Abstract
Background
Japanese encephalitis virus (JEV) is the principal cause of mosquito-borne encephalitis in human populations within Asia. If introduced into new geographic areas, it could have further implications for public and animal health. However, potential mosquito vectors for virus transmission have not been fully investigated. The Asian tiger mosquito, Aedes albopictus, has emerged in Europe and is now expanding its geographical range into more northerly latitudes. Culex quinquefasciatus, although absent from Europe, has been detected in Turkey, a country with territory in Europe, and could act as a vector for JEV in other regions. To assess the risk of these invasive species acting as vectors for JEV and therefore potentially contributing to its geographical expansion, we have investigated the vector competence of Ae. albopictus and Cx. quinquefasciatus.
Methods
Two colonised lines of Ae. albopictus (Italy and Spain) and a line of Cx. quinquefasciatus (Tanzania) were compared for susceptibility to infection by oral feeding with JEV strain SA-14, genotype III at 106 PFU/ml and maintained at 25 °C. Specimens were processed at 7 and 14 days post-inoculation (dpi). Rates of infection, dissemination and transmission were assessed through detection of viral RNA by real-time polymerase chain reaction (RT-PCR) in mosquito body, legs and saliva, respectively, at each time point. Where possible, infection and dissemination were confirmed by immunohistochemical (IHC) detection of the JEV envelope protein.
Results
Aedes albopictus from Italy showed no susceptibility to infection with JEV strain SA-14. Conversely, Ae. albopictus colonised in Spain was susceptible and 100% of infected mosquitoes that were subjected to saliva screening expressed viral RNA at 14 dpi. Culex quinquefasciatus was highly susceptible to infection as early as 7 dpi and 50% of infected mosquitoes that were subjected to saliva screening expressed viral RNA at 14 dpi. Infection and dissemination were confirmed in Cx. quinquefasciatus by IHC detection of JEV envelope protein in both the mid-gut and salivary glands.
Conclusions
Aedes albopictus from two different locations in Europe range from being susceptible to JEV and capable of transmission through to being resistant. Culex quinquefasciatus also appears highly susceptible; therefore, both species could potentially act as vectors for JEV and facilitate the emergence of JEV into new regions.
Graphical Abstract

Similar content being viewed by others
Background
Flaviviruses have a global distribution and many species can be transmitted by arthropods such mosquitoes, ticks and sandflies [1, 2]. Japanese encephalitis virus (JEV) (Family Flaviviridae, Genus Flavivirus) is the main aetiological agent for human viral encephalitis in the Far East and Southeast Asia. The World Health Organisation estimates that there are 68,000 clinical cases every year, but that over 3 billion people are at risk of exposure in Asia. Five genotypes are currently recognised, all endemic in Asia [3, 4].
Japanese encephalitis virus is maintained in an enzootic cycle between mosquito vectors and avian hosts, especially wading birds [5], although recent infection studies have demonstrated that domestic birds and pigs can act as amplifying hosts for JEV [6]. Some mosquito species can act as bridge vectors transmitting the virus to humans and livestock. Humans and other mammals such as horses are considered dead-end hosts due to low levels of viraemia, although recent studies have shown that pig-to-pig transmission can occur by the oronasal infection route [5, 7]. Due to a combination of climate change, movement of people and livestock, migratory birds, and the introduction of invasive vectors into new areas, geographic expansion of JEV into Europe could occur in the future [8, 9].
Culex tritaeniorhynchus is the main mosquito vector of JEV in regions where JEV is endemic, but other species, particularly within the genus Culex, can act as vectors [3, 10]. In Europe, Cx. tritaeniorhynchus has only been reported in Greece [11], and JEV RNA has been detected in a pool of Cx. pipiens in northern Italy [12], although this has not been associated with infection in either humans or swine. Recent experimental studies have demonstrated the competence of European populations of mosquitoes to transmit JEV, including Cx. pipiens from the UK and France, as well as Culiseta annulata and Aedes detritus from the UK [13,14,15]. Vector competence studies carried out by de Wispelaere et al. [16] demonstrated that a French population of the invasive species Ae. albopictus was vector competent for JEV genotypes III and V, and Jansen et al. [17] showed that infected Ae. japonicus japonicus from Germany expressed JEV in saliva at 14 days post-inoculation (dpi). However, there are many unassessed indigenous mosquito vectors with endemic European populations that may be able to transmit JEV and facilitate its emergence in the continent. In addition, non-native species to Europe have the potential to act as JEV vectors. These include Cx. quinquefasciatus, which has been found in Turkey [18]. Also, Ae. albopictus and Ae. japonicus japonicus are now distributed in several European countries [19, 20]. The detection of Cx. quinquefasciatus in Turkey could indicate that this mosquito species might expand its geographical range into Europe in the near future. Globally, different populations of Ae. albopictus have been identified as competent vectors of JEV, including Australia and Taiwan [21,22,23], and Cx. quinquefasciatus in North America [24], Brazil [13], India [25] and a colony from Queensland in Australia [26] were also competent to transmit JEV. However, other studies have shown that wild caught populations of Cx. quinquefasciatus from Australia and New Zealand were not competent to transmit JEV [26, 27], and two strains of Ae. albopictus (Yungho and Liyang, Taichung County) were less efficient vectors compared with a strain originating from Sanhsia (Taipei County) from Taiwan.
Due to the continued risk of invasive mosquito species globally, and the potential emergence of JEV into new areas [15], this study assessed the vector competence of two populations of Ae. albopictus (originating from Italy and Spain) and Cx. quinquefasciatus as a control for JEV genotype III. In this study, we selected to use JEV genotype III to inoculate mosquitoes as it is one of the most prevalent JEV genotypes along with genotype I, and it is associated with temperate climates [4]. In addition, immunohistochemistry (IHC) techniques were utilised to assess the presence of JEV antigen in mosquito histological sections, which facilitated the visualisation of JEV distribution within the context of specific mosquito tissues.
Methods
Colonisation of mosquitoes
Laboratory colonies comprised Ae. albopictus (Padua, Italy) (year of colonisation unknown and donated by Entostudio, Italy), Ae. albopictus (Barcelona, year of colonisation 2009 and donated by Universidad de Zaragoza, Spain) and Cx. quinquefasciatus (established at the Tropical Pesticides Research Institute (TPRI), Arusha, East Tanzania) (year of colonisation at London School of Hygiene and Tropical Medicine 2010 and donated by London School of Hygiene and Tropical Medicine, UK). A colonised line of Cx. quinquefasciatus originating from Africa was included for comparison as the species is known to be vector competent for JEV. Maintenance of Culex and Aedes mosquitoes in an insectary within biosecurity level 3 laboratories followed previously published protocols [19, 28].
Virus stocks
Japanese encephalitis virus genotype III (strain SA-14, isolated from Cx. pipiens larvae, China 1954) was donated by Dr. Jonas Schmidt-Chanasit, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany). Virus stocks were propagated in Vero cells as previously described [15, 19, 28]. Briefly, virus was propagated in Vero E6 cells in 25 ml of a culture medium consisting of Eagles minimal essential medium (E-MEM-Sigma Aldrich, UK), with 10% foetal bovine serum (FBS) and penicillin-streptomycin-nystatin solution (1% Thermo Fisher Scientific) at 37 °C and 5% CO2 for 3 days in T75 flasks. Infection of the cell monolayer was confirmed by light microscopy to assess the cytopathic effect (CPE).
Assessment of vector competence
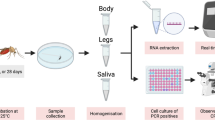
Adult females of Ae. albopictus (Italy and Spain) and Cx. quinquefasciatus (Tanzania) were tested for their vector competence for JEV genotype III at 25 °C, which is representative of peak summer temperatures in the UK. Mosquitoes were provided with an infectious blood meal, composed of defibrinated horse blood, adenosine 5′-triphosphate (final concentration 0.02 mM) and virus stock to give a final virus concentration of 1.8 × 106 PFU/ml (plaque forming units), using a Hemotek membrane feeding system (Hemotek Ltd Accrington, Lancashire, UK). Five- to ten-day-old adult female mosquitoes of both species were first starved of sucrose for 5 h and then allowed to feed on the infectious blood meal (as described above) in Bugdorm insect cages of 22 × 22 × 22 cm (Bugzarre.co.uk, Suffolk, UK) from 16:00, for a minimum of 16 h. The following day they were anaesthetised with triethylamine (TEA) FlyNap® (Blades Biological Limited, Edenbridge, UK) and separated into groups of blood-fed and non-blood-fed specimens. Only blood-fed mosquitoes were used to assess vector competence. For the processing of specimens and assessment of vector competence (infection, dissemination and transmission rates), a modified protocol adapted from [15, 19, 28] was followed. The transmission efficiency (TE) was calculated only at dpi 14, and it was defined as the number of virus-positive saliva samples per total number of fed females. Briefly, at 7 and 14 dpi, mosquitoes were immobilised at − 80 °C for 2 min and held in a plastic pot embedded in ice to ensure that they remained immobile during processing. Legs and wings were removed, saliva samples taken and the bodies, legs and wings, and saliva retained at − 80 °C for downstream analysis.
Processing of samples for molecular detection of JEV RNA
The protocol of [15] was used for detection of JEV RNA in tissues using previously published primers [29], which target and amplify a fragment of the NS1 gene. A sample was considered positive for JEV RNA at a cycle threshold (ct) value of 39 or lower, based on validation trials of the JEV PCR against positive and negative samples. Instead of using a standard to calculate RNA copies per mosquito, we opted to use ct values, which would enable direct comparison with previous studies [15].
Immunohistochemistry
The presence of JEV antigen in mosquitoes was determined by immunohistochemistry (IHC) in histological sections. All segments of the mosquito, head, thorax and abdomen, were examined by light microscopy. A sufficient number of blood-fed specimens was available to assess by IHC for Cx. quinquefasciatus only. Unfortunately, fewer female Ae. albopictus took an infectious blood meal; therefore, the number of mosquitoes available for the vector competence experiment was lower, and we were not able to retain any specimens for IHC. Briefly, 12 infected blood-fed and 3 non-infected control female mosquitoes were placed in 10% neutral buffered formalin for fixation for 48 h. After fixation the wings and extremities were removed and the body was placed in sagittal plane prior to routine processing to paraffin blocks. Serial 3-μm-thick sections of the formalin-fixed paraffin-embedded (FFPE) mosquitoes were cut and placed on silane-coated slides (3-trietoxysilyl-propylamine). Proteinase enzyme buffer (DAKO, Ely, Cambridgeshire, UK) applied for 15 min at 20 °C was used as the antigen retrieval method. A mouse monoclonal anti-Flavivirus E-glycoprotein antibody (ab155882, Abcam, Cambridge, UK) applied at 1 in 50 dilution in Tris-buffered saline with 0.05% Tween 20 (TBST, VWR, Leicestershire, UK) at 4 °C for 18–20 h (overnight) was used as primary antibody to detect JEV. Parallel sections were tested with a protein concentration matched mouse immunoglobulin G class 2a (Abcam, Cambridge, UK) as isotype controls to identify any non-specific immunolabelling. Slides were then washed in purified water and assembled into coverplates for immunolabelling. DAKO mouse EnVision™ + System, HRP Peroxidase (DAKO, Ely, Cambridgeshire, UK) was used as a secondary antibody and incubated for 30 min at 20 °C combined with swine and goat immune serum (Vector Laboratories, Peterborough, UK). Antibody binding was visualised using the chromogen 3,3′-diaminobenzidine (DAB) + substrate-chromogen, which results in a brown-colored precipitate at the antigen site after 10-min incubation. Finally, sections were counterstained with Mayer’s haematoxylin (HE) and mounted in Distyrene Plasticiser Xylene (DPX) mounting medium (TCS Bioscience, Buckingham, UK) for light microscopy. Sections of West Nile virus-infected mouse brain and JEV-infected Vero cells were used as positive controls for flavivirus immunostaining.
Virus titration
Titrations of both stock virus and virus in the infected blood meal were performed by plaque assay as previously described [19, 28].
Statistical analysis
The graphical output was carried in the R programme (http://www.R-project.org). A t-test was performed comparing the ct values for RT-PCR to measure relative levels of virus infection between Cx. quinquefasciatus and Ae. albopictus.
Results
A total of 81 females (28.9%) from Ae. albopictus originating from Italy successfully fed following an infectious blood meal containing JEV, while 106 females (52.7%) of Ae. albopictus originating from Spain fed on the bloodmeal (Table 1). Conversely, only 79 females (37.4%) of Cx. quinquefasciatus fed (Table 2). In general, mortality was observed between 1 and 2 dpi, then the survival of mosquitoes was relatively stable until 6 dpi before declining towards 13–14 dpi, which is typical for vector competence experiments (Additional file 1: Fig. S1).
Virus infection, dissemination and transmission were initially determined by RT-PCR. Aedes albopictus originating from Italy did not show evidence of infection, dissemination or transmission at either 7 or 14 dpi (Table 1). However, Ae. albopictus originating from Spain did show infection at 7 dpi (6%), but neither dissemination nor transmission was detected. However, at 14 dpi infection (17%), dissemination (75%) and transmission (100%, n = 3) were detected (Table 1). The overall transmission efficiency of Ae. albopictus from Spain at dpi 14 was 2.83%.
Culex quinquefasciatus showed higher prevalence of infection (50%), dissemination (20%) and transmission (100%, n = 1) at 7 dpi. In addition, higher prevalence of infection (79%), dissemination (17%) and transmission (50%, n = 2) were observed at 14 dpi (Table 2). The transmission efficiency of Cx. quinquefasciatus at dpi 14 was 2.53%.
To compare relative amounts of virus genome in the tissue we analysed, the threshold values from each amplification cycle (ct) were evaluated, in particular at 14 dpi (Fig. 1). The ct values detected from Cx. quinquefasciatus were significantly lower compared to Ae. albopictus, suggesting a higher level of viral RNA detection in these samples (t = 2163, P = 0.019).
Boxplot comparing the cycle threshold (ct) values for Ae. albopictus and Cx. quinquefasciatus from RNA extractions of specimens infected with Japanese encephalitis virus and maintained at 25 °C. Culex quinquefasciatus ct values were significantly lower compared to Ae. albopictus, suggesting that quantity of viral RNA was higher in these samples. Significance (P < 0.05) denoted by a double asterisk (**)
Cellular distribution of JEV infection in Cx. quinquefasciatus was determined by IHC on sections of FFPE whole mosquitoes to detect JEV envelope antigen. JEV-immunolabeled cells were observed in the posterior midgut of seven infected females of Cx. quinquefasciatus (Fig. 2; Additional file 2: Fig. S2). Immunolabelling was present in clusters of epithelial cells, predominantly ciliated pseudostratified intestinal cells, located in the posterior midgut region, as characterised by dark brown pigment deposition within the cytoplasm. The levels of midgut epithelial cells infection were highly variable with labelling ranging from a continuous row of cells to single cells (Additional file 2: Fig. S2). Positive intracytoplasmatic immunolabelling was observed in the salivary gland of one specimen of Cx. quinquefasciatus, defined by the presence of secretory masses corroborating the detection of virus by this and other methods such as PCR (Table 2). Isotype control immunolabelled sections did not show any non-specific staining on infected mosquitoes.
Japanese encephalitis virus infection at 25 °C of posterior midgut epithelial cells in Cx. quinquefasciatus. a Head (H), thorax (T), abdomen (Ab). b Intracytoplasmic immunolabelling in the distal lobes of salivary gland, defined by the presence of secretory masses (SM); intense antigen labelling particularly in the basal region of the epithelium (red arrow). c Antigen labelling in the apical ciliated cells (arrow) and basal epithelial cells (arrowhead) of the posterior midgut; lumen of the midgut (L). Scale bar: 500 µm (a); 20 µm (b, c)
Discussion
Our results show that Ae. albopictus originating from Spain and a line of Cx. quinquefasciatus originating from Tanzania were susceptible to infection by JEV genotype III. In addition, our study demonstrates that after 14 days at 25 °C, JEV virus was able to disseminate throughout Ae. albopictus originating from Spain and Cx. quinquefasciatus with viral RNA being detected in saliva by RT-PCR, and also in the salivary glands of Cx. quinquefasciatus by IHC. This suggests that the studied populations may be competent vectors of JEV genotype III under our experimental conditions, corroborating previous findings from populations of Ae. albopictus and Cx. quinquefasciatus [13, 16, 21, 23, 24, 26, 30]. Of the mosquito populations assessed, Cx. quinquefasciatus appeared the more competent vector for JEV, as expected, demonstrating the highest levels of dissemination and transmission, despite blood feeding at a lower rate than Ae. albopictus from Spain. However, these results demonstrated that a population of Ae. albopictus originating from Spain may also act as a competent vector for JEV; this is also supported by the TE for JEV in both species.
Both Ae. albopictus and Cx. quinquefasciatus are indigenous in tropical areas, where temperatures > 30 °C can be encountered, and they are well known vectors for many arboviruses [24, 31,32,33,34]. Although Cx. quinquefasciatus has not yet been detected in Europe and its ability to successfully transmit JEV in Europe would require the establishment of high-density populations, its morphological, ecological and phylogenetic similarity to Cx. pipiens and its ability to colonise new areas via ship and airline vessels [35] make it a potentially important invasive species for studying the transmission of re-emerging zoonotic viruses such as JEV. The origin of Cx. quinquefasciatus is thought to be West Africa from where it colonised other regions through trade and migration. The species reached the Americas during the 1800s, spreading to Asia and the Pacific via whaling and merchant vessels [36, 37]. It is a common species in Africa; thus, our finding that the population from Tanzania is a highly competent vector for JEV is epidemiologically relevant in the event of JEV spreading in the African continent.
Conversely, Ae. albopictus is now distributed in many countries in Europe and the Mediterranean Basin, with a few sporadic recorded incursions into more temperate regions such as the UK [38]. Several populations of Ae. albopictus are considered the main drivers for outbreaks of dengue and chikungunya fever in Europe [39]; it is also a known secondary vector of Zika virus in Latin America [28]. The species has spread rapidly throughout Europe, being mainly transported by road vehicles, where it can be considered a biting pest together with Ae. japonicus japonicus [40]. In our study, the Italian population of Ae. albopictus from Padua, Northern Italy, was not a competent vector for JEV genotype III, although it is an efficient vector of other arboviruses such as chikungunya virus [41]. Previous reports have suggested that the different origins of the Italian Ae. albopictus populations, which were introduced separately from different tropical and subtropical areas over the past 3 decades [41], could be the basis for differences in their vector competence. It is worth noting that the experimental conditions in this study maintained constant heat and humidity with a 24-h day-night photoperiod, which are standard conditions during vector competence studies [15, 19]. However, these conditions are not representative of natural conditions. Given that previous studies suggest that temperature appears to be a critical factor for both vector competence and vector mortality in this experimental system, we suggest that future studies incorporate variation between minimum and maximum temperature/humidity means to represent what occurs naturally [42]. Infection experiments carried out in Cx. pipiens have shown that a limiting factor at which this species becomes unable to transmit JEV genotype III is temperature, with higher temperatures (25 °C) causing increased mortality in infected mosquitoes compared to mosquitoes held at 20 °C [15]. However, no increased mortality was observed for Ae. albopictus or Cx. quinquefasciatus at 25 °C in the present study, suggesting that under our experimental conditions an elevated temperature and infection with JEV strain SA-14 did not cause additional mosquito mortality. This may be a consequence of the mosquito species and virus strain used as both naturally encounter higher temperatures than our experimental paradigm [3].
The labelling of virus antigen in Cx. quinquefasciatus confirmed that at 25 °C, JEV was able to infect the posterior midgut epithelial cells such as ciliated pseudostratified intestinal cells, which corroborates detection of virus in the mosquito body by molecular means. This supports a previous study showing that midgut epithelial cells are a major site of viral replication [15]. In addition, viral antigen was observed in mid-gut and salivary glands by IHC, which demonstrated that at 25 °C and by 14 dpi, the virus was able to overcome the midgut barrier and to infect secondary organs such as the salivary glands. Previous studies found that JEV present in the midgut appeared viable by the recovery of live virus in vitro from homogenised mosquito bodies [15]. However, it was unclear whether the restriction of JEV to the midgut was a result of active anti-viral control by the mosquitoes or the lower experimental temperature restricting virus replication. The authors suggested that an increase in temperature, or an increase in the duration of the experiment, could potentially trigger further virus replication and escape from the midgut; our results suggest that temperature may be a contributing factor to full viral dissemination.
Conclusions
Of the mosquito populations studied, there was no evidence that the virus could infect or disseminate within the Ae. albopictus line originating from Italy at 25 °C at either 7 or 14 dpi. By contrast, Ae. albopictus originating from Spain and Cx. quinquefasciatus originating from Tanzania proved to be susceptible to infection as early as 7 dpi. Dissemination occurred in a proportion of infected mosquitoes and JEV was detected in the saliva of these mosquitoes. This suggests the potential of these mosquito populations to transmit JEV genotype III (strain SA-14). Considering that several mosquito species have been shown to be competent vectors for a number of arboviruses, our results contribute to this expanding dataset and indicate that if JEV were to emerge in new areas, there would be a number of mosquito populations that could facilitate its transmission and persistence.
Availability of data and materials
All data generated by this study and used is presented within this published article.
References
Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microbes Infect. 2015;4:e18. https://doi.org/10.1038/emi.2015.18.
Braack L, Gouveia de Almeida AP, Cornel AJ, Swanepoel R, de Jager C. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasites Vectors. 2018;11:29. https://doi.org/10.1186/s13071-017-2559-9.
Auerswald H, Maquart P-O, Chevalier V, Boyer S. Mosquito vector competence for Japanese encephalitis virus. Viruses. 2021;13:1154. https://doi.org/10.3390/v13061154.
Schuh AJ, Ward MJ, Brown AJL, Barrett ADT. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoS Negl Trop Dis. 2014;7:e2411.
Mansfield KL, Hernández-Triana LM, Banyard AC, Fooks AR, Johnson N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol. 2017;201:85–92.
Hameed M, Wahaab A, Nawaz M, Khan S, Nazir J, Liu K, et al. Potential role of birds in Japanese encephalitis virus zoonotic transmission and genotype shift. Viruses. 2021;13:357. https://doi.org/10.3390/v13030357.
Ricklin ME, García-Nicolás O, Brechbühl D, Python S, Zumkehr B, Nougairede A, et al. Vector free transmission and persistence of Japanese encephalitis virus in pigs. Nat Commun. 2016;7:10832.
Gould EA, Higgs S, Buckley A, Gritsun TA. Potential arbovirus emergence and implications for the United Kingdom. Emerg Infect Dis. 2006;12:549–55.
Pearce JC, Learoyd TP, Langendorf BJ, Logan JG. Japanese encephalitis: the vectors, ecology and potential for expansion. J Travel Med. 2018;25:S16–26.
Oliveira ARS, Strathe E, Etcheverry L, Cohnstaedt LW, McVey DS, Piaggio J, et al. Assessment of data on vector and host competence for Japanese encephalitis virus: a systemic review of the literature. Prev Vet Med. 2018;54:71–89.
Lytra I, Emmanouel N. Study of Culex tritaeniorhynchus and species composition of mosquitoes in a rice field in Greece. Acta Trop. 2014;134:66–71.
Ravanini P, Huhtamo E, Ilaria V, Crobu MG, Nicosia AM, et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Euro Surveill. 2012;17:20221. https://doi.org/10.2807/ese.17.28.20221-en.
Mackenzie-Impoinvil L, Impoinvil DE, Galbraith SE, Dillon RJ, Ranson H, Johnson N, et al. Evaluation of a temperate climate mosquito, Ochleratutus detritus (=Aedes detritus), as a potential vector of Japanese encephalitis virus. Med Vet Entomol. 2015;29:1–9. https://doi.org/10.1111/mve.12083.
Chapman GE, Sherlock K, Hesson JC, Blagrove MS, Lycett GJ, Archer D, et al. Laboratory transmission potential of British mosquitoes for equine arboviruses. Parasites Vectors. 2020;13:413. https://doi.org/10.1186/s13071-020-04285-x.
Folly AJ, Dorey-Robinson D, Hernández-Triana LM, Ackroyd S, Vidana B, Lean FZX, et al. Temperate conditions restrict Japanese encephalitis virus infection to the mid-gut and prevents systemic dissemination in Culex pipiens mosquitoes. Sci Rep. 2021;11:6133. https://doi.org/10.1038/s41598-021-85411-2.
de Wispelaere M, Desprès P, Choumet V. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Negl Trop Dis. 2017;11:e0005294. https://doi.org/10.1371/journal.pntd.0005294.
Jansen S, Heitmann A, Lühken R, Jöst H, Helms M, Vapalahti O. Experimental transmission of Zika virus by Aedes japonicus japonicus from southwestern Germany. Emerg Microbes Infect. 2018;7:192. https://doi.org/10.1038/s41426-018-0195-x.
Gunay F, Alten B, Simsek F, Aldemir A, Linton Y-M. Barcoding Turkish Culex mosquitoes to facilitate arbovirus vector incrimination studies reveals hidden diversity and new potential vectors. Acta Trop. 2015;143:112–20.
Hernández-Triana LM, Barrero E, Delacour-Estrella S, Ruiz-Arrondo I, Lucientes J, Fernández de Marco MM, et al. Evidence for infection but not transmission of Zika virus by Aedes albopictus (Diptera: Culicidae) from Spain. Parasites Vectors. 2019;12:204. https://doi.org/10.1186/s13071-019-3467-y.
Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013;19:685–92.
Nicholson J, Ritchie SA, van den Hurk AF. Aedes Albopictus (Diptera: Culicidae) as a potential vector of endemic and exotic arboviruses in Australia. J Med Entomol. 2014;51:661–9.
Chen W-J, Dong C-F, Chiou L-Y, Chuang W-L. Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-Chiu Islet, Taiwan. J Med Entomol. 2000;37:108–13. https://doi.org/10.1603/0022-2585-37.1.108.
Weng MH, Lien JC, Wang YM, Wu HL, Chin C. Susceptibility of three laboratory strains of Aedes albopictus (Diptera: Culicidae) to Japanese encephalitis virus from Taiwan. J Med Entomol. 1997;34:745–7.
Huang YJ, Harbin JN, Hettenbach SM, Maki E, Cohnstaedt LW, Barret ADT, et al. Susceptibility of a North American Culex quinquefasciatus to Japanese encephalitis virus. Vector-Borne Zoonotic Dis. 2015;15:709–11. https://doi.org/10.1089/vbz.2015.1821.
Banerjee K, Deshmukh PK, Ilkal MA, Dhanda V. Comparative susceptibility of three species of mosquitoes to infection with Japanese encephalitis virus. Indian J Med Res. 1983;78:603–6.
van den Hurk AF, Nisbet DJ, Hall RA, Kay BH, Mackenzie JS, Ritchie SA. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J Med Entomol. 2003;40:82–90.
Kramer LD, Chin P, Cane RP, Kauffman EB, Mackereth G. Vector competence of New Zealand mosquitoes for selected arboviruses. Am J Trop Med Hyg. 2011;85:182–9.
Hernández-Triana LM, Fernández de Marco M, Mansfield KL, Thorne L, Lumley S, Marston D, et al. Assessment of vector competence of UK mosquitoes for Usutu virus of African origin. Parasites Vectors. 2018;11:381. https://doi.org/10.1186/s13071-018-2959-5.
Pyke TA, Smith IL, van den Hurk AF, Northill JA, Chuan TF, Westacott AJ, et al. Detection of Australasian flavivirus encephalitic viruses using rapid fluorogenic TaqMan RT-PCR assays. J Virol Methods. 2004;117:161–7.
Hanna JN, Ritchie SA, Phillips DA, Lee JM, Hills SL, van den Hurk AF, et al. Japanese encephalitis in North Queensland, Australia, 1998. Med J Aust. 1999;170:533–6.
Bhattacharya S, Basu P. The southern house mosquito, Culex quinquefasciatus: profile of a smart vector. J Entomol Zool Stud. 2016;4:73–81.
Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–85. https://doi.org/10.1016/j.meegid.2011.08.013.
Mitchell CJ. The role of Aedes albopictus as an arbovirus vector. Parassitologia. 2020;37:108–13.
Vega-Rúa A, Marconcini M, Madec Y, Manni M, Carraretto D, Gomulski LM, et al. Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun Biol. 2020;3:326. https://doi.org/10.1038/s42003-020-1046-6.
Bataille A, Cunningham AA, Cedeño V, Cruz M, Eastwood G, Fonseca DM, et al. Evidence for regular ongoing introductions of mosquito disease vectors into the Galápagos Islands. Proc Biol Sci. 2009;276:3769–75.
Belkin JN, Heinemann SJ. Collection records of the project “Mosquitoes of Middle America”. 3. Bahama Is. (BAH), Cayman Is. (CAY), Cuba (CUB), Haiti (HAC, HAR, HAT) and Lesser Antilles (LAR). Mosq Syst. 1975;7:367–93.
Vinogradova EB. Culex pipiens pipiens mosquitoes, taxonomy, distribution, ecology physiology genetics, applied importance and control. Sofia: Pensoft; 2000. p. 646.
Medlock JM, Vaux AGC, Cull B, Schaffner F, Gillingham E, Pfluger V, et al. Detection of the invasive mosquito species Aedes albopictus in southern England. Lancet Infect Dis. 2017;17:140.
Jourdain F, Roiz D, de Valk H, Noël H, L’Ambert G, Franke F, et al. From importation to autochthonous transmission: drivers of chikungunya and dengue emergence in a temperate area. PLoS Negl Trop Dis. 2020;14:e0008320. https://doi.org/10.1371/journal.pntd.0008320.
Werner D, Kampen H. The further spread of Aedes japonicus japonicus (Diptera, Culicidae) towards northern Germany. Parasitol Res. 2013;112:3665–8. https://doi.org/10.1007/s00436-013-3564-3.
Severini F, Boccolini D, Fortuna C, Di Luca M, Toma L, Amendola A, et al. Vector competence of Italian Aedes albopictus populations for the chikungunya virus (E1–226V). PLoS Negl Trop Dis. 2018;12:e0006435. https://doi.org/10.1371/journal.pntd.0006435.
Perry A, Golding N. Range of environmental temperature conditions in the United Kingdom. Met office report for transport for London; 2021. p. 63. www.kipdf.com. Accessed 3 May 2022.
Acknowledgements
The authors thank Shahida Begum (London School of Hygiene and Tropical Medicine, United Kingdom) for the provision of the Cx. quinquefasciatus colony. Thanks are also giving to Jonas-Schmidt-Chanasit (Bernhard Nocht Institute, Hamburg, Germany) for providing the JEV (strain SA-14).
Funding
Funding was provided by the Department for Environment, Food and Rural Affairs (Defra), The Scottish Government and Welsh Government through grant SV3045 and SE4116. Further funding was obtained through the European Union’s Horizon 2020 research and innovation program under grant agreement no. 871029 EVA-GLOBAL.
Author information
Authors and Affiliations
Contributions
NJ obtained funding for the study. LMHT, AJF, SS, FZXL, SA, AN, conceived and designed experiments. LMHT, AJF, SS, FZXL performed the experiments. LMHT, AJF, SS, FZXL, SA, AN, SB, AD, PV, KLM, and NJ analysed the data. LMHT wrote the first draft. NJ revised the draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Survival of Ae. albopictus (Italy and Spain) and Cx. quinquefasciatus (Tanzania) at 25 °C following a blood meal containing Japanese encephalitis genotype III over 14 days post-infection; DPI, days post-infection.
Additional file 2: Figure S2.
Japanese encephalitis virus infection of the midgut in seven specimens of Cx. quinquefasciatus maintained at 25 °C. (a–e) Strong immunolabelling. (f, g) Moderate immunolabelling . Scale bar: 20 µm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hernández-Triana, L.M., Folly, A.J., Sewgobind, S. et al. Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasites Vectors 15, 210 (2022). https://doi.org/10.1186/s13071-022-05329-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05329-0