Abstract
Background
The lack of information on behavioural patterns of Aedes aegypti and Aedes albopictus has become a significant limitation in vector control and disease management programmes. Therefore, the current study was focused on determining some bionomics aspects: breeding, resting, host-seeking and feeding preferences of Ae. aegypti and Ae. albopictus in Sri Lanka.
Methods
Larval and adult surveys were conducted from April 2017 to April 2019 monthly in six selected Medical Officer of Health (MOH) areas in Gampaha Distinct, Western province, Sri Lanka, representing urban, suburban and rural settings. Resting preferences of adult mosquitoes were observed from indoor and outdoor places using a Prockopack aspirator. The information on resting height, surface, material and locality was recorded. Human-baited double-net traps were used to determine the host-seeking time of Aedes mosquitoes. Statistical differences in the spatial distribution of mosquitoes in selected MOH areas and prevalence of vectors were analysed using general linear model (GLM). A chi-square test was used to analyse the resting behaviour.
Results
Total of 19,835 potential breeding sites were examined at 13,563 premises, and 18.5% (n = 1856) were positive for Aedes larvae. Distribution of Ae. aegypti and Ae. albopictus was statistically significant at species level (df = 1; F = 137.134; P < 0.05 GLM) and study setting (df = 2; F = 8.125; P < 0.05). Aedes aegypti breeding was found mainly in temporary removals (18.8%; n = 34), discarded non-degradables (12.15%; n = 22) and tyres (9.95%; n = 18). Natural (14.7%; n = 246) and temporary removals (13.6%; n = 227) and discarded non-reusable items were the key ovipositing sites for Ae. albopictus. In the adult mosquito survey, the majority was comprised of Ae. albopictus (54.5%; n = 999), which denoted exophilic nature (90.8%; n = 758), and 45.5% (n = 835) represented by Ae. aegypti mosquitoes who were mainly endophilic (84.3%; n = 842). Aedes aegypti rested on cloth hangings and curtains, followed by the furniture, while Aedes albopictus was predominant in outdoor vegetation. In both vectors, biting patterns denoted a typical diurnal pattern with two peaks of host-seeking and biting activity in the morning and afternoon.
Conclusions
The majority (80%) of the larval habitats were artificial containers. The use of larvicides for vector control as the prominent measure is questionable since applying these chemicals may target only 20% of the total breeding grounds, which are permanent. The resting places of adult mosquitoes are mainly indoors. Therefore, using thermal space spraying of insecticide may not be appropriate, and indoor residual spraying is recommended as a suitable intervention to target adult mosquitoes. This study warrants a holistic vector control approach for all medically important mosquitoes and insects, ensuring the rational use of finance and resources.
Graphical Abstract

Similar content being viewed by others
Background
Some species of the Genus Aedes are medically important for transmitting arboviruses which cause dengue, chikungunya, yellow fever and Zika to humans [1,2,3,4]. Of the recorded species, Aedes aegypti and Aedes albopictus received immediate attention since these two species have been identified as vectors of dengue transmission [5]. According to the records, Aedes aegypti originated in Africa as tree-hole forest mosquitoes [6]. In contrast, the Asian tiger mosquito Aedes albopictus is native to tropical and subtropical areas of Southeast Asia and breeds in natural habitats, including tree holes, bamboo stumps and bromeliads at the edges of tropical forests [7]. However, with time they spread to other regions worldwide through travel and trade [8] and reformed to breed in human-made containers/micro-breeding habitats in urban setup [6,7,8].
In the Southeast Asian region, Ae. aegypti is considered as the principal vector of dengue. Aedes albopictus has been recognized as the secondary vector of the dengue, which is also important in the maintenance of the virus [9]. Furthermore, Ae. albopictus has become one of the most invasive species globally because of its strong ability to adapt to new environments [7, 10]. These mosquitoes can be infected by the virus during their feeding process and, once infected, the mosquito can retain the virus throughout its adult life [11].
In the case of insect-borne disease transmission, biology, bionomics and the life history of vectors are important aspects that could influence the efficiency to transmit diseases. There can be changes in the biology and bionomics of vector species in different regions. Furthermore, the availability and composition of vectors may also vary with different spatial setups. Aedes aegypti is a strongly anthropophilic mosquito adapted to live around humans at a domestic setup. Therefore, the mosquito is more predominant in urban settings than in rural areas as its abundance is positively correlated with increasing urbanization. It is considered the most efficient vector of the dengue virus even at low densities [12, 13]. In some countries, Ae. albopictus leads to an outbreak situation of dengue incidence. The outbreak of dengue and chikungunya in Madagascar (Toamasina) during 2006 showed that Ae. albopictus is the only urban vector [14]. It is evidenced that the biology and behavioural aspects of dengue vectors vary in different regions of the globe [5].
Unplanned urbanization, globalization of the world with travel and trade, human population growth and suitable climatic conditions directly correlates with the expansion of dengue vector distribution and dengue transmission, especially in low- and middle-income countries in tropical and subtropical regions [15,16,17]. Therefore, understanding the prevalence of vector species, their behaviours and important bionomic aspects at different urban, suburban and rural settings would provide more practical and reliable information to develop more effective, precise and focused vector control strategies. Resting and feeding behaviours of vectors are important facts since they are prerequisites to determine their role in disease transmission in endemic settings [17]. The behavioural and physiological processes that may account for the presence of resting behaviour of Ae. aegypti inside houses and their implications for dengue outbreak interventions have been revealed [18].
In Sri Lanka, insecticidal space-spraying is extensively used as a routine dengue control activity [19]. The efficacy of this method depends on targeted mosquito species, their susceptibility to insecticides, indoor penetration capacity of the insecticides, frequency/timing of applications and targeting of appropriate sites [20]. Most importantly, the application of space-spraying also should be related to the behaviour of the targeted species [20]. However, investigations on such behavioural, biological and bionomic aspects of dengue vectors in Sri Lanka are scarce. This has become a significant limitation in vector control and disease management programmes. Therefore, this study aimed to investigate the biology, bionomics and behavioural aspects of dengue vectors in rural, suburban and urban areas in the Gampaha District; the western province of Sri Lanka contributes the second-highest number of cases of dengue in the country.
Methods
Study area
Gampaha district of Sri Lanka covers an area of 1387 km2. It has a human population of 2,574,324, recorded as the highest residential population in Sri Lanka. The annual rainfall is about 2500 mm, mainly during two monsoonal periods from April to June and October to December [21]. The District of Gampaha comprises 16 Medical Officer of Health (MOH) areas. The present study was conducted in six selected Medical Officer of Health (MOH) areas (Fig. 1), covering an estimated human population of 1.2 million. In selecting sites, areas with different environmental settings (urban, suburban and rural) were selected randomly. Geographic areas located inside towns and cities were described as urban. In contrast, rural areas are located outside towns and cities and are usually less developed with significant land cover under agriculture and natural vegetation. Areas with mixed characteristics were considered suburban [12]. Accordingly, Negombo (population density per km2: 4292/km2) and Wattala (4191/km2) MOH areas were identified as urban and Attanagalla (1447/km2) and Gampaha (2357/km2) MOH areas as suburban. Dompe (1052/km2) and Divulapitiya (836/km2) MOH areas represent rural setups.
Entomological investigations and bionomics aspects of Aedes mosquitoes
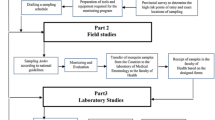
Entomological surveys for both larval and adult stages of Aedes mosquitoes and the biology/bionomics aspects were conducted from April 2017 to December 2019 monthly using standard entomological techniques according to the guidelines specified by the World Health Organization and National Dengue Control Unit of Sri Lanka [22, 23]. At each sampling attempt, a locality was selected, considering a central point for entomological surveillance. The survey was conducted within 200–300 m at each selected locality.
Collection of mosquito larvae from breeding habitats
The larval collections were performed, covering all potential permanent and temporary breeding sites using standard dipping, siphoning and pipetting methods depending on the nature in the breeding habitat [24]. All positive and potential breeding sites were recorded and categorized under 20 different types including water storage containers (water storage barrels, water storage cement tanks, water storage other containers), concrete slabs, gutters, tyres, ornamentals (flower vases, fish tanks), natural breeding places (leaf axils, tree holes, bamboo stumps), ponds, shallow wells, tube wells, A/C and refrigerator trays, temporary removal items (household utensils, machinery, machinery parts stored in backyard/outside of premises without shade for future usage), covering items/polythene, discarded degradable (damaged clay pots, coconut shells), discarded non-reusable items (damaged ceramic items, tin, can, non-reusable plastic containers), discarded reusable items (glass bottles, tyres, reusable plastic containers), pet feeding cups, non-used commodes and cisterns. All other larval habitats were classified as other.
Field-collected larvae were classified into early (1st and 2nd) and late (3rd and 4th) instars and transferred into transparent plastic vials (5 ml) using a pasture pipette. The larvae were transferred safely to the laboratory. Larvae of 3rd and 4th instars were directly taken for species identification using morphological taxonomic keys [25]. Early instars were reared under optimized laboratory conditions with larval food until 3rd instar stage to confirm the species identification. The prevalence of mosquitoes in different breeding habitat categories was calculated as a percentage of containers positive for each species out of the total number of positive containers.
Adult mosquito collection from resting places
Adult mosquitoes were collected in both outdoor and indoor places of 15 randomly selected premises once in 2 months in each selected MOH area using a Prokopack aspirator (John W. Hock Co., Gainesville, FL, USA, Model: 140) (Fig. 2). The surveys were conducted for 2 years, from September 2017 to 2019.
The adult collections were performed from 08:00 to 15:00 h, and collections lasted for 15 min at each selected location. Mosquito collections from the selected houses/bedrooms/outdoor places were performed according to the WHO guidelines using a Prokopack aspirator. The collection began at the entry point and continued in a clockwise direction. The collection was made in four sessions, ensuring the minimum disturbance using four collection cups separately. First, the collections were made targeting resting places that were closer to the approximate height of < 1 m from the ground level such as underneath furniture, the bottom part of hangings, etc., at indoor resting places and small shrubs, grass, outdoor walls and other possible resting surfaces at outdoor sites. Later, the resting site at the next level of heights (1–2 m, 2–3 m and > 3 m) were reached by approximation of height from the floor. A total of 1482 premises were surveyed during the surveillance period. All collected mosquitoes were identified to the species level by referring to the morphological taxonomic key for Aedes mosquitoes [25].
Detection of host-seeking hours of adult mosquitoes
Human-baited double-net traps were used to determine the host-seeking time of Aedes mosquitoes using the standard traps according to the specified guidelines [26], and the trial was repeated three times. A trained Field Assistant volunteered, and written informed consent was obtained from the participants. The participants rested on a metal-framed bed net (20 × 200 × 70 cm). They were fully protected from mosquitoes by an internal polyester bed net (97 × 200 × 100 cm, mesh size 1.5 mm), which was not treated with any insecticide. It was hung over the bed to the ground. A larger untreated bed net (100 × 250 × 150 cm, mesh size 1.5 mm), also not treated with insecticide, was hung over the smaller net. It was raised 30 cm above the ground. The mosquitoes caught in the ± 20 cm gap between the two nets were collected using a mouth aspirator every 10 min per hour. Mosquito collections were continued from 05:00 to 19:00 h. Atmospheric temperature and relative humidity were recorded hourly with mosquito collections. The mosquitoes collected each hour were transferred into different paper cups covered with a nylon mesh (1.5 mm). The mosquitoes collected from genus Aedes were authenticated to species level using standard morphological keys [25].
Data analysis
The prevalence of Ae. aegypti and Ae. albopictus immature stages (larvae and pupae) in different breeding sites were used to calculate the container index (CI), which refers to the percentage of positive containers over the total number of water-holding containers inspected [22]. All recorded data were analysed using Statistical Package for Social Sciences (SPSS), version 21. The difference in the distribution of Ae. aegypti and Ae. albopictus at different environmental setups was analysed using the univariate general linear model (GLM) followed by Tukey’s HSD (honest significant difference) at a 5% significance level. The variation in the oviposition site preferences in terms of CI for both species was analysed by multivariate GLM at a 5% significance level. The difference between exophilic and endophilic resting behaviour of adult mosquitoes was analysed using the chi-squared test of independence at a 95% of confidence level. The mean number of adult female mosquitoes was calculated. Pearson’s correlation was used to analyse the relationship between mean numbers of female mosquitoes per hour, with mean atmospheric temperature and relative humidity at 5% significant intervals.
Results
Oviposition preferences of Aedes mosquitoes at different environmental setup
A total of 19,829 potential breeding sites (water-holding containers/wet containers; n = 10,032, dry potential containers; n = 9797), both temporary and permanent, were examined at 13,563 premises. Of them, 18.5% (n = 1856) of water-holding containers were positive for Aedes larvae. Aedes albopictus was observed from all spatial setups (rural, suburban and urban), denoted as the leading species over Ae. aegypti. However, urban areas were more conducive for Ae. aegypti breeding. The container index (CI) for Ae. albopictus was highest in rural areas, followed by suburban setup. Aedes aegypti was identified mainly from urban and suburban setups (Table 1). According to GLM, the distribution of Ae. aegypti and Ae. albopictus was statistically significant at species level (df = 1; F = 137.134; P < 0.05) and spatially in selected areas in the Gampaha district (df = 2; F = 8.125; P < 0.05). Figure 3 represents the distribution of Ae. aegypti and Ae. albopictus in terms of mean container index at different environmental settings representing urban, suburban and rural areas.
Breeding site preference of Aedes mosquitoes
A total of 20 breeding site categories for Aedes mosquitoes were identified. Aedes aegypti was recorded in 16 larval habitat types, and Ae. albopictus was recorded from all categories. The statistics of GLM showed that the percentage positivity of Aedes mosquitoes varied significantly, among MOH areas (Ae. aegypti; df = 5; F = 47.9; P < 0.05, Ae. albopictus; df = 5; F = 28.261; P < 0.05) and breeding site categories (Ae.aegypti; df = 19; F = 48.1; P < 0.05, Ae. albopictus: df = 19; F = 20.171, P < 0.05).
The breeding of Ae. aegypti was more conducive in temporary removals (19.0%; n = 34), discarded non-reusable items (12.0%; n = 21), tyres (10.1%; n = 18) and covering items (10.1%; n = 18). Aedes albopictus mosquitoes were predominant in natural breeding places (14.7%; n = 246), temporary removals (13.6%; n = 227), discarded non-reusable items (12.0%; n = 198), covering items/polythene (11.5%; n = 192) and ornamentals (7.10%; n = 119) (Fig. 4). A pictorial collection of breeding sites identified in the field surveys is included as supplementary material (Additional file 1: Fig. S1).
Percentage occurrence of each positive breeding site out of the total number of positive breeding sites inspected. a Study areas representing all environmental settings, b urban environmental setting, c suburban environmental setting and d rural environmental setting. C1, water storage barrels; C2, water storage cement tank; C3, water storage other; C4, concrete slabs; C5, gutters; C6, tyres; C7, ornamentals; C8, natural; C9, ponds; C10, wells; C11, tube wells; C12, AC/refrigerator trays; C13, temporary removals; C14, covering items/polythene; C15, discarded degradable items; C16, discarded reusable items; C17, discarded non-degradable items; C18, pet feeding cups; C19, non-use cisterns and commode; C20, other
Although temporary removal items and discarded items were recognized as the most frequent breeding sites with higher percentage occurrence for Ae. aegypti, tyres (6.6%), non-used cisterns/commode (6%) and concrete slabs (5.4%) were identified as the preferable breeding places for Ae. aegypti indicating higher container indices. The highest container type indices for Ae. albopictus were also reported for non-used cisterns/commode (36.8%), tyres (27.9%) and covering items/polythene (22.7%) (Table 1).
Resting preferences of Ae. aegypti and Ae. albopictus adult mosquitoes
A total of 1834 adult mosquitoes were collected from indoor and outdoor resting locations inspected. Of the collections, 80.8% (n = 1482) represented by females. The majority of the collection comprised of Ae. albopictus (54.5%; n = 999). Aedes aegypti adult mosquitoes were detected mostly from indoor resting places, and outdoor resting places were more prominent for Ae. albopictus (Table 2). The chi-square analysis showed that the observed difference of two vectors for indoor and outdoor resting preferences was statistically significant at 95% confidential intervals (χ2 = 1025; df = 1; P < 0.001). In terms of resting places, Ae. aegypti was mostly found in resting places such as bedrooms (18.4%; n = 337) followed by living rooms (11.3%; n = 207) and kitchens (4.7%; n = 86). Outdoor resting places were least preferred by Ae. aegypti. On the other hand, Ae. albopictus was conducive to rest on the vegetation (25.1%; n = 460) in peri-domestic and house backyard (Table 2).
Aedes aegypti female mosquitoes rested on cloth hangings and curtains, followed by furniture (13.2%; n = 243). Outside walls were the least preferable resting surface for Ae. aegypti (1.0%; n = 21). The highest percentages of Ae. albopictus mosquitoes were resting on vegetation in outdoor dwellings (25.1%; n = 460). According to the chi-square analysis, the preferences in resting surface among two mosquito species were statistically significant (χ2 = 1049; df = 1; P < 0.001).
It was interesting to note that the highest percentage of Aedes mosquitoes were found resting on wooden surfaces in both indoor and outdoor sites (44.2%; n = 811), clothes/curtains (22.8%; n = 418) and cement surfaces (22.6%; n = 415). Among two species, Ae. aegypti preferred cloths and hangings (16.8%; n = 308). Aedes albopictus mosquitoes rested on wooden surfaces (29.4%; n = 540). Furthermore, it was observed that the majority (45.7%; n = 839) of the resting population of Aedes mosquitoes was identified on the resting places closer to the ground level (< 1 m) followed by surfaces at 1–2 m height and 2–3 m (Table 3). Only 3.7% of the places > 3 m of height were identified as the least preferred resting sites for Aedes adult mosquitoes. However, no statistical differences were observed in terms of resting height with two species or gender of the mosquito species.
Biting behaviours and peak biting hours of Aedes mosquitoes
A total of six mosquito species were recorded from human-baited double-net traps, namely, Ae. aegypti, Ae. albopictus, Culex gelidus, Culex quinquefasciatus, Mansonia uniformus and Armigeres subalbatus. However, 73.2% of the collection (n = 161/273) represented the mosquitoes under genus Aedes (Ae. aegypti—25.6%, n = 70; Ae. albopictus—33.3%; n = 91). Both Ae. aegypti and Ae. albopictus exhibited a typical diurnal pattern with two host-seeking and biting activity peaks, one in the morning and one in the afternoon.
The host-seeking cycle of Ae. albopictus was bimodal with two equally dominated peaks; morning peak occurred at 05:00–11:00 h and afternoon peak between 14:00 and 19:00 h. Aedes aegypti also demonstrated the same bimodal host-seeking cycle. A minor peak occurred between 05:00 and 09:00 h in the morning and a major peak between 13:00 and 19:00 h in the afternoon. It is interesting to note that Ae albopictus is more active outdoors in the morning and afternoon (Fig. 5). In contrast, Ae aegypti is more active indoors in the afternoon peak (Fig. 6).
The host-seeking activity of Ae albopictus is prominent in the early afternoon from 2 to 5 p.m. for a broader period outdoors. Aedes aegypti was active in a narrower period from 3 to 5 p.m. late evening (Figs. 5 and 6). Results indicate that both mosquito species shift their host-seeking locations only from outdoors to indoors but not vice versa in both periods. There was no significant correlation between the mean numbers of female mosquitoes caught with mean atmospheric temperature (r = 0.1; 30.7 ℃ ± 1.7 ℃) or relative humidity (r = − 0.3; 72.5% ± 5.4) according to the Pearson’s correlation.
Discussion
The results of the present study document the oviposition/breeding site preferences, resting, biting and peak biting hours of Ae. aegypti and Ae. albopictus mosquitoes in Sri Lanka. Identifying vector distribution, density and percentage abundance, breeding habitats and behavioural aspects plays a critical role in evaluating current vector control strategies and further facilitating localized interventions that are specific to a particular region [27]. Therefore, the present investigation adds some fundamental information on the bionomics of dengue vector mosquitoes which can aid the designing of appropriate vector control interventions.
The current study’s findings showed that urban areas with higher dengue incidence were more conducive to Ae. aegypti breeding than suburban and rural areas. However, the present study also identified Ae. albopictus as the most abundant vector in all spatial setups in Gampaha District, including rural, suburban and urban areas. The study’s findings are supported by a few similar studies on the distribution and prevalence of these vectors in Sri Lanka [28, 29].
Reports from different countries, including Africa [30], Southeast Asia [31] and Australia [32], have described the elusive resting habits of Ae. aegypti [33]. However, some studies conducted in Panama [34], Costa Rica [35], Dominican Republic [36], Puerto Rico [37] and Mexico [38] evidenced the seclusive resting behaviour of Ae. aegypti within houses [18]. Furthermore, some investigations have indicated the presence of dengue infected Ae. aegypti in and around houses [39, 40]. Therefore, it is shown that houses provide a suitable environment for human-vector contact, dengue transmission and suitable resting sites for adult mosquitoes [18]. In addition, identifying the resting behaviour and resting sites of vectors would be beneficial to targeting vector control interventions such such as indoor residual spraying (IRS) or intra-domiciliary spraying and insecticide-treated curtains as emergency control measures against Ae. aegypti [18, 41].
The present study revealed that resting behaviour varied between the two Aedes vector species, where Ae. aegypti adult mosquitoes had a highly endophilic nature, while Ae. albopictus demonstrated exophilic behaviour confirming previous findings [18, 34]. In this study Ae. aegypti was mainly found resting in bedrooms, living rooms and kitchens. However, Ae. albopictus was found resting especially in outdoor vegetation. Results of the present study are similar to those of previous studies conducted in Trinidad [18], Panama [34] and Mexico [39]. They suggest that a domestic environment with high human-vector contact, especially in urbanized areas, provides suitable breeding and resting sites for Ae. aegypti mosquitoes. On the other hand, Ae. albopictus is mainly found among the vegetation in rural and suburban areas.
A higher proportion of Ae. aegypti resting was observed in indoor, dark and hidden surfaces such as cloths and cloth hangings and under furniture in houses kept at 2 m elevation from the ground. Previous studies have emphasized that around 67% of the dengue-positive houses harboured Ae. aegypti mosquitoes [42]. Furthermore, some studies have shown the presence of dengue virus-positive mosquitoes inside houses even 27 days after the initial clinical diagnosis of a patient [43]. Therefore, the presence of these mosquitoes in houses could be directly associated with disease incidence and transmission in these areas. Hence, appropriate control interventions should be focused on suppressing the adult densities based on evidence on the resting behaviour of vector mosquitoes.
Some research studies have indicated that insecticide applications targeting Ae. aegypti mosquitoes may be more effective in controlling dengue transmission [18]. However, some studies have identified that thermal fogging has limited success in controlling dengue outbreak situations, giving the reason that insecticide droplets may not reach up to adult Aedes mosquitoes at rest mainly indoors in hidden localities [44,45,46,47,48]. Simultaneously, thermal space fogging is associated with socio-economic and environmental problems because of high cost, difficulty of application and harm to non-targeted beneficial insects such as bees [34, 46,47,48].
Several studies have highlighted that Ae. aegypti females remain indoors and stay longer at resting sites [18, 49]. This behaviour of Ae. aegypti leads to failure of conventional space spraying of insecticide targeting the suppression of adult mosquitoes since there is a minimal possibility of insecticide droplets reaching indoors, especially in bedrooms [18]. Therefore, it shows the resting behaviour of Ae. aegypti mosquitoes is primarily responsible for the success of vector control programmes [18, 34, 50]. Many countries, including Trinidad [51], Suriname [52], Dominican Republic [36] and Panama [34], reiterated that the location of these resting sites is also a primary reason for the failure of Ae. aegypti control. Therefore, health authorities should focus on more appropriate vector interventions based on biology and behavioural aspects of dengue vectors.
Considering the limitation in conventional ultra-low-volume (ULV) spraying or thermal space spraying, indoor residual spraying (IRS) could be more viable since this method allows the insecticide to contact the resting mosquito population on insecticide-treated surfaces. This may provide evidence on the efficacy of IRS programmes and indicates why some countries, including Sri Lanka, in the past controlled malaria [18], with no dengue and leishmaniasis, with considerable public health importance. Therefore, the health authorities should re-introduce the IRS programme to control disease vectors rationally, targeting one disease vector. It is also important to elaborate that all these activities should be initiated based on scientific evidence and surveillance-guided integrated vector control programmes.
Host-seeking and -biting activities were also examined in this study to determine the biting cycles of the two dengue vectors [53] according to the habitat settings in the study area to plan appropriate vector control and bite prevention [54]. Adding to this, Chaves et al. [53] reported that the difference in the feeding behaviour of vectors affects the transmission pattern of vector-borne diseases during different seasons around the globe [55]. The present study demonstrated that both dengue vectors showed diurnal feeding behaviour, where the host-seeking cycle of Ae. albopictus was bimodal with two equally dominant peaks: morning peak occurred at 05:00–11:00 h and afternoon peak between 14:00 and 19:00 h. Meanwhile, Ae. aegypti exhibited a typical diurnal pattern with a minor peak occurring between 05:00 and 09:00 h in the morning and a significant peak between 13:00 and 19:00 h in the afternoon. The results were consistent with similar studies done in Trinidad, West Indies [56], Cameroon [57] and Malaysia [54], and Ae. aegypti and Ae. albopictus adult females showed endophagic and exophagic feeding behaviour, respectively.
Breeding site preference and oviposition behaviour are important aspects of vector bionomics. The primary vector for dengue transmission, Ae. aegypti, originated in Africa but now has spread to other continents [7]. Mosquitoes in the genus Aedes show different choices for oviposition ranging from indoors to outdoors and natural vegetation to human-made containers [12]. Aedes aegypti is a highly domesticated urban mosquito that prefers to live with humans in their homes, feeds on humans and oviposits in human-made artificial containers [16].
The results of the present study corroborate that urban areas are favoured by Ae. aegypti over suburban or rural areas where females can obtain their blood meals, rest and oviposit more easily and frequently because of high human population density and higher availability of human-made breeding habitats. Since Ae.aegypti has a relatively short flight range [27], this drastic urbanization provides the ideal ecological conditions to support higher densities of Ae.aegypti, assuring close contact with crowded human populations, leading to dengue epidemic situations in urban areas [16, 58]. Another study conducted by Noordeen et al. [28] in the central province of Sri Lanka quoted similar findings.
Aedes mosquitoes breed in both artificial and natural breeding sites, which provide clear and unpolluted water indoors and outdoors [23, 59], at ground level and above in places such as roof gutters, overhead tanks, concrete slabs and receptacles on roofs and slab areas at any type of premises [23]. Furthermore, Aedes mosquitoes have been recorded in brackish and saline water in Sri Lanka [60, 61]. The present research reports refrigerator trays, ant traps, ornamental items including flower vases, abandoned fish tanks and ponds, water storage containers in toilets/bathrooms, and cisterns and squatting pans of non-used toilets as the major indoor breeding sites for Aedes mosquitoes. Discarded items including degradables (coconut shells, clay pots) and non-degradables (plastics, glass, metal), water storage tanks and barrels, temporary removal items (toys, buckets, cooking utensils, etc.), ponds, tube wells and shallow cement wells, used tyres, roof gutters, natural breeding places (leaf axils, bamboo stumps and tree holes), covering polythenes, concrete slabs and cement floors and pet feeders were identified as the major outdoor breeding sites.
Even though Aedes mosquitoes breed in different types of breeding sites, the productivity varies based on the living standards of the population, and it is seasonal and area-specific [19, 23, 62]. In an area with an irregular water supply and shortage of water, water storage tanks and barrels have been identified as the major breeding sites for dengue vectors [60]. Simultaneously, discarded containers are the most productive breeding sites in areas with poor solid waste management, especially in urban settings [61]. Discarded containers and domestic wells are the major breeding places for Aedes [29]. Findings of the present study indicated that Ae. aegypti mainly breed in discarded items, temporary removals and covering items in urban areas because of higher availability of breeding sites with poor solid waste management, agreeing with Louis et al. [62], while Ae. albopictus mosquitoes were more predominant in discarded items followed by natural breeding places. In the present investigation, since > 80% of the breeding places for Ae. aegypti larvae were human-made artificial containers, larvicidal-driven vector control interventions such as the application of temephos and biological controlling methods may not be successful. It was also noted that Aedes populations are maintained in indoor breeding sites during the dry season. Therefore, well-planned integrated vector management programmes should be initiated with the collaboration of all the multiple stakeholders, including local government bodies and other related ministries, targeting source reduction by improving garbage collection and disposal systems, law enforcement and, most importantly, enhancing the awareness of the community about the elimination of mosquito breeding places in a domestic environment to control ongoing dengue outbreaks in the district.
Conclusions
Aedes aegypti prefers to rest indoors, especially in dark and shady areas such as cloth hangings and under furniture. Aedes albopictus showed mainly exophilic behaviour; they are mostly resting among vegetation in the peridomestic environment. The majority of the vector population was identified at indoor resting places. Therefore, the efficacy of using thermal space spraying of insecticides could be questionable. Hence, re-introduction of indoor residual spraying for vector control could be recommended. The biting time was 05:00–11:00 h in the morning and between 13:00 and 19:00 in the afternoon. Therefore, preventive measures and attention to minimizing vector biting are recommended during the peak biting hours. Discarded items, temporary removals and covering items were identified as critical breeding places for Ae. aegypti, and discarded items followed by natural breeding places were identified as the key breeding places for Ae. albopictus mosquitoes. This study shows the need for scientific-based surveillance, monitoring and decision making in vector control approaches in Sri Lanka. It could be advantageous to have a holistic vector control and surveillance programme targeting medically important disease vectors in Sri Lanka.
Availability of data and materials
The study datasets are available from the corresponding author upon reasonable request.
Abbreviations
- GLM:
-
Generalized linear model
- IRS:
-
Indoor residual spraying
- MOH:
-
Medical officer of health
- ULV:
-
Ultra-low volume
- CI:
-
Container index
References
Thonnon J, Fontenille D, Tall A, Diallo M, Renaudineau Y, Baudez B, et al. Re-emergence of yellow fever in Senegal in 1995. Am J Trop Med Hyg. 1998;59:108–14.
Farraudière L, Sonor F, Crico S, Étienne M, Mousson L, Hamel R, et al. First detection of dengue and chikungunya viruses in natural populations of Aedes aegypti in Martinique during the 2013–2015 concomitant outbreak. Rev Panam Salud Publica. 2017;41:e63.
Cigarroa TN, Blitvich B, Cetina TR, Talavera AL, Baak BC, Torres CO, et al. Chikungunya virus in febrile humans and Aedes aegypti mosquitoes, Yucatan, Mexico. Emerg Infect Dis. 2016;22:1–5.
Ferreira-de-Brito A, Ribeiro IP, Miranda RM, Fernandes RS, Campos SS, Silva KA, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. 2016;111:655–8.
Higa Y. Dengue vectors and their spatial distribution. Trop Med Health. 2011;39:17–27.
Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—a review. Mem Inst Oswaldo Cruz. 2013;108:11–7.
Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29:460–8.
Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;30:e08347.
World Health Organization. Dengue vector management: report of a regional workshop Colombo, Sri Lanka. Geneva: World Health Organization; 2013.
Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7.
Ndenga BA, Mutuku FM, Ngugi HN, Mbakaya JO, Aswani P, Musunzaji PS, et al. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE. 2017;12:e0189971.
Yalla S, Clark J, Oullo D, Ngonga D, Abuom D, Wanja E, et al. Comparative efficacy of existing surveillance tools for Aedes aegypti in Western Kenya. J Vector Ecol. 2015;40:301–7.
Ratsitorahina M, Harrison J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, et al. Outbreak of dengue and Chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–7.
World Health Organization. Dengue hemorrhagic fever diagnosis, treatment, prevention and control. 2nd ed. Geneva: World Health Organization; 1997.
Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health. 2011;39:3–11.
Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309.
Chadee DD. Resting behaviour of Aedes aegypti in Trinidad: with evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasit Vectors. 2013;6:255.
Karunaratne SH, Weeraratne TC, Perera MD, Surendran SN. Insecticide resistance and efficacy of space spraying and larviciding in the control of dengue vectors Aedes aegypti and Aedes albopictus in Sri Lanka. Pestic Biochem Physiol. 2013;107:98–105.
Bonds JA. Ultra-low-volume space sprays in mosquito control: a critical review. Med Vet Entomol. 2012;26:121–30.
Alahacoon N, Edirisinghe M. Spatial variability of rainfall trends in Sri Lanka from 1989 to 2019 as an indication of climate change. Int J Geo Inf. 2021;10:84.
World Health Organization. Entomological surveillance for Aedes spp. in the context of Zika virus: interim guidance for entomologists. Geneva: World Health Organization; 2016.
National Control Unit, Sri Lanka. Standard operational procedures for Aedes vector surveillance in Sri Lanka. Sri Lanka: National Dengue Control Unit; 2019.
Service MW. Mosquitoes ecology. Field sampling methods. 2nd ed. London: Elsevier and Chapman & Hall; 1993.
Rueda L. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa. 2004. https://doi.org/10.11646/zootaxa.589.1.1.
Tangena JAA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: an alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS ONE. 2015;10:e0138735.
World Health Organization. Dengue haemorrhagic fever Diagnosis, treatment, prevention and control. 2nd ed. Geneva: World Health Organisation; 1997.
Noordeen F, Raza M, Pitchai F, Saranga W, Sandeepani L, Sadamali L, et al. Distribution of dengue vectors, Aedes aegypti and Aedes albopictus, in a few selected semi-urban areas of the Central Province of Sri Lanka. Sri Lankan J Infect Dis. 2018;8:36–9.
Sirisena PD, Noordeen F. Evolution of dengue in Sri Lanka-changes in the virus, vector, and climate. Int J Infect Dis. 2014;19:6–12.
Trips M, Hausermann W. Demonstration of differential domesticity of Aedes aegypti (L.) (Diptera, Culicidae) in Africa by mark-release-recapture. Bull Entomol Res. 2009;65:199–208.
Pant CP, Yasuno M. Field studies on the gonotrophic cycle of Aedes aegypti in Bangkok, Thailand. J Med Entomol. 1973;10:219–23.
Muir LE, Kay BH. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998;58:277–82.
Schoof HF. Mating, resting habits and dispersal of Aedes aegypti. Bull World Health Organ. 1967;36:600–1.
Perich MJ, Davila G, Turner A, Garcia A, Nelson M. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J Med Entomol. 2000;37:541–6.
Perich MJ, Rocha NO, Castro AL, Alfaro AW, Platt KB, Solano T, et al. Evaluation of the efficacy of lambda-cyhalothrin applied by three spray application methods for emergency control of Aedes aegypti in Costa Rica. J Am Mosq Control Assoc. 2003;19:58–62.
Perich MJ, Tidwell MA, Williams DC, Sardelis MR, Pena CJ, Mandeville D, et al. Comparison of ground and aerial ultra-low volume applications of malathion against Aedes aegypti in Santo Domingo, Dominican Republic. J Am Mosq Control Assoc. 1990;6:1–6.
Clark GG, Seda H, Gubler DJ. Use of the “CDC backpack aspirator” for surveillance of Aedes aegypti in San Juan, Puerto Rico. J Am Mosq Control Assoc. 1994;10:119–24.
Eisen L, Beaty BJ. Innovative decision support and vector control approaches to control dengue. In: Vector-borne diseases: understanding the environmental, human health and ecological connections. Washington: Workshop Summary, The National Academies Press; 2008.
Halstead SB, Scanlon JE, Umpaivit P, Udonsakdi A. Dengue and chickungunya virus infection in man in Thailand 1962–1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am J Trop Med Hyg. 1969;18:997–1033.
Race MW, Fortune RAJ, Agostini CAM, Varma MGR. Isolation of dengue viruses in mosquito cell cultures under field conditions. Lancet. 1978;8054:48–9.
Cardé R, Gibson G. Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues. Ecol Vector Borne Dis. 2010;2:115–41.
Chadee DD, Doon R, Severson DW. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: the cardinal points approach. Acta Trop. 2007;104:1–7.
Chadee DD. Key premises, a guide to Aedes aegypti (Diptera: Culicidae) surveillance and control. Bull Entomol Res. 2004;94:201–7.
Abeyasuriya K, Nugapola N, Perera M, Karunaratne W, Karunaratne S. Effect of dengue mosquito control insecticide thermal fogging on non-target insects. Int J Trop Insect Sci. 2017;37:11–8.
Usuga AF, Zuluaga-Idárraga LM, Alvarez N, Rojo R, Henao E, Rúa-Uribe GL. Barriers that limit the implementation of thermal fogging for the control of dengue in Colombia: a study of mixed methods. BMC Public Health. 2019;19:669.
World Health Organization. Space spray application of insecticides for vector and public health pest control A practitioner’s guide. Geneva: World Health Organization; 2003.
World Health Organization. Guidelines for dengue surveillance and mosquito control. 2nd ed. Geneva: World Health Organization; 2003.
Mani TR, Arunachalam N, Rajendran R, Satyanarayana K, Dash AP. Efficacy of thermal fog application of deltacide, a synergized mixture of pyrethroids, against Aedes aegypti, the vector of dengue. Trop Med Int Health. 2005;10:1298–304.
Chadee DD. Studies on the post-oviposition blood-feeding behaviour of Aedes aegypti (L.) (Diptera: Culicidae) in the laboratory. Pathog Glob Health. 2012;106:413–7.
Chadee DD. An evaluation of Malathion ULV spraying against natural and caged populations of Aedes aegypti in Trinidad, West Indies. Cah Orstom Ser Ent Méd Parasitol. 1985;23:71–4.
Rohani A, Chan ST, Abdullah AG, Tanrang H, Lee HL. Species composition of mosquito fauna in Ranau, Sabah, Malaysia. Trop Biomed. 2008;25:232–6.
Chen CD, Wan-Norafikah O, Nurin-Zulkifli IM, Lee HL, Faezah K, Izzul AA, et al. Biting behaviour of medically important mosquitoes (Diptera: Culicidae) in Peninsular Malaysia. Trop Biomed. 2017;34:199–211.
Chaves LF, Cohen JM, Pascual M, Wilson ML. Social exclusion modifies climate and deforestation impacts on a vector-borne disease. PLoS Neg Trop Dis. 2008;2:e176.
Chadee DD, Martinez R. Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies. J Vector Ecol. 2000;25:158–63.
Kamgang B, Nchoutpouen E, Simard F, Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit Vectors. 2012;5:57.
Rodrigues Mde M, Marques GR, Serpa LL, Arduino Mde B, Voltolini JC, Barbosa GL, et al. Density of Aedes aegypti and Aedes albopictus and its association with number of residents and meteorological variables in the home environment of dengue-endemic area, São Paulo, Brazil. Parasit Vectors. 2015;8:115.
Surendran SN, Jayadas TTP, Thiruchenthooran V, Raveendran S, Tharsan A, Santhirasegaram S, et al. Aedes larval bionomics and implications for dengue control in the paradigmatic Jaffna peninsula, northern Sri Lanka. Parasit Vectors. 2021;14:162.
Kusumawathie P, Siyambalagoda R. Distribution and breeding sites of potential dengue vectors in Kandy and Nuwara Eliya districts of Sri Lanka. Ceylon J Med Sci. 2008;48:43–52.
World Health Organization. Comprehensive guideline for prevention and control of dengue and dengue hemorrhagic fever—revised and expanded edition. Geneva: World Health Organization; 2011.
Jude PJ, Tharmasegaram T, Sivasubramaniyam G, Senthilnanthanan M, Kannathasan S, Raveendran S, et al. Salinity-tolerant larvae of mosquito vectors in the tropical coast of Jaffna, Sri Lanka and the effect of salinity on the toxicity of Bacillus thuringiensis to Aedes aegypti larvae. Parasit Vectors. 2012;5:269.
Surendran SN, Veluppillai T, Eswaramohan T, Sivabalakrishnan K, Noordeen F, Ramasamy R. Salinity tolerant Aedes aegypti and Ae. albopictus infection with dengue virus and contribution to dengue transmission in a coastal peninsula. J Vector Borne Dis. 2018;55:26–33.
Louis VR, Montenegro Quiñonez CA, Kusumawathie P, Palihawadana P, Janaki S, Tozan Y, et al. Characteristics of and factors associated with dengue vector breeding sites in the city of Colombo, Sri Lanka. Pathog Glob Health. 2016;110:79–86.
Acknowledgements
We would like to acknowledge the entomological field staff who helped conduct field surveys. Furthermore, the support provided by the residents in conducting investigations is greatly appreciated.
Funding
The authors received no funds for the present research work.
Author information
Authors and Affiliations
Contributions
RD: Conducting the field surveys, data entry, data analysis and writing of the manuscript; DA: Designing the research, supervision and review of the manuscript; NG: Supervision of the research work and writing of the manuscript; NA: Facilitating the research work and field activities. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance for the present study was obtained from the Ethics Review Committee of the Institute of Biology, Sri Lanka (IOBSL161 09 17). Written consents were obtained from all adults who participated in human-baited double-net traps. Verbal permission was also obtained from household heads for conducting entomological investigations at their houses and compounds.
Consent for publication
Not applicable.
Completing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Breeding site categories observed during the field surveys: a water storage barrel, b ornamental item, c concrete slab, d tyre, e bamboo hole, f natural leaf axil, g covering polythene, h non-used commode /cistern, i temporary removals, j gutter, k discarded degradables/non-degradables, l pet feeders, m refrigerator tray.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dalpadado, R., Amarasinghe, D., Gunathilaka, N. et al. Bionomic aspects of dengue vectors Aedes aegypti and Aedes albopictus at domestic settings in urban, suburban and rural areas in Gampaha District, Western Province of Sri Lanka. Parasites Vectors 15, 148 (2022). https://doi.org/10.1186/s13071-022-05261-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05261-3