agro-industrial complex in the South of Cameroon. J Entomol Acarol Res. 2016;48:380–7.
Tchuandom SB, Tchouangueu TF, Antonio-Nkondjio C, Lissom A, Djang JON, Atabonkeng EP, et al. Seroprevalence of dengue virus among children presenting with febrile illness in some public health facilities in Cameroon. Pan Afr Med J. 2018;31:177.
Akono PN, Tonga C, Lehman LG, Kekeunou S. Mosquito species diversity and malaria transmission in ayos, an area of degraded forest targeted for universal long-lasting insecticidal net distribution in Southern Cameroon. Afr Entomol. 2014;22:602–10.
Akono PN, Mbida AM, Tonga C, Kayoum AY, Youmbi LE, Lehman LG. Données préliminaires sur Le Paludisme Humain En Zones Rurale et Sémi-Urbaine Du Département Du Nkam (Littoral-Cameroun). J Appl Biosci. 2017;115:11441–52.
Akono PN, Tonga C, Mbida JA, Hondt OE, Ambene PA, Ndo C, et al. Anopheles Gambiae, major malaria vector in Logbessou, a peri-urban area of Douala (Cameroon). Bull Soc Pathol Exotique (1990). 2015;108:360–8.
Akono PN, Mbida JAM, Tonga C, Belong P, Ngo Hondt OE, Magne GT, et al. Impact of vegetable crop agriculture on anopheline agressivity and malaria transmission in urban and less urbanized settings of the South Region of Cameroon. Parasit Vectors. 2015;8:293.
Amvongo-Adjia N, Wirsiy EL, Riveron JM, Chounna Ndongmo WP, Enyong PA, Njiokou F, et al. Bionomics and vectorial role of anophelines in wetlands along the volcanic chain of Cameroon. Parasit Vectors. 2018;11:471.
Antonio-Nkondjio C, Simard F, Awono-Ambene P, Ngassam P, Toto J-C, Tchuinkam T, et al. Malaria vectors and urbanization in the equatorial forest region of South Cameroon. Trans R Soc Trop Med Hyg. 2005;99:347–54.
Atangana J, Fomena A, Tamesse JL, Fondjo E. Pratiques agricoles et épidémiologie du paludisme en zone Soudano-Sahélienne du Cameroun. Bull Soc Pathol Exot. 2012;105:23–9.
Bamou R, Mbakop LR, Kopya E, Ndo C, Awono-Ambene P, Tchuinkam T, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasit Vectors. 2018;11:464.
Bigoga JD, Manga L, Titanji VP, Coetzee M, Leke RG. Malaria vectors and transmission dynamics in coastal South-Western Cameroon. Malar J. 2007;6:5.
Bigoga JD, Nanfack FM, Awono-Ambene PH, Patchoké S, Atangana J, Otia VS, et al. Seasonal Prevalence of malaria vectors and entomological inoculation rates in the rubber cultivated area of Niete, South Region of Cameroon. Parasit Vectors. 2012;5:1–7.
Bouba T, Saotoing P, Souké A, Ngatarang C, Ouang-Yang B, Njan Nlôga AM. Diversity of Culicidae, determination of entomological parameters of the transmission of Plasmodium spp. in Maga, Far North Cameroon. Int J Mosq Res. 2017;4:27–31.
Cheteug G, Elanga-Ndille E, Donkeu C, Ekoko W, Oloume M, Essangui E, et al. Preliminary validation of the use of IgG antibody response to anopheles GSG6-P1 salivary peptide to assess human exposure to malaria vector bites in two endemic areas of Cameroon in Central Africa. PLoS One. 2020;15:e0242510.
Djamouko-Djonkam L, Nkahe DL, Kopya E, Talipouo A, Ngadjeu CS, Doumbe-Belisse P, et al. Implication of Anopheles Funestus in malaria transmission in the city of Yaoundé, Cameroon. Parasite. 2020;27:10.
Doumbe-Belisse P, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, Kopya E, et al. High malaria transmission sustained by Anopheles Gambiae s.l. Occurring both indoors and outdoors in the city of Yaoundé, Cameroon. Wellcome Open Res. 2018;3:164.
Ekoko WE, Awono-Ambene P, Bigoga J, Mandeng S, Piameu M, Nvondo N, et al. Patterns of anopheline feeding/resting behaviour and Plasmodium infections in North Cameroon, 2011–2014: implications for malaria control. Parasit Vectors. 2019. https://doi.org/10.1186/s13071-019-3552-2.
Eyong EEJ, Kengne-Ouafo AJ, Chounna PW, Datchoua-Poutcheu FR, Wanji S. Altitudinal variation in the parasitological and entomological indices of malaria around Mount Cameroon, South West Region of Cameroon. J Parasitol Vector Biol. 2016;8:74–85.
Feufack-Donfack LB, Sarah-Matio EM, Abate LM, Bouopda Tuedom AG, Ngano Bayibéki A, Maffo Ngou C, et al. Epidemiological and entomological studies of malaria transmission in Tibati, Adamawa Region of Cameroon 6 years following the introduction of long-lasting insecticide nets. Parasit Vectors. 2021;14:247.
Fils EMB, Ntonga PA, Belong P, Messi J. Contribution of mosquito vectors in malaria transmission in an urban district of Southern Cameroon. J Entomol Nematol. 2010;2:13–7.
Galani BRT, Ayangma EN, Wouatedem S, Nguele HCM. Prospective case-control study of dengue infection in some malaria and non-malaria patients consulting at the bertoua regional Hospital. medRxiv. 2021. https://doi.org/10.1101/2021.09.03.21263073.
Antonio-Nkondjio C, Awono-Ambene P, Toto J-C, Meunier J-Y, Zebaze-Kemleu S, Nyambam R, et al. High malaria transmission intensity in a village close to Yaoundé, the capital city of Cameroon. J Med Entomol. 2002;39:350–5.
Anchang-Kimbi JK, Nkweti VN, Ntonifor HN, Apinjoh TO, Tata RB, Chi HF, et al. Plasmodium falciparum parasitaemia and malaria among pregnant women at first clinic visit in the Mount Cameroon Area. BMC Infect Dis. 2015;15:439.
Atangana J, Bigoga JD, Patchoké S, Ndjemaï MH, Tabue RN, Nem TE, et al. Anopheline Fauna and malaria transmission in four ecologically distinct zones in Cameroon. Acta Trop. 2010;115:131–6.
Kimbi HK, Nana Y, Sumbele IN, Anchang-Kimbi JK, Lum E, Tonga C, et al. Environmental factors and preventive methods against malaria parasite prevalence in rural Bomaka and Urban Molyko, Southwest Cameroon. J Bacteriol Parasitol. 2013;4:4172.
Léger OEM, Noël NTF, Rachel N, Nasser NI, Roméo M, Pauline TK, et al. Malaria transmission and insecticide susceptibility of the anophelian fauna in the Njombé-Penja Health District. Pan African Mosquito Control Association Conference;2021.
Mbakop LR, Awono-Ambene PH, Mandeng SE, Ekoko WE, Fesuh BN, Antonio-Nkondjio C, et al. Malaria transmission around the memve’ele hydroelectric dam in South Cameroon: a combined retrospective and prospective study, 2000–2016. Int J Environ Res Public Health. 2019;16:1618.
Mbohou CN, Foko LPK, Nyabeyeu HN, Tonga C, Nono LK, Kangam L, et al. Malaria screening at the workplace in Cameroon. PLoS ONE. 2019;14:e0225219.
Mieguim Ngninpogni D, Ndo C, Ntonga Akono P, Nguemo A, Nguepi A, Metitsi DR, et al. Insights into factors sustaining persistence of high malaria transmission in forested areas of sub-saharan Africa: the case of Mvoua, South Cameroon. Parasit Vectors. 2021;14:2.
Nkenfou CN, Fainguem N, Dongmo-Nguefack F, Yatchou LG, Kameni JJK, Elong EL, et al. Enhanced passive surveillance dengue infection among febrile children: prevalence, Co-infections and associated factors in Cameroon. PLoS Negl Trop Dis. 2021;15:e0009316.
Nlinwe NO, Singong YC, Florentine TMR. Evaluation of malaria preventive measures among adult patients attending the Bamendjou and Foumbot District hospitals of the West Region of Cameroon. Malar J. 2021;20:60.
Ntonga PA, Bakwo E-FM, Kekeunou S, Belong P, Messi J. Impact of extensive rice cultivation on the culicid fauna and the transmission of malaria in Tonga, West Cameroon. J Animal Plant Sci. 2010;7:841–51.
Nyasa RB, Fotabe EL, Ndip RN. Trends in malaria prevalence and risk factors associated with the disease in Nkongho-Mbeng; a typical rural setting in the equatorial rainforest of the south west region of Cameroon. PLoS ONE. 2021;16:e0251380.
Oyono MG, Lehman LG, Fosso S, Bilong CB. Multiparasitism among schoolchildren of Akonolinga, Nyong et Mfoumou Division, Centre Region of Cameroon. BioRxiv. 2019. https://doi.org/10.1101/584318.
Saotoing P, Tchuenguem FF, Nlôga AN. Study of entomological parameters involved in the transmission of plasmodium parasite in Anopheles Gambiae in the City of Maroua, Far North Region Cameroon. J Entomol Zool Stud. 2014;2:381–6.
Tabue RN, Awono-Ambene P, Etang J, Atangana JCA-N, Toto J-C, Patchoke S, et al. Role of anopheles (Cellia) Rufipes (Gough, 1910) and other local anophelines in human malaria transmission in the Northern Savannah of Cameroon: a cross-sectional survey. Parasit Vectors. 2017;10:22.
Tchuinkam T, Simard F, Lélé-Defo E, Téné-Fossog B, Tateng-Ngouateu A, Antonio-Nkondjio C, et al. Bionomics of Anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. BMC Infect Dis. 2010;10:1–12.
Teh RN, Sumbele IUN, Asoba Nkeudem G, Meduke DN, Ojong ST, Kimbi HK. Concurrence of CareStartTM Malaria HRP2 RDT with microscopy in population screening for Plasmodium falciparum infection in the Mount Cameroon area: predictors for RDT positivity. Trop Med Health. 2019;47:17.
Fokam EB, Levai LD, Guzman H, Amelia PA, Titanji VPK, Tesh RB, et al. Silent circulation of arboviruses in Cameroon. East Afr Med J. 2010;87:262–8.
Ndip LM, Bouyer DH, Da Rosa APT, Titanji VPK, Tesh RB, Walker DH. Acute spotted fever rickettsiosis among febrile patients, Cameroon. Emerg Infect Dis. 2004;10:432.
Gake B, Vernet MA, Leparc-Goffart I, Drexler JF, Gould EA, Gallian P, et al. Low Seroprevalence of Zika virus in Cameroonian blood donors. Braz J Infect Dis. 2017;21:481–3.
Demanou M, Pouillot R, Grandadam M, Boisier P, Kamgang B, Hervé JP, et al. Evidence of dengue virus transmission and factors associated with the presence of anti-dengue virus antibodies in humans in three major towns in Cameroon. PLoS Negl Trop Dis. 2014;8:e2950.
Sadeuh-Mba SA, Yonga Wansi GM, Demanou M, Gessain A, Njouom R. Serological evidence of rift valley fever phlebovirus and crimean-congo hemorrhagic fever orthonairovirus infections among pygmies in the East Region of Cameroon. Virol J. 2018;15:63.
Nana-Djeunga HC, Tchouakui M, Njitchouang GR, Tchatchueng-Mbougua JB, Nwane P, Domche A, et al. First evidence of lymphatic filariasis transmission interruption in cameroon: progress towards elimination. PLoS Negl Trop Dis. 2017;11:e0005633.
Simo Tchetgna H, Sado Yousseu F, Kamgang B, Tedjou A, McCall PJ, Wondji CS. Concurrent circulation of dengue serotype 1, 2 and 3 among acute febrile patients in Cameroon. PLoS Negl Trop Dis. 2021;15:e0009860.
Atangana J, Fomena A, Tamesse JL, Fondjo E. Agricultural activities and epidemiology of malaria in Soudano-Sahelian zone in Cameroon. Bull Soc Pathol Exotique (1990). 2012;105:23–9.
Djamouko-Djonkam L, Mounchili-Ndam S, Kala-Chouakeu N, Nana-Ndjangwo SM, Kopya E, Sonhafouo-Chiana N, et al. Spatial distribution of Anopheles Gambiae Sensu Lato Larvae in the urban environment of Yaoundé, Cameroon. Infect Dis Poverty. 2019;8:84.
Nyasa RB, Zofou D, Kimbi HK, Kum KM, Ngu RC, Titanji VPK. The current status of malaria epidemiology in Bolifamba, atypical Cameroonian Rainforest Zone: an assessment of intervention strategies and seasonal variations. BMC Public Health. 2015;15:1105.
Santé, E. Le Ministère de la Santé annonce la distribution de plus de 14 millions de MILDA en 2019. Le Bled Parle: L’actualité africaine de dernière minute du jour. https://www.lebledparle.com/fr/societe/1106665-cameroun-le-ministere-de-la-sante-annonce-la-distribution-de-plus-de-14-millions-de-milda-en-2019. Accessed 22 Jul 2022.
Ministère de la Santé Publique. Cameroon-Enquête Démographique et de Santé 2018. https://microdata.worldbank.org/index.php/catalog/3717. Accessed 22 Jul 2022.
Ministère de la Sante Publique. Plan Stratégique National de Lutte Contre Le Paludisme Au Cameroun 2007–2010. 2011.
Antonio-Nkondjio C, Defo-Talom B, Tagne-Fotso R, Tene-Fossog B, Ndo C, Lehman LG, et al. High mosquito burden and malaria transmission in a district of the city of Douala Cameroon. BMC Infect Dis. 2012;12:275.
Ebai CB, Ebongue FNE, Lum OKT, Musoro JZ, Yamssi C, Kimbi HK. Plasmodium falciparum parasitaemia during pregnancy and the use of malaria prevention methods by women attending antenatal consultation at the regional hospital Bamenda, Northwest Cameroon. Int J Trop Dis Health. 2020. https://doi.org/10.9734/ijtdh/2020/v41i2030393.
Mandeng SE, Awono-Ambene HP, Bigoga JD, Ekoko WE, Binyang J, Piameu M, et al. Spatial and temporal development of deltamethrin resistance in malaria vectors of the Anopheles gambiae complex from North Cameroon. PLoS ONE. 2019;14:e0212024.
National Program for the Fight Against Malaria (PNLP). Rapport annuel 2019 lutte contre le paludisme. 2019 Cameroon. http://onsp.minsante.cm/fr/publication/230/rapport-annuel-2019-lutte-contre-le-paludisme. Accessed 22 July 2022.
Nopowo F. Évaluation de l’efficacité Des Moustiquaires Imprégnées 36 Mois Après Leur Distribution Dans Le Sud Cameroun. Bull Soc Pathol Exot. 2020;113:289–97.
Antonio-Nkondjio C, Sandjo NN, Awono-Ambene P, Wondji CS. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: key parameters for success. Parasit Vectors. 2018;11:57.
Boyce MR, Katz R, Standley CJ. Risk factors for infectious diseases in urban environments of sub-saharan africa: a systematic review and critical appraisal of evidence. Trop Med Infect Dis. 2019;4:123.
Batoure A, Michel T. Logiciels libres et gestion des données à référence spatiale : cas des données urbaines de la ville de Ngaoundéré au Cameroun Free software's and spatial data management: case study of urban data of Ngaoundere in Cameroon. Master Thesis. University of Ngaoudere. 2015.
Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, et al. Urban malaria in Sub-Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20:364.
Ndo C, Menje Djantio B, Antonio-Nkondjio C. Awareness, attitudes and prevention of malaria in the cities of Douala and Yaoundé (Cameroon). Parasit Vectors. 2011;4:181.
Enama ML, Mbida AM, Takap NN, Ntonga PA, Mbiada B, Hondt OE, et al. Le paludisme: connaissances, attitudes et pratiques des chefs de ménage de la region de l’ouest-Cameroun. J Appl Biosci. 2020. https://doi.org/10.35759/JABS.147.5.
Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: Technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019;12:501.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LDA conceived and designed the study. LDA and HO performed the literature search. LDA and JE, with the contributions of others authors, interpreted and analysed data and wrote the paper. JE, LRM, PAA, PW, WE, SM, ENB, JCT and MP critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alenou, L.D., Nwane, P., Mbakop, L.R. et al. Burden of mosquito-borne diseases across rural versus urban areas in Cameroon between 2002 and 2021: prospective for community-oriented vector management approaches. Parasites Vectors 16, 136 (2023). https://doi.org/10.1186/s13071-023-05737-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05737-w