Abstract
Background
Ticks and tick-borne diseases play a major role in companion animal health. Additionally, the European tick fauna is changing, for instance due to the spread of Dermacentor reticulatus, displaying a higher likelihood of winter activity than Ixodes ricinus. Therefore, we investigated current tick infestations in dogs and cats in Germany and in parts of Austria and the seasonal infestation risk.
Methods
Overall, 219 veterinary practices were invited to collect ticks from cats and dogs on a monthly basis. Ticks were morphologically identified and female I. ricinus specimens were measured to estimate attachment duration.
Results
In total, 19,514 ticks, 17,789 (91.2%) from Germany and 1506 (7.7%) from Austria, were received between March 2020 and October 2021, with 10,287 specimens (52.7%) detached from dogs, 8005 from cats (41.0%) and 1222 from other species (6.3%). In Germany, the most common tick species collected from dogs were I. ricinus (78.0%) and D. reticulatus (18.8%), while cats mainly harboured I. ricinus (91.3%) and I. hexagonus (5.5%) and only few D. reticulatus (0.6%). In Austria, collected I. ricinus reached similar proportions in dogs (90.4%) and cats (95.3%), followed by D. reticulatus in both dogs (5.2%) and cats (1.5%), with I. hexagonus (0.9%) collected only marginally from cats. The average infestation intensity amounted to 1.62 ticks/dog and 1.88 ticks/cat. The single to multiple infestation ratio was 79.1% to 20.9% in dogs and 69.0% to 31.0% in cats, with cats being significantly more often multiple infested than dogs, while the proportion of mixed-species infestations was 2.0% for both dogs and cats. The average attachment duration of female I. ricinus specimens amounted to 78.76 h for dogs and 82.73 h for cats. Furthermore, year-round tick exposure was confirmed, with 108 D. reticulatus and 70 I. ricinus received on average per month during December 2020 to February 2021.
Conclusions
The study shows a year-round tick infestation risk, with activity of both D. reticulatus and I. ricinus during winter, and confirms the widespread occurrence of D. reticulatus in Germany. Additionally, long average attachment durations and frequent multiple infestations underline the need for adequate year-round tick control, even during the winter months.
Graphical Abstract

Similar content being viewed by others
Background
Ixodes ricinus, known as the sheep or castor bean tick, and Dermacentor reticulatus, known as the meadow or ornate dog tick, are the most common tick species in temperate Europe and act as vectors for various diseases of veterinary importance. Among the most frequent tick-borne diseases (TBDs) of dogs and cats in central Europe is granulocytic anaplasmosis, transmitted by I. ricinus. Furthermore, I. ricinus acts as a vector for Borrelia spirochaetes, causing Lyme borreliosis, and for tick-borne encephalitis virus (TBEV), which may be pathogenic to dogs [1]. Moreover, potentially fatal canine babesiosis transmitted by D. reticulatus represents a major threat for dogs [2, 3].
During the last decades, the central European tick fauna has undergone significant changes, with an increasing distribution of ticks across Europe as well as altered activity patterns with a tendency towards year-round activity [4, 5]. While the latitudinal and altitudinal range limit of I. ricinus is continuously increasing [6, 7], D. reticulatus has undergone a rapid and dramatic range expansion in central Europe, e.g. in Poland [8], the Czech Republic [9] and Germany [5, 10]. The reasons for the geographical spread are not yet fully elucidated and may include global climate change as well as changes in land use and biodiversity [10, 11]. The role of re- and deforestation is currently subject to debate [4, 12], whereas different studies mention an increasing wildlife population as a driving force for the spread of D. reticulatus [10, 13]. As D. reticulatus prefers open landscapes [14], increasing deforestation and a decrease of agricultural diversity may have contributed to the successful establishment of this tick species in many areas, while increased temperatures facilitate the completion of the tick’s life cycle within 1 year [15]. In addition, more and more dogs are traveling with their owners or are imported from different countries, promoting a spread of tick species across country borders [4, 13]. Related to this expansion of D. reticulatus, it is alarming that the incidence of canine babesiosis has increased in Germany and neighbouring countries in recent years [16,17,18].
In addition to changes in the geographic occurrence of ticks, altered tick activity patterns may result in an increased risk of TBD transmission. Particularly, increasingly mild winters may lead to year-round tick activity [19, 20]. It is commonly assumed that I. ricinus starts to be active at soil temperatures above approximately 5–7 °C [21], while D. reticulatus is active at a wider temperature range, starting from an air temperature of 0.7 °C and an even lower soil temperature of − 0.2 °C [22], while an increased level of activity has been recorded from 3.8 °C air temperature [23]. In addition, D. reticulatus displays high survival rates and activity during winter in the temperate climatic zone [24].
In light of the above-mentioned changes, a nation-wide update regarding tick exposure of dogs and cats is needed to adequately assess the infection risk and improve recommendations for veterinarians and pet owners. Previous studies investigating patterns of tick infestation in dogs and cats in Germany were mostly performed on a local scale and/or date several years back [10, 25, 26]. In the first of these studies, domestic and wild animals from north Baden, in the southwest of Germany, were examined over a 1-year period from 1993 to 1994. Overall, 434 ticks were collected, consisting of 88.7% I. ricinus (62.2% from cats and 25.6% from dogs) and 11.3% Ixodes hexagonus (3.2% from cats and 1.4% from dogs) [25]. Considering the northern German region of Berlin/Brandenburg, which has been known as D. reticulatus-endemic for several years, a study from 2003 indicated that the most frequently collected tick species from dogs was I. ricinus (60.8%), followed by a proportion of 11.2% D. reticulatus as well as 4.1% I. hexagonus [10]. The proportion of D. reticulatus on dogs increased to 45.0% in the same area in 2010/2011, while 46.0% of collected ticks were I. ricinus and 8.8% I. hexagonus [26]. These data indicate that a change in the tick population infesting dogs and cats occurred during the past decades, resulting in an increased or expanded risk of infection with TBDs, respectively. Meanwhile, D. reticulatus has been detected all over Germany [5], but the relative frequency on dogs and cats as compared to I. ricinus as well as the proportion of other possibly infesting tick species remains unknown on a national level. Therefore, the present study analysed geographic and seasonal patterns of tick exposure of dogs and cats in Germany and Austria based on ticks collected by participating veterinary practices.
Methods
Tick collection and morphological identification
Tick collection from infested dogs and cats was designed as convenience sampling by recruiting veterinary practices via sales representatives of Intervet Deutschland GmbH. In each of 27 sales areas, 10 practices were contacted with a focus on an even distribution over rural and urban areas. The contacted veterinary practices received an information folder and a registration form for participation in the study. Recruiting took place 1 month prior to study start and over the course of the first 5 months of the study. Of the contacted practices, a total of 225 agreed to participate, of which 219 participated actively (197 from Germany and 22 from Austria). Tick collection kits containing tick removal tools, 10 tick collection tubes (one tube to be used per animal) and 10 questionnaires each were sent to the participating veterinarians once a month from May 2020 to June 2021, i.e. over a 14-month period. In case > 10 animals were sampled per month, it was possible to reorder kits. For reasons of data protection, it was not possible to record the locations of the participating veterinarians, but the ticks’ geographic origin was obtained via a questionnaire in form of the owner’s postal zip code along with space for further specifications of the area of origin. Questionnaires were matched to the tick collection tubes with a unique identification number. Travel history during the last 2 weeks before tick collection was also documented. In addition, the date of tick detachment was indicated. After every month, collected ticks were sent to the Institute for Parasitology, University of Veterinary Medicine Hannover, where they were morphologically identified under a stereomicroscope (ZEISS Stemi SV 11) according to keys by A.M. Estrada-Peña, D. Andrei and T. Petney [14].
Determination of tick attachment duration
Female I. ricinus specimens were measured using the OLYMPUS cellSens Entry (v. 3.2) software paired with an OLYMPUS SC50 camera adapter to determine the coxal index and estimate the duration of attachment via the formula described by J. Gray, G. Stanek, M. Kundi and E. Kocianova [27]. Values > 245 h were assessed as not reliable and excluded. Due to a lack of data concerning the correlation of morphometric indices with engorgement time for D. reticulatus, calculation was not possible for this species and the attachment time was estimated visually. Here, the size increase of the idiosoma was used to classify three stages of engorgement: unengorged (0–24 h), partially engorged (> 24–144 h) and fully engorged (> 144 h), following Fielden et al. [28].
Tick distribution mapping and statistical analyses
Obtained data were documented in an Excel® spreadsheet (Microsoft Office Professional Plus 16). The geographic distribution of received ticks was plotted using R. v. 4.1.0 [29] with geographic data distributed by OpenStreetMap under the Open Database License (www.openstreetmap.org/copyright).
The distribution of the three most common tick species (I. ricinus, D. reticulatus and I. hexagonus) as well as the proportion of single and multiple infestations was compared between dogs and cats via Chi-square test or Fisher’s exact test if counts in any category were < 5. To examine seasonal differences in the ratio of single and multiple I. ricinus infestations between dogs and cats, separate Chi-square tests were carried out for ticks collected from December to February (winter), March to May (spring), June to August (summer) as well as September to November (autumn). P-values were corrected for multiple comparisons using the Bonferroni method. Tick attachment duration was compared between dogs and cats via Mann-Whitney U-test.
Results
Received tick species
In total, 19,514 tick specimens were collected along with respective documentation via questionnaire, 18,126 of them between May 2020 and June 2021, during the originally envisioned 14-month study period. The remaining 1388 ticks were additionally collected from March–April 2020 (10/1,388) and July–October 2021 (1193/1388), or no date of collection was documented on the questionnaire (185/1388).
Most received ticks were identified as I. ricinus (15,943/19,514, 81.70%), followed by D. reticulatus (2,013/19,514, 10.32%) and I. hexagonus (1012/19,514, 5.19%). Furthermore, Dermacentor marginatus (38/19,514, 0.19%), Rhipicephalus sanguineus (8/19,514, 0.04%), Haemaphysalis concinna (9/19,514, 0.05%), Ixodes canisuga (7/19,514, 0.04%) and Ixodes frontalis (6/19,514, 0.03%) were among the received ticks. The remaining 452 ticks (2.32%) were only identified to genus level because of a deteriorated condition or the difficulty to differentiate between larval and nymphal I. hexagonus and I. canisuga (precisely 258 I. hexagonus/I. canisuga, 161 Ixodes spp., 43 Dermacentor spp. and 4 Rhipicephalus spp.).
In terms of host species, 8095 (78.69%) of the ticks found on dogs were identified as I. ricinus, 1860 (18.08%) as D. reticulatus and 166 (1.61%) as I. hexagonus (Fig. 1). Further tick species detected on dogs were R. sanguineus, H. concinna, I. canisuga, I. frontalis and D. marginatus (Table 1). Of all the ticks found on cats, 7344 (91.74%) were identified as I. ricinus, 398 (4.97%) as I. hexagonus and 56 (0.70%) as D. reticulatus (Fig. 1). This distribution differed significantly compared to dogs (χ2 = 1555.5, df = 2, P < 0.001). Apart from dogs and cats, various other host species contributed a total of 1222 ticks, as summarised in Table 1. Regarding tick developmental stages, only adult D. reticulatus specimens were sent in. For I. ricinus, 1.07% (87/8095) of specimens found on dogs and 1.67% (123/7344) of specimens from cats were immature stages (larvae or nymphs), respectively (Fig. 2). Regarding I. hexagonus, 18.67% (31/166) of the specimens detached from dogs and 43.72% (174/398) of those from cats were immature (Fig. 2). Immature stages of I. ricinus (larvae: 30/15,913; 1.89%; nymphs: 347/15,913; 2.18%) and I. hexagonus (larvae: 12/1012; 1.19%; nymphs: 226/1012; 22.33%) were mainly sent in from Germany, while only 2 larvae and 10 nymphs of I. ricinus were received from Austria.
Geographical distribution of received ticks
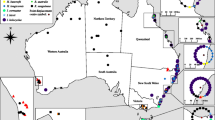
Most of the collected ticks were sent in from Germany (17,789/19,514; 91.16%) and Austria (1,506/19,514; 7.72%). In addition, some sporadic submissions from France (15/19,514; 0.08%), The Netherlands (3/19,514; 0.02%) and Switzerland (3/19,514; 0.02%) were received, while the country and postal code of 198/19,514 submissions (1.01%) was not indicated. The distribution of I. ricinus and D. reticulatus per postal code area is shown in Fig. 3. As expected, I. ricinus was received from all participating veterinarians. Due to the location of recruited practices, some clustering (> 50 ticks per postal code area) in the German federal states of Lower Saxony and Bavaria was observed. Regarding the federal state of Mecklenburg-Western Pomerania, only few ticks were received because only a few veterinarians from the northeast of this federal state participated in the study.
Geographical distribution (zip code areas) and number of all ticks (A), I. ricinus (B) and D. reticulatus (C) detached from dogs and cats in Germany and Austria. A map of the federal states of Germany and Austria is shown in the upper right corner (Germany: BW = Baden-Württemberg, BY = Bavaria, BE = Berlin, BB = Brandenburg, HB = Bremen, HH = Hamburg, HE = Hesse, LS = Lower Saxony, MV = Mecklenburg-Vorpommern, NW = North Rhine-Westphalia, RP = Rhineland-Palatinate, SL = Saarland, SN = Saxony, ST = Saxony-Anhalt, SH = Schleswig–Holstein, TH = Thuringia; Austria: BU = Burgenland, CA = Carinthia, LA = Lower Austria; UA = Upper Austria, SA = Salzburg; ST = Styria, TY = Tyrol, VA = Vorarlberg, VI = Vienna)
Except for Schleswig-Holstein and the two city states Hamburg and Bremen in the northernmost part of Germany, from where fewer ticks were received overall because of few participating veterinarians, D. reticulatus was also sent in from all German federal states. Primarily, D. reticulatus specimens were sent in from the east of Germany, where the proportion of D. reticulatus among ticks found on dogs reached up to 66.67% (federal state of Saxony-Anhalt, Table 2, Fig. 4). Among the remaining federal states, this proportion varied between 0.99 and 63.59%. Regarding cats, the proportion of D. reticulatus varied between 0 and a maximum of 1.81% (federal state of Saxony-Anhalt, Table 2). The infestation rates with the most frequently collected tick species among tick-infested dogs and cats in the different German and Austrian federal states are given in Additional file 1: Table S1.
Proportions of the three most frequent tick species detached from dogs per German federal state. A map of the federal states of Germany is shown in the upper right corner (Germany: BW = Baden-Württemberg, BY = Bavaria, BE = Berlin, BB = Brandenburg, HB = Bremen, HH = Hamburg, HE = Hesse, LS = Lower Saxony, MV = Mecklenburg-Vorpommern, NW = North Rhine-Westphalia, RP = Rhineland-Palatinate, SL = Saarland, SN = Saxony, ST = Saxony-Anhalt, SH = Schleswig-Holstein, TH = Thuringia)
Due to the lower number and uneven distribution of recruited veterinary practices in Austria, ticks were mostly sent in from five federal states, namely Burgenland, Salzburg, Styria, Lower Austria and Tyrol, with sporadic collections in other areas. Regarding D. reticulatus, 91.15% (43/47) of the Austrian specimens were received from the state of Burgenland, with 22 specimens (46.81%) found in only one postal code area (7023). Most I. ricinus submissions came from Salzburg (284/1,375; 20.65%), Styria (271/1,375; 19.71%), Burgenland (261/1,375; 18.98%) and Lower Austria (223/1,375; 16.22%), so that other parts of Austria may be underrepresented, since only a few veterinarians participated in Austria. An overview of the distribution of the most frequently collected tick species from cats and dogs over the Austrian federal states is given in Additional file 2: Table S2.
Characterisation of tick origin
Only a few owners gave specific information on animal husbandry, namely that the animal only had access to their yard or garden (280/18,295; 1.53%) or lives near the coast (43/18,295; 0.23%).
Most of the ticks were probably acquired in the declared postal code area, as the host animals had not left this region during the last 2 weeks before tick collection (16,178/19,514; 82.90%). While 6.90% (1,347/19,514) of host animals had a travel history beyond the given postal code area or even beyond the German border, in 10.19% (1,989/19,514) of cases, no data on travel history were available.
Infestation intensity and co-infestations in dogs vs. cats
In total, 6335 dogs were part of this submission study and provided a total of 10,287/19,514 ticks, so that the average infestation was 1.62 ticks per dog. Of all dogs, 5011 (79.10%) were infested with a single tick only and 1324 (20.90%) with more than one tick, whereby the maximum number of detached ticks from one dog amounted to 96. This dog was reported as a Labrador-Husky mix co-infested with 91 adult I. ricinus and 5 adult D. reticulatus.
Concerning cats, 4248 animals were sampled and provided a sample size of 8,005/19,514 ticks, so that the average infestation was 1.88 ticks per cat. The number of single infestations amounted to 2932 (69.02%), while 1316 cats (30.98%) were infested with multiple ticks. The proportion of multiple infestations on cats was significantly higher compared to dogs (χ2 = 137.45, df = 1, P < 0.001). The highest infestation intensity was recorded on a European shorthair, which was infested with 54 I. hexagonus specimens, including 50 nymphs and four females.
The seasonal pattern of infestations with multiple vs. single specimens of the most frequently detected tick species (i.e. I. ricinus and D. reticulatus for dogs and I. ricinus and I. hexagonus for cats) is visualised in Fig. 5. Regarding I. ricinus, both single and multiple infestations peaked during the period of main tick activity, which was from May to July (Fig. 5A, C). Concerning D. reticulatus, also both infestation types peaked during the tick’s main activity periods, e.g. September to October 2020 as well as in March to April 2021 (Fig. 5B). In contrast, single infestations with I. hexagonus in cats occurred throughout the study period without distinct peaks, while infestations with multiple specimens peaked in May 2021 (Fig. 5D). While in dogs the number of single infestations was always above the level of multiple infestations, cats were infested with multiple ticks more often in the times of species-related activity peaks (Fig. 5C, D). Cats had a significantly higher proportion of multiple infestations with I. ricinus than dogs in each season of the year (Table 3).
The seasonal comparison of single and multiple infestations between the two most frequently collected tick species per host showed significant differences in dogs. The proportion of multiple infestations by D. reticulatus was significantly higher compared to I. ricinus in the autumn of 2020 (χ2 = 47.092, df = 1, P < 0.001), winter of 2020/21 (χ2 = 21.886, df = 1, P < 0.001) and spring 2021 (Fisher’s exact test, P < 0.001), while in summer 2021 the opposite pattern occurred (χ2 = 9.3044, df = 1, P < 0.001). For cats, no significant difference between seasonal rates of multiple infestations with I. ricinus and I. hexagonus was recorded (P > 0.05).
Most multiple infestations were limited to one tick species, while fewer mixed-species infestations were also observed, namely in 128 (2.02%) dogs and 84 (1.98%) cats. These showed a similar pattern in dogs and cats throughout the study and peaked between March and June 2021 (Fig. 6). Regarding the different tick species in these mixed infestations, in dogs almost as many D. reticulatus (40.1%) as I. ricinus (48.5%) specimens were found, followed by some I. hexagonus (3.2%). In cats, the dominating species was I. ricinus (59.3%), followed by I. hexagonus (17.1%) and not further determinable Ixodes sp. (9.9%).
Attachment duration
Attachment duration was calculated for 10,871 female I. ricinus, 5175 of which were collected from dogs and 5544 from cats. For another 1421 specimens collected from dogs (21.54%) and 674 of those from cats (10.84%), the attachment time was not determinable because of calculation limits resulting in extreme values; therefore, these ticks were excluded. Dogs infested with female I. ricinus specimens harboured these for 78.76 h on average (standard deviation [SD] = 45.22 h, median = 76.85 h). In cats, the average attachment time was slightly higher (Mann-Whitney U-test, χ2 = 13,300,506, P < 0.001), with an average of 82.73 h (SD = 41.65 h, median = 82.94 h; Fig. 7).
Among the 1159 female D. reticulatus specimens collected from dogs, 42.02% (487/1159) were fully engorged, 21.92% (254/1159) were partially engorged and 35.98% (417/1159) were detached before engorgement started, while for one specimen the stage of engorgement was impossible to determine because of the tick’s condition. In cats, the distribution was similar although the sample size was considerably lower. Of the 33 collected females, 45.45% (15/33) were fully engorged, 21.21% (7/33) were partially engorged and 33.33% (11/33) were unengorged.
Temporal course of I. ricinus and D. reticulatus collection
To account for the fact that the number of actively participating veterinary practices varied between individual months, the monthly number of received ticks was divided by the number of active participants in that month (Fig. 8a). The study started in May 2020 with 86 participating veterinarians and resulted in a maximum number of 219 recruited participants in September 2020, 197 from Germany and 22 from Austria. After September 2020, no new participants were recruited and the number of actively participating veterinarians, meaning all participants that did not sign off for the month and got a new collection kit, never dropped below 200 during the predetermined study period up to June 2021 (Fig. 8). In July 2021, just 160 veterinarians were supplied with tick collection boxes because only remaining kits were sent out. However, veterinarians may have used remaining boxes from the preceding months, as evidenced by the fact that some ticks were still sent in after the previously determined study period from July to October 2021 (1193/19,514; 6.11%). Therefore, the number of ticks per actively participating veterinarian was not calculated as of July 2021. Concerning the main study period, it cannot be excluded that some veterinarians were not taking part during some months despite having ordered boxes. Nevertheless, normalising tick numbers by the recorded number of participants per month was regarded to yield a more accurate estimate of tick activity than normalising by incoming boxes because empty boxes may not have been sent to our institute.
Collected I. ricinus and D. reticulatus specimens per actively participating veterinarian over the course of the study period (A) and total number of received ticks per month (B). The number of specimens per active participant is not indicated for March to April 2020 or July to October 2021, as these periods were outside the 14-month study period and no collection kits were sent out for these months
Both I. ricinus and D. reticulatus were collected throughout the year (Fig. 8). Regarding the temporal distribution of received I. ricinus ticks, two major peaks were evident, namely in June 2020 (2007 ticks received in total, 13.93 per participant) and May 2021 (3300 ticks received in total, 15.71 per participant). A smaller peak was also observed in September 2020 (524 ticks received in total, 2.00 per participant). Over the winter period from December 2020 to February 2021, an average number of 70 ticks per month was sent in (0.34 ticks per participant).
Regarding D. reticulatus, collections peaked in September 2020 (345 ticks received in total, 1.56 per participant) and in March 2021 (300 ticks received in total, 1.49 per participant). Over the winter period (December 2020–February 2021), an average of 108 D. reticulatus specimens was sent in per month (0.53 ticks per participant).
Discussion
The present study constitutes the first large-scale investigation of tick infestation of cats and dogs from all over Germany and parts of Austria. Previous studies have investigated patterns of tick infestation on a local scale and mainly concentrated on dogs, e.g. in southwestern Germany [25] or in the Berlin/Brandenburg area, focusing on the regional importance of D. reticulatus [10, 26]. In the light of climate change and altered tick activity patterns/species distributions, a large-scale study on the year-round tick infestation risk was urgently needed. Therefore, the present study also included the winter period. The fact that almost 20,000 ticks were obtained during the 14-month study period indicates that many dogs and cats are inadequately protected. Therefore, recommendations for tick control as well as counselling of animal owners need to be improved to protect cats and dogs from infection with TBDs as well as to slow the spread and increasing local abundance of D. reticulatus on a national and European scale.
Frequently collected tick species and their geographic distribution
As expected, most ticks collected from dogs and cats (81.7%) were identified as I. ricinus. However, D. reticulatus represented the second most common tick species, accounting for one in 10 ticks (10.3%) in total and for almost every fifth tick (18.1%) collected from dogs. This is in line with the fact that this tick species is now widely spread over Germany, with some highly endemic areas in eastern Germany [5]. Accordingly, D. reticulatus was received from all federal states, except for the northernmost part of the country (federal state of Schleswig-Holstein, city states of Bremen and Hamburg). These northernmost areas together comprise only approximately 6.5% of the German population [30] and were therefore sampled less intensely, with only 273 ticks collected from dogs compared to other northern federal states like Lower Saxony, represented by 1384 ticks collected from dogs. Therefore, further local investigations are necessary to assess the abundance and distribution of tick species in these most northern areas. Most D. reticulatus specimens originated from the eastern federal states (Saxony-Anhalt, Saxony, Brandenburg and Berlin) and the Rhine Valley, which represent the original distribution areas of D. reticulatus in Germany [31]. In 2013, a study in Brandenburg already determined a proportion of 45.0% D. reticulatus specimens among all ticks detached from dogs, which almost corresponded to the percentage of I. ricinus (46.0%) [26]. In the present study, the proportion of D. reticulatus on dogs amounted to 55.0% in Brandenburg and ranged from 1.0% in the federal state of North Rhine-Westphalia to 66.0% in Saxony-Anhalt. In the highly D. reticulatus-endemic regions, this tick species thus seems to displace I. ricinus as the most common tick species parasitising dogs, which is alarming in light of the vector function for Babesia canis. An increasing canine babesiosis incidence has already been observed in the Berlin/Brandenburg area [32] and in the federal state of Hesse [18], underlining the need for proper tick control. In this context, differences in the duration of action of acaricides against different tick species need to be considered. For example, several permethrin-based products have a 4-week duration of action against I. ricinus but only 3 weeks against D. reticulatus, so more frequent treatment in D. reticulatus-endemic areas is necessary. Some fipronil- or deltamethrin-based products are not licensed against D. reticulatus at all. A thorough counselling of pet owners by veterinarians concerning the different acaricides is therefore necessary to achieve adequate protection.
Regarding Austria, only 22 veterinary practices participated in the current study, so it was not possible to assess a nation-wide geographic distribution pattern for Austria. Nevertheless, only 3.1% of all ticks from Austria were identified as D. reticulatus compared to every 10th tick from Germany (10.9%). These numbers are comparable with German areas characterised by low D. reticulatus abundance as well as with numbers from Switzerland [33]. Most of the Austrian D. reticulatus specimens originated from the eastern part of the country, which is already known as a D. reticulatus-endemic area [34, 35].
The comparably low proportion of D. reticulatus obtained from cats (0.70%) reflects the affinity of D. reticulatus for canine hosts. Similarly, only 0.6% of 2535 respectively 0.4% of 1960 D. reticulatus specimens from two citizen science studies were detached from cats compared to 47.8% respectively 66.9% from dogs [5, 36]. Consequently, I. hexagonus, with a share of 5.0%, was the second most common tick species after I. ricinus (91.7%) found on cats and the third most common species found on dogs (1.6%). Previous studies also identified these three tick species as the most frequently detached ticks from dogs in Germany [10, 26] and further central European countries [33, 37,38,39], while cats have only rarely been studied in this regard [25, 39].
In cats, the comparably high I. hexagonus proportion in relation to dogs is probably driven by its species-specific behaviour [40]. Due to their nocturnal activity, often unrestricted hunting drive and smaller body size, cats probably come into contact with hedgehogs and their nests more often than dogs. Interestingly, in a study by Dautel et al. [10], 4.1% of ticks detached from dogs were identified as I. hexagonus, while this proportion amounted to only 1.6% in the current study. This may be due to the increased proportion of D. reticulatus on dogs in the present study, which amounted to only 9.1% in the study by Dautel et al. [10].
Related to the differences in tick species distribution, mixed-species infestations were approximately detected as often in dogs (2.02%) as in cats (1.98%). For both cats and dogs, the distribution of mixed-species infestations throughout the year followed the activity peak of I. ricinus. In dogs, an additional increase during the main activity period of D. reticulatus in October and November was observed.
Further tick species
Apart from I. ricinus, D. reticulatus and I. hexagonus, several other Ixodidae were sporadically sent in. These included ticks primarily associated with wildlife, such as I. frontalis, I. canisuga and H. concinna, which were rarely detected on dogs and cats. Of note, three of seven received H. concinna specimens were detached from hunting dogs. Furthermore, D. marginatus was received primarily from horses, humans, sheep and cattle. The predominant association with hoofed animals was not surprising as these are known as preferred hosts for adult D. marginatus [5, 9, 14]. Similarly, 35.0% of D. marginatus received in the frame of a citizen science study were detached from horses but only 3.04% from dogs [5].
Moreover, R. sanguineus, known as the brown dog tick, plays a role as an imported tick species in Germany and Austria [41]. As expected, R. sanguineus was found exclusively on dogs, mainly on those with a travel history to Bosnia, Bulgaria and Hungary, where this tick species is widely distributed [14, 42]. Although only few R. sanguineus specimens were received, these findings show that import by travelling dogs occurs on a regular basis. In central Europe, R. sanguineus may reproduce inside buildings, which should be prevented by adequate tick prophylaxis and monitoring of travelling dogs or dogs imported by animal welfare organisations or private individuals, respectively.
Tick developmental stages
Regarding the tick developmental stages, the results are similar to previous tick submission studies from Germany and other European countries with the same collected tick species [26, 38]. While for I. ricinus only 2.3% of all received specimens were nymphs or larvae, this proportion amounted to 23.5% for I. hexagonus collected from dogs and even to 43.7% of those collected from cats. Similarly, foxes also harbour a much larger proportion of I. ricinus adults than nymphs [43], in contrast to I. hexagonus and I. canisuga [44], indicating that host preference may play a role regarding this pattern. In addition, single infestations with immature stages of I. ricinus may be harder to detect because of the ticks’ small size, while infestations with immature stages of nidicolous ticks are more likely to result in higher infestation intensities. Such high-intensity infestations are less likely to be missed; as a result, the proportion of I. ricinus immature stages present on dogs and cats may be underestimated compared to I. hexagonus/I. canisuga. In case of D. reticulatus, only adult stages were sent in. This is consistent with the fact that juvenile stages are nidicolous, live in close association with small mammals and have a relatively short seasonal activity period in summer with an engorgement period of 12–19 days for larvae and 3–4 days for nymphs [14].
Infestation intensity and attachment duration
Every third cat (31.0%), but only every fifth dog (20.9%), was infested with multiple ticks. As half of the infested cats (2294/4248; 54.0%) were described as mousers, they probably spend more time in contact with vegetation and wildlife compared to dogs typically being walked by their owner for a limited amount of time. The maximum number of reported ticks from one cat was 54 specimens, all identified as I. hexagonus (4 females and 50 nymphs). This seems credible as contact with a hedgehog or its nest can result in such high-level infestation, especially concerning nymphs [14]. Nevertheless, the proportion of multiple infestations caused by I. hexagonus was never significantly larger compared to I. ricinus, which may have been expected because of the species’ biology, i.e. the nest adapted way of life.
Regarding multiple infestations of dogs, a Labrador-Husky mix was infested with 96 specimens (93 adult I. ricinus and 3 adult D. reticulatus). This very high infestation intensity was unexpected as, in contrast to high tick burdens caused by R. sanguineus in southern European countries [45], such a high infestation intensity of dogs, especially concerning only adult stages, is uncommon for the sampling region and was an exception in our dataset. It cannot entirely be ruled out that the sender added ticks from multiple animals into the same tube, but due to the extremely dense undercoat of this particular breed it is also conceivable that the ticks were missed by the owner and accumulated over time.
While cats had more multiple infestations during the periods of high tick activity, in dogs single infestations dominated the whole year round. In addition to the differences in host behaviour mentioned above, another possible reason for this pattern may be that owners are recognising tick infestations in dogs faster than in cats, resulting in removal of many ticks. This may be related to the often closer physical relationship of owners and dogs, including long and intensive grooming that often is not possible with free-roaming cats because of their more independent way of life. However, the average attachment time of 82.73 h for I. ricinus females on cats was only slightly higher compared to dogs (78.76 h), so the often more intimate relationship of owners to dogs did not reduce tick attachment time to an acceptable level compared to cats. These long attachment durations indicate a substantial risk of TBD transmission. Most pathogens, including Anaplasma phagocytophilum and Borrelia spp., are transmitted after 24–48 h of tick attachment [46, 47]. Regarding B. canis, the process of sporogony in the salivary glands takes about 48 h before transmission can occur [48]. However, an experimental approach showed that earlier transmission with pre-activated D. reticulatus specimens is possible within 8 h of infestation [49]. Thus, the long attachment durations observed in the current study show that visual inspection is an inadequate measure of tick control. Only for animals with minimal infection risk, meaning animals with limited or no outdoor access, may visual examination and acaricide treatment only after proven infestation be sufficient. Since no experimental data exist on the relationship between attachment duration and morphometric dimensions of D. reticulatus, the average engorgement time of D. reticulatus could not be determined in the present study. Nevertheless, a similar average engorgement duration as for I. ricinus can be assumed, considering the fact that almost 60% of specimens were visually assessed as partially or fully engorged.
Seasonal abundance
Regarding seasonality, most I. ricinus specimens were collected in May/June of both sampled years. The lower absolute number of ticks received in May/June 2020 compared to 2021 is related to the fact that the study started in April 2020 and recruiting of participants was still ongoing until October 2020. Nevertheless, the average monthly number of ticks per actively participating veterinarian correlates with the total number of received ticks per month. The observed spring peak represents the well-known activity pattern of I. ricinus [50,51,52]. Dermacentor reticulatus collections showed two peaks, one in autumn 2020 and a slightly smaller one in early spring 2021. This pattern also corresponds to the literature [51,52,53], although some studies also report a higher spring than autumn peak [35, 54, 55].
Overall, there is a complementary activity pattern of I. ricinus and D. reticulatus, i.e. D. reticulatus generally shows high activity in the cooler months of the year, when activity of I. ricinus is rather low. Combined with the spread of D. reticulatus, this leads to an increased risk of tick infestation in the colder months of the year, especially for dogs. Nevertheless, winter activity of both tick species was evident. In particular, it was apparent for D. reticulatus, which is known to be more cold-tolerant than I. ricinus [20]. For I. ricinus, winter activity has been reported more sporadically, especially under mild circumstances [19]. According to data from the German weather service [56], the meteorological winter 2020/2021 and the preceding winters were unusually mild, with an average temperature of 1.8 °C, deviating by + 1.6 °C from the previous long-term mean (1961–1990). These mild conditions were probably conducive to a constant winter activity of both species, with a lack of, delayed or early termination of diapause [57]. In February 2021, a cold period lasting approximately 2 weeks with temperatures around − 20 °C and heavy snowfall affected large parts of Germany, which however did not seem to affect the activity of I. ricinus and D. reticulatus in the following spring, given the observed spring peak of submissions. In the face of future climate scenarios with increasingly mild winters, it is important to stress that tick winter activity no longer reflects sporadic or coincidental findings. Indeed, both questing D. reticulatus and I. ricinus were found on the vegetation during all winter months in 2021/2022 (unpublished own data). This finding in combination with the observed year-round tick infestation of dogs and cats in the study presented here shows that the traditional “standard treatment period” from April to October, covering the main activity period of I. ricinus, is outdated. Therefore, since both tick species transmit relevant pathogens, effective tick control in dogs and cats should no longer be limited to certain months but should be practiced all year round.
Conclusions
Compared to previous surveys, the present large-scale study revealed marked differences in tick exposure between dogs and cats in Germany and Austria. While I. hexagonus remains the second most frequent tick species affecting cats, almost every fifth tick collected from dogs was identified as D. reticulatus. Therefore, the dramatic range expansion of D. reticulatus is confirmed once more, leading to an increasing risk of infection with B. canis. Adequate tick control, considering the shorter duration of action of several acaricides against D. reticulatus, is therefore indispensable and may limit the further spread of canine babesiosis, which is already on the rise in Germany. Moreover, the high percentage of multiple infestations and long average attachment duration of more than 3 days indicate a substantial risk of TBD transmission and the inadequacy of visual tick control in animals with regular outdoor access. Although the infestation risk still seems to be highest in the species-specific periods of main activity, winter activity of D. reticulatus, and to a lesser extent also I. ricinus, was observed. Because of the complementary and expanding activity patterns of these two tick species, no time of the year can be regarded as a period of negligible tick infestation risk anymore and a year-round use of licensed acaricides is therefore advocated in dogs and cats. Thorough counselling of pet owners by veterinarians regarding product choice, correct administration and duration of action is essential to protect animals successfully against health-threatening TBDs.
Availability of data and materials
Data supporting reported results are contained within the article. Further data can be obtained from the corresponding author on reasonable request.
Abbreviations
- TBD:
-
Tick borne diseases
- SD:
-
Standard deviation
References
Springer A, Glass A, Topp A-K, Strube C. Zoonotic tick-borne pathogens in temperate and cold regions of Europe—a review on the prevalence in domestic animals. Front Vet Sci. 2020. https://doi.org/10.3389/fvets.2020.604910.
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336. https://doi.org/10.1186/s13071-016-1596-0.
Bajer A, Beck A, Beck R, Behnke JM, Dwużnik-Szarek D, Eichenberger RM, et al. Babesiosis in Southeastern, Central and Northeastern Europe: an emerging and re-emerging tick-borne disease of humans and animals. Microorganisms. 2022;10:945.
Karbowiak G. The occurrence of the Dermacentor reticulatus tick - its expansion to new areas and possible causes. Ann Parasitol. 2014;60:37–47.
Drehmann M, Springer A, Lindau A, Fachet K, Mai S, Thoma D, et al. The Spatial distribution of Dermacentor ticks (Ixodidae) in Germany - evidence of a continuing spread of Dermacentor reticulatus. Front Vet Sci. 2020;7:578220. https://doi.org/10.3389/fvets.2020.578220.
De Pelsmaeker N, Korslund L, Steifetten Ø. High-elevational occurrence of two tick species, Ixodes ricinus and I trianguliceps, at their northern distribution range. Parasit Vectors. 2021;14:161. https://doi.org/10.1186/s13071-021-04604-w.
Hvidsten D, Frafjord K, Gray JS, Henningsson AJ, Jenkins A, Kristiansen BE, et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020;11:101388. https://doi.org/10.1016/j.ttbdis.2020.101388.
Zając Z, Woźniak A, Kulisz J. Density of Dermacentor reticulatus ticks in eastern Poland. Int J Environ Res Public Health. 2020;17:2814. https://doi.org/10.3390/ijerph17082814.
Daněk O, Hrazdilová K, Kozderková D, Jirků D, Modrý D. The distribution of Dermacentor reticulatus in the Czech Republic re-assessed: citizen science approach to understanding the current distribution of the Babesia canis vector. Parasit Vectors. 2022;15:132. https://doi.org/10.1186/s13071-022-05242-6.
Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp RpA4. Int J Med Microbiol. 2006;296:149–56. https://doi.org/10.1016/j.ijmm.2006.01.013.
Medlock JM, Hansford KM, Vaux AGC, Cull B, Abdullah S, Pietzsch ME, et al. Distribution of the tick Dermacentor reticulatus in the United Kingdom. Med Vet Entomol. 2017;31:281–8. https://doi.org/10.1111/mve.12235.
Mierzejewska E, Estrada-Peña A, Bajer A. Spread of Dermacentor reticulatus is associated with the loss of forest area. Exp Appl Acarol. 2017;72:399–413. https://doi.org/10.1007/s10493-017-0160-8.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314. https://doi.org/10.1186/s13071-016-1599-x.
Estrada-Peña AM, Andrei D, Petney T. Ticks of Europe and North Africa: a guide to species identification. New York: Springer; 2018.
Nosek J. The ecology, bionomics, behaviour and public healh importance of Dermacentor marginatus and D. reticulatus ticks. Wiad Parazytol. 1972;18:5–6.
Dwużnik-Szarek D, Mierzejewska EJ, Rodo A, Goździk K, Behnke-Borowczyk J, Kiewra D, et al. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasit Vectors. 2021;14:267. https://doi.org/10.1186/s13071-021-04758-7.
Kohn CW, Helm C, Schäfer I, Pachnicke S, Müller E, von Samson-Himmelstjerna G, et al. Autochthonous Babesia canis infections in 25 dogs in Berlin/Brandenburg (Germany). J Vet Intern Med. 2021;35:3198.
Seibert S, Rohrberg A, Stockinger A, Schaalo S, März I. Vorkommen von kaniner Babesiose bei Hunden im Rhein-Main-Gebiet in Hessen – eine Fallstudie mit 81 Hunden. Tierarztl Prax Ausg K Klientiere Heimtiere. 2022;50:162–72. https://doi.org/10.1055/a-1704-6604.
Dautel H, Dippel C, Kämmer D, Werkhausen A, Kahl O. Winter activity of Ixodes ricinus in a Berlin forest. Int J Med Microbiol. 2008;298:50–4. https://doi.org/10.1016/j.ijmm.2008.01.010.
Kiewra D, Czułowska A, Lonc E. Winter activity of Dermacentor reticulatus (Fabricius, 1794) in the newly emerging population of Lower Silesia, south-west Poland. Ticks Tick Borne Dis. 2016;7:1124–7. https://doi.org/10.1016/j.ttbdis.2016.08.012.
Pretzmann G, Radda A, Loew J. Studien zur Ökologie von Ixodes ricinus L. in einem Endemiegebiet der Frühsommermeningoenzephalitis (FSME) im Bezirk Neunkirchen (Niederösterreich). Z Morphol Oekol Tiere. 1964;54:393–413.
Kaszewski B. Climatic conditions in Lublin region. Wydawnictwo UMCS Lublin. 2008:1–60.
Hubálek Z, Halouzka J, Juricova Z. Host-seeking activity of ixodid ticks in relation to weather variables. J Vector Ecol. 2003;28:159–65.
Zając Z, Bartosik K, Kulisz J, Woźniak A. Ability of Adult Dermacentor reticulatus ticks to overwinter in the temperate climate zone. Biol. 2020. https://doi.org/10.3390/biology9070145.
Beichel E, Petney TN, Hassler D, Brückner M, Maiwald M. Tick infestation patterns and prevalence of Borrelia burgdorferi in ticks collected at a veterinary clinic in Germany. Vet Parasitol. 1996;65:147–55. https://doi.org/10.1016/0304-4017(96)00943-0.
Beck S, Schreiber C, Schein E, Krücken J, Baldermann C, Pachnicke S, et al. Tick infestation and prophylaxis of dogs in northeastern Germany: a prospective study. Ticks Tick Borne Dis. 2014;5:336–42. https://doi.org/10.1016/j.ttbdis.2013.12.009.
Gray J, Stanek G, Kundi M, Kocianova E. Dimensions of engorging Ixodes ricinus as a measure of feeding duration. Int J Med Microbiol. 2005;295:567–72. https://doi.org/10.1016/j.ijmm.2005.05.008.
Fielden LJ, Jones RM, Goldberg M, Rechav Y. Feeding and respiratory gas exchange in the American dog tick. Dermacentor variabilis J Insect Physiol. 1999;45:297–304. https://doi.org/10.1016/S0022-1910(98)00127-9.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021.
BPB: Federal Agency for Civic Education. https://www.bpb.de/kurz-knapp/zahlen-und-fakten/soziale-situation-in-deutschland/61535/bevoelkerung-nach-bundeslaendern/ (2022). Accessed 11 Nov 2022.
Rubel F, Brugger K, Monazahian M, Habedank B, Dautel H, Leverenz S, et al. The first German map of georeferenced ixodid tick locations. Parasit Vectors. 2014;7:477. https://doi.org/10.1186/s13071-014-0477-7.
Helm CS, Weingart C, Ramünke S, Schäfer I, Müller E, von Samson-Himmelstjerna G, et al. in a recent local outbreak in Berlin/ Brandenburg. Germany Transbound Emerg Dis. 1895. https://doi.org/10.1111/tbed.14617.
Eichenberger RM, Deplazes P, Mathis A. Ticks on dogs and cats: A pet owner-based survey in a rural town in northeastern Switzerland. Ticks Tick Borne Dis. 2015;6:267–71. https://doi.org/10.1016/j.ttbdis.2015.01.007.
Hodžić A, Zörer J, Duscher GG. Dermacentor reticulatus, a putative vector of Babesia cf.. microti (syn. Theileria annae) piroplasm. Parasitol Res. 2017;116:1075–7. https://doi.org/10.1007/s00436-017-5379-0.
Duscher GG, Feiler A, Leschnik M, Joachim A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit Vectors. 2013;6:76. https://doi.org/10.1186/1756-3305-6-76.
Springer A, Lindau A, Probst J, Drehmann M, Fachet K, Thoma D, et al. Update and prognosis of Dermacentor distribution in Germany: Nationwide occurrence of Dermacentor reticulatus. Front Vet Sci. 2022. https://doi.org/10.3389/fvets.2022.1044597.
Król N, Obiegala A, Pfeffer M, Lonc E, Kiewra D. Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration. South-West Poland Parasit Vectors. 2016;9:351. https://doi.org/10.1186/s13071-016-1632-0.
Abdullah S, Helps C, Tasker S, Newbury H, Wall R. Ticks infesting domestic dogs in the UK: a large-scale surveillance programme. Parasit Vectors. 2016;9:391. https://doi.org/10.1186/s13071-016-1673-4.
Geurden T, Becskei C, Six RH, Maeder S, Latrofa MS, Otranto D, et al. Detection of tick-borne pathogens in ticks from dogs and cats in different European countries. Ticks Tick Borne Dis. 2018;9:1431–6. https://doi.org/10.1016/j.ttbdis.2018.06.013.
Ogden NH, Cripps P, Davison CC, Owen G, Parry JM, Timms BJ, et al. The ixodid tick species attaching to domestic dogs and cats in Great Britain and Ireland. Med Vet Entomol. 2000;14:332–8. https://doi.org/10.1046/j.1365-2915.2000.00244.x.
Buczek A, Buczek W. Importation of ticks on companion animals and the risk of spread of tick-borne diseases to non-endemic regions in Europe. Animals. 2021;11:6.
Lorusso V, Dantas-Torres F, Lia RP, Tarallo VD, Mencke N, Capelli G, et al. Seasonal dynamics of the brown dog tick, Rhipicephalus sanguineus, on a confined dog population in Italy. Med Vet Entomol. 2010;24:309–15. https://doi.org/10.1111/j.1365-2915.2010.00885.x.
Kahl O, Geue L: Laboratory study on the role of the european fox, Vulpes vulpes, as a possible reservoir of Borrelia burgdorferi sl. In: Proceedings, 2nd International Conference on Tick-Borne Pathogens at the Host-Vector Interface: a global perspective, vol. 281995: 239.
Meyer-Kayser E, Hoffmann L, Silaghi C, Pfister K, Mahling M, Passos LMF. Dynamics of tick infestations in foxes in Thuringia. Germany Ticks Tick Borne Dis. 2012;3:232–9. https://doi.org/10.1016/j.ttbdis.2012.05.004.
Brianti E, Falsone L, Napoli E, Prudente C, Gaglio G, Giannetto S. Efficacy of a combination of 10% imidacloprid and 4.5% flumethrin (Seresto®) in slow release collars to control ticks and fleas in highly infested dog communities. Parasit Vectors. 2013;6:210. https://doi.org/10.1186/1756-3305-6-210.
Cook MJ. Lyme borreliosis: a review of data on transmission time after tick attachment. Int J Gen Med. 2014;8:1–8. https://doi.org/10.2147/IJGM.S73791.
Fourie JJ, Evans A, Labuschagne M, Crafford D, Madder M, Pollmeier M, et al. Transmission of Anaplasma phagocytophilum (Foggie, 1949) by Ixodes ricinus (Linnaeus, 1758) ticks feeding on dogs and artificial membranes. Parasit Vectors. 2019;12:136. https://doi.org/10.1186/s13071-019-3396-9.
Schein E, Mehlhorn H, Voigt WP. Electron microscopical studies on the development of Babesia canis (Sporozoa) in the salivary glands of the vector tick Dermacentor reticulatus. Acta Trop. 1979;36:229–41.
Varloud M, Liebenberg J, Fourie J. Early Babesia canis transmission in dogs within 24 h and 8 h of infestation with infected pre-activated male Dermacentor reticulatus ticks. Parasit Vectors. 2018;11:41. https://doi.org/10.1186/s13071-018-2637-7.
Daniel M, Malý M, Danielová V, Kříž B, Nuttall P. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasit Vectors. 2015;8:478. https://doi.org/10.1186/s13071-015-1092-y.
Kohn M, Krücken J, McKay-Demeler J, Pachnicke S, Krieger K, von Samson-Himmelstjerna G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): Activity patterns and associated pathogens. Ticks Tick Borne Dis. 2019;10:191–206. https://doi.org/10.1016/j.ttbdis.2018.10.003.
Zając Z, Kulisz J, Woźniak A, Bartosik K, Khan A. Seasonal activity of Dermacentor reticulatus ticks in the era of progressive climate change in eastern Poland. Sci Rep. 2021;11:20382. https://doi.org/10.1038/s41598-021-99929-y.
Bartosik K, Wiśniowski L, Buczek A. Abundance and seasonal activity of adult Dermacentor reticulatus (Acari: Amblyommidae) in eastern Poland in relation to meteorological conditions and the photoperiod. Ann Agric Environ Med. 2011;18:340–4.
Széll Z, Sréter-Lancz Z, Márialigeti K, Sréter T. Temporal distribution of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna in Hungary. Vet Parasitol. 2006;141:377–9. https://doi.org/10.1016/j.vetpar.2006.06.008.
Hornok S. Allochronic seasonal peak activities of Dermacentor and Haemaphysalis spp. under continental climate in Hungary. Vet Parasitol. 2009;163:366–9. https://doi.org/10.1016/j.vetpar.2009.03.048.
DWD: German Weather Service. https://www.dwd.de/DE/presse/pressemitteilungen/DE/2021/20210226_deutschlandwetter_winter2020_2021_news.html (2021). Accessed 14 Sep 2022.
Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George J-C, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. https://doi.org/10.1186/1756-3305-6-1.
Acknowledgements
The authors are grateful to Intervet Deutschland GmbH for recruiting the participating veterinary practices and providing them with the study's tick collection kits. Furthermore, we thank all veterinarians who supported this study by collection and sending of ticks.
Funding
This study was financially supported by Intervet Deutschland GmbH. This Open Access publication was partially funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 491094227 “Open Access Publication Funding” and the University of Veterinary Medicine Hannover, Foundation. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CS designed and coordinated the study and acquired the funding. JP conducted all morphological analysis, evaluated the questionnaires, analysed the data and drafted the manuscript supervised by CS. AS supported the statistical analyses. AS and CS participated in data interpretation and discussion by critically editing and revising the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not required for this study. Informed consent was obtained from all veterinarians involved in this study.
Consent for publication
Not applicable.
Competing interests
CS declares that she repeatedly has lectured for and acted as consultant for (veterinary) pharmaceutical companies and has previous and ongoing research collaborations with various (veterinary) pharmaceutical companies. JP declares that she has been employed as a PhD student in the project, which was included in the financial support by Intervet Deutschland GmbH. AS declares that she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Infestation rates with the most frequently collected tick species among tick-infested dogs and cats in the different German and Austrian federal states (number of infested hosts/% of all examined).
Additional file 2: Table S2.
Overview of the distribution of the most frequently collected tick species from cats and dogs over the Austrian federal states (number per tick species/% of total ticks).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Probst, J., Springer, A. & Strube, C. Year-round tick exposure of dogs and cats in Germany and Austria: results from a tick collection study. Parasites Vectors 16, 70 (2023). https://doi.org/10.1186/s13071-023-05693-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05693-5