Abstract
Background
Clip domain serine proteases (CLIPs), a very diverse group of proteolytic enzymes, play a crucial role in the innate immunity of insects. Innate immune responses are the first line of defense in mosquitoes against the invasion of pathogenic microorganisms. The Toll pathway, immunodeficiency (IMD) pathway and melanization are the main processes of innate immunity in Aedes aegypti. CLIPS are classified into five subfamilies—CLIPA, CLIPB, CLIPC, CLIPD, and CLIPE—based on their sequence specificity and phylogenetic relationships. We report the functional characterization of the genes that code for two CLIPs in Ae. aegypti (Ae): Ae-CLIPB15 and Ae-CLIPB22.
Methods
Clustal Omega was used for multiple amino acid sequence alignment of Ae-CLIPB15 and Ae-CLIPB22 with different CLIP genes from other insect species. The spatiotemporal expression profiles of Ae-CLIPB15 and Ae-CLIPB22 were examined. We determined whether Ae-CLIPB15 and Ae-CLIPB22 respond to microbial challenge and tissue injury. RNA interference (RNAi) was used to explore the function of Ae-CLIPB15 and Ae-CLIPB22 in the defense of Ae. aegypti against bacterial and fungal infections. The expression levels of nuclear factor kappa B (NF-κB) transcription factors REL1 and REL2 in the Toll pathway and IMD pathway after bacterial infection were investigated. Finally, the change in phenoloxidase (PO) activity in Ae-CLIPB15 and Ae-CLIPB22 knockdown adults was investigated.
Results
We performed spatiotemporal gene expression profiling of Ae-CLIPB15 and Ae-CLIPB22 genes in Ae. aegypti using quantitative real-time polymerase chain reaction. These genes were expressed in different stages and tissues. The messenger RNA (mRNA) levels for both genes were also up-regulated by Gram-negative bacteria Escherichia coli, Gram-positive bacteria Staphylococcus aureus and fungal Beauveria bassiana infections, as well as in the tissue injury experiments. RNAi-mediated knockdown of Ae-CLIPB15 led to a significant decrease of PO activity in the hemolymph of Ae. aegypti, while other RNAi experiments revealed that both Ae-CLIPB15 and Ae-CLIPB22 were involved in immune defense against bacterial and fungal infections. The mRNA expression of NF-κB transcription factors REL1 and REL2 in the Toll pathway and IMD pathway differed between Ae-CLIPB15 and Ae-CLIPB22 knockdown mosquitoes infected with bacteria and wild type mosquitoes infected with bacteria.
Conclusions
Our findings suggest that Ae-CLIPB15 and Ae-CLIPB22 play a critical role in mosquito innate immunity, and that they are involved in immune responses to injury and infection. Their regulation of transcription factors and PO activity indicates that they also play a specific role in the regulation of innate immunity.
Graphical Abstract

Similar content being viewed by others
Background
Mosquito-borne diseases are a major public health problem throughout most of the world [1]. For instance, there are more than 100 million annual cases of dengue fever, a viral disease transmitted by Aedes aegypti. Despite the burden of mosquito-borne diseases on human populations, our understanding of the relationships between pathogens and their mosquito hosts remains limited [2]. Studies have shown that innate immunity plays a key role in the interaction between pathogens and their vectors, and that pathogens face multiple barriers in the innate immune system of mosquitoes. Therefore, the study of vector immunity and its interaction with pathogens is important for the development of new vector disease control strategies [3].
Mosquitoes lack acquired immunity, but their innate immune system can destroy various prokaryotic and eukaryotic pathogens [4]. In arthropods, innate immunity plays an important role in limiting pathogen infection through the production of molecules such as antimicrobial peptides (AMPs), through phagocytosis and encapsulation, and by the secretion of physical barriers and melanization [5]. Melanization is an immune response of arthropods to a wide range of viruses [6], bacteria [7, 8], fungi [9], nematodes associated with chromogenic bacteria [10] and other eukaryotic parasites [11,12,13]. This immune response is regulated by prophenoloxidase (proPO), which mediates the conversion of tyrosine to melanin; recognition of a pathogen triggers a serine protease cascade in which activated serine proteases cleave proPO to produce phenoloxidase (PO) [14]. The Toll pathway and immunodeficiency (IMD) pathway are two major pathways of the innate immune response in arthropods, and are responsible for the production of AMPs and other effectors [15]. In Ae. aegypti, the activation of genes coding for AMPs and other immune effectors is achieved by releasing nuclear factor kappa B (NF-κB) transcription factors REL1 and REL2, which are homologous to those in Drosophila melanogaster [16].
Clip domain serine proteases (CLIPs), non-digestible serine proteases found in the hemolymph of insects and other arthropods, are key components of the insect immune response. CLIPs have been identified in arthropods and mollusks, and the genes coding for them comprise a large gene family in the insect genome [17,18,19,20,21,22]. CLIPs are secreted into hemolymph in the form of a zymogen and need to be hydrolyzed and activated by cleavage [14]. CLIPs have a N-terminal with disulfide bonds and a C-terminal domain with protease activity. Infection can stimulate the activation of CLIP protease in hemolymph, which can specifically cleave at a site in the N terminal domain of the protease, resulting in the creation of a double-stranded enzyme in which the CLIP domain and the protease domain are connected by disulfide bonds. Once CLIP proteases are activated, they are regulated by serine inhibitors in hemolymph plasma [23,24,25,26]. The functions of CLIPs include proteolytic activation of the Spätzle cytokine to form active Toll ligands for the synthesis of AMPs and the specific activation of proPO that is needed for melanization. However, some CLIPs have a non-catalytic protease-like domain and N-terminal clip domain, which are called clip domain serine protease homologs (CSPHs). Despite their lack of enzymatic activity, CSPHs seem to play an essential role in the immune response of mosquitoes. In melanization, the cleavage and activation of proPO require CSPHs as cofactors [27,28,29]. Two CSPHs, serine protease homolog (SPH)1 and SPH2 in Manduca sexta, are cofactors of proPO activating enzymes (PAP)1 and PAP3, which effectively cleave and activate proPO [29,30,31]. The precursors of SPH1 and SPH2 cannot activate proPO [29, 32] and need to be treated with PAP3 and PAP1 to generate activated SPH1 and SPH2 [31, 33]. Tenebrio molitor CSPH1 [28] and Holotrichia diomphalia proPO activating factor II [34] have also been proven to be indispensable CSPHs for the activation of proPO. CSPHs are also involved in cell adhesion and bacterial conditioning [35].
In Ae. aegypti, the family of CLIP proteases can be classified into five subfamilies: CLIPA, CLIPB, CLIPC, CLIPD and CLIPE [17]. Of the 82 CLIPs, 62 belong to CLIPB; CLIPC and CLIPD are expected to show serine protease activity. CLIPA and CLIPE comprise 17 CSPHs. In addition, 15 CLIPs and two CSPHs have special domains and are considered to play essential regulatory roles in immune responses.
Through evolutionary analysis we found that there are two members of the CLIPB subfamily in Ae. aegypti (Ae): Ae-CLIPB15 and Ae-CLIPB22. These CLIPs are similar to those of other organisms such as Drosophila melanogaster, Bombyx mori and Manduca sexta [3]. However, compared with the CLIPs of different species of mosquitoes, such as Aedes albopictus and Anopheles gambiae, they are highly conserved, indicating that they may play an important role in the regulation of innate immunity in Ae. aegypti. Thus, we decided to examine their role in immune response and immune pathways, and especially their role in the proPO activation pathway. Here, we report the involvement of Ae-CLIPB15 and Ae-CLIPB22 in the defense of Ae. aegypti against bacterial and fungal infections. The results showed that these two serine proteases enable Ae. aegypti to resist infection by exogenous pathogenic microorganisms. They are also involved in regulating PO activity and transcription factors of innate immunity such as the Toll and IMD pathways. Our study shows that Ae-CLIPB15 and Ae-CLIPB22 are regulatory factors of the mosquito immune system, and thus contributes to a better understanding of the mechanisms of immune regulation in insects.
Methods
Mosquito rearing
The Ae. aegypti Rockefeller strain was maintained in the insectary at Hainan University. Eggs of Ae. aegypti were placed in a 30 × 25 × 8-mm plastic feeding pot and maintained at 27 °C and 90 ± 5% relative humidity. Upon hatching, the larvae were reared in water with fish food provided ad libitum. The adult mosquitoes were kept in the insectary at 26 ± 1 ℃ and 80 ± 5% relative humidity under a 16-h light:18-h dark cycle with access to water and 8% sugar ad libitum. Three-day-old mosquitoes were used for the experiments.
Multiple amino acid sequence alignment
We retrieved protein sequences of CLIPs from the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/) that have been studied in other insect species. Clustal Omega software (https://www.ebi.ac.uk/Tools/msa/clustalo/) was used for multiple amino acid sequence alignment of Ae-CLIPB15 and Ae-CLIPB22 with different CLIPs from other insect species.
Sampling of different stages and tissue samples of mosquitoes
To examine the temporal expression of Ae-CLIPB15 and Ae-CLIPB22, we used different stages of the mosquito, including eggs, first-instar to fourth-instar larvae, white and black female pupae, white and black male pupae, and female and male adults. To examine the spatial expression of Ae-CLIPB15 and Ae-CLIPB22, different tissues of female adults, including those of the thorax, legs, fat body, midgut, ovary, and Malpighian tubule were collected. Adult female mosquitoes were anesthetized at low temperature and placed in a Petri dish. Each tissue sample comprised material that was collected from about 30 mosquitoes. A total of 50 first- and second-instar larvae and ten larvae at other instar stages were collected from each tube. There were three repeats for each life cycle stage and type of tissue sample.
Bacterial and fungal infection and tissue injury experiments
For bacterial infection, Escherichia coli (DH5α) [36] and Staphylococcus aureus (ATCC43300) were obtained from Hainan University. Both were cultured in 37 ℃ Luria–Bertani liquid medium, and their growth monitored by the absorbance reading at 600 nm until the optical density reached about 1. Pellets were collected after centrifugation, then suspended in sterile double-distilled H2O to obtain a final E. coli cell suspension of 2.0 mg/mL and S. aureus cell suspension of 0.8 mg/mL. Adult female mosquitoes were anesthetized on ice for 5 min and then gradually injected with 2 µL of bacterial suspension. The injected adult female mosquitoes were divided into three groups of 30 mosquitoes each for use in the follow-up experiment.
Beauveria bassiana (strain 242) was obtained from the microbial bank of Hainan University. Beauveria bassiana was cultured on potato glucose agar plates at 28 ℃ for 10 days until the plate was covered with hyphae and conidia. Then eight to 10 fungal colonies were added to 100 ml potato dextrose broth and cultured at 28 ℃, 240 r.p.m. for several days. After the medium was turbid, it was filtered through aseptic neutral filter paper and the filtrate aspirated into aseptic 1.5-ml Eppendorf tubes for centrifugation. The harvested spores were suspended in 0.05% Tween 80. Fungal spores were counted under a hemocytometer with an inverted microscope and diluted to 2.65 × 106 spores/mL with 0.05% Tween 80. An appropriate amount of spore suspension was dipped into a ground aseptic capillary tube, which was used to pierce the ventral tip of the frozen anesthetized adult female mosquitoes, to infect them with B. bassiana spores. The infected adult female mosquitoes were divided into three groups comprising 30 mosquitoes each, which were used in the follow-up experiment.
For the tissue injury experiment, the thorax of freeze-anesthetized adult female mosquitoes was punctured with a ground aseptic capillary tube to cause effective physical damage. The adult female mosquitoes were divided into three groups comprising 10 mosquitoes each. Three independent repeated experiments were carried out for each of the above treatments.
RNA extraction and quantitative polymerase chain reaction
To analyze the spatiotemporal expression profiles of Ae-CLIPB15 (GenBank accession number AAEL014349) and Ae-CLIPB22 (GenBank accession number AAEL008668) and their expression profiles after microbial infection and tissue injury, the total RNA was extracted from the different tissues of female adult mosquitoes (those of the thorax, fat body and other parts), and from different developmental stages, as well as from adult female mosquitoes after microbial infection and tissue injury. Total RNA was extracted from adult mosquitoes for each of the three independent repeats. Quantitative real-time polymerase chain reaction (qPCR) was performed as described previously [37] in a LightCycler 480 system (Roche Applied Science, Mannheim, Germany) using SYBR Green Master I (Roche) according to the manufacturer’s instructions, with the following cycling conditions: initial denaturation at 95 ℃ for 30 s followed by 40 cycles of 95 ℃ for 5 s, and 60 ℃ for 30 s. Primers (forward, 5'-CTGTAAGGTCCTGTGAATACG-3'; reverse, 5'-GGTTTATCAGGGAGTTCACC-3') were used to amplify the 109-bp DNA fragment of Ae-CLIPB15. Primers (forward, 5'-GATCCTGTCAAAGGCTTCC-3'; reverse, 5'-GTACTGTCAGTGCGTATTGG-3') were used to amplify the 236-bp DNA fragment of Ae-aaCLIP22. The Ae. aegypti ribosomal protein S17 gene (GenBank accession number AAEL025999) was used as a reference gene. Messenger RNA (mRNA) expression was quantified using the comparative cross threshold (Ct; number of PCR cycles required for the fluorescent signal to cross the signal threshold) method. The relative 2−ΔΔct method was used to analyze the relative gene expression data [38].
Double-stranded RNA preparation
Total RNA was extracted from adult mosquitoes with the SPARKeasy Cell RNA Kit (Sparkjade) and then subjected to qPCR using the PrimeScript RT Reagent Kit with the genomic DNA Eraser Kit (TaKaRa). Primers (forward, 5'-GGAACTCCCTGATAAACC-3'; reverse, 5'-AACGCACATATTCTAACG-3') were used to amplify the fragment of Ae-CLIPB15 from the cDNA. Primers (forward, 5'-TGTGGCACTGCTTCCGATTT-3'; reverse, 5'-GAGTATTGCGTAGTCCTTGTA-3') were used to amplify the fragment of Ae-CLIPB22 from the cDNA. The β-glucuronidase gene (gus) (KY848224), a bacterial gene specific to E. coli, was used as the negative control, as reported previously [39]. The PCR products were collected and purified. The gene fragments were cloned into PMD19-T (TaKaRa), and NotI and XhoI restriction enzymes used to excise target fragments from PMD19-T, which were then ligated into plasmid pL4440. The recombinant plasmid was transformed into competent cells of the RNase-III-deficient E. coli strain HT115(DE3), following a previously described method [39]. The cells were grown in 2× Luria–Bertani medium (10 g/l tryptone, 5 g/l yeast extract, 10 g/l NaCl) containing ampicillin and tetracycline at 37 ℃ for 12–14 h. The bacterial solution was grown until optical density measured at a wavelength of 600 nm reached 0.5. Isopropyl-β-D-thiogalactopyranoside was added to a final concentration of 0.6 mM to induce T7 polymerase activity. The expressed double-stranded RNA (dsRNA) was extracted and confirmed by electrophoresis on 1% agarose gel.
RNA interference
The adult mosquitoes were collected 3–5 days after hatching and 24 h after they had fed on sugar water. A moderate number of mosquitoes were placed in a refrigerator at − 20 ℃ for a few minutes. The frozen mosquitoes were then put on ice and injected with purified dsRNA by using a manual microinjection device (Eppendorf, Hamburg, Germany). Meanwhile, four groups of females were injected in the thorax with 2 μl of one of the following: the dsRNA of gus (control group); diethyl pyrocarbonate (DEPC)-treated water (control group); dsRNA of Ae-CLIPB15 (dsAe-CLIPB15); dsRNA of Ae-CLIPB22 (dsAe-CLIPB22). Thirty female adults were injected in each group, and all the experiments were repeated three times. After injection, the mosquitoes were transferred to mosquito cages and collected 24 h later for the preparation of RNA. RNA interference (RNAi) efficiency was verified by qPCR.
Mosquito survival assays and analysis of transcription factor expression
Twenty-four hours after dsRNA injection, the four groups of mosquitoes were infected with either E. coli, S. aureus, or B. bassiana. Twenty adults from each group were used in the survival assays, which were carried out at 1-day intervals during the following week. Three independent biological repeat experiments were carried out for each treatment. To explore whether the expression of transcription factors Ae-REL1 (GenBank accession number AAEL012164) and Ae-REL2 (GenBank accession number AAEL007624) would be affected when Ae-CLIPB15 and Ae-CLIPB22 were knocked down in the adults 24 h after dsRNA injection, four groups of mosquitoes were infected with one of the above bacteria. After 24 h, total RNA was extracted for three independent recombination experiments. Primers (forward, 5'-ATAGGCGAGATCAACATCAGCAGC-3'; reverse, 5'-CGTTGCTGTTCCTGCTTCATATCG-3') were used to amplify the fragment of Ae-REL1. Primers (forward, 5'-TTTGAATGTGCTGTTGGGTC-3'; reverse, 5'-GAATGTTGTTTCCGTGCTTA-3') were used to amplify the fragment of Ae-REL2 using cDNA as a template. All the analyses were repeated three times.
PO activity assay
To determine PO activity, adult mosquitoes were injected with dsRNA 2 days prior to the experiment. The thoraces of ten mosquitoes from each group were added to a 1.5-ml Eppendorf tube with 200 µl HEPES buffer (50 mmol/L), and then crushed with a grinding machine and centrifuged at 12,000 r.p.m. for 20 min at 4 ℃ to collect the hemolymph. A total volume of 75 µl tissue fluid containing hemolymph and 125 µl of L-dopamine (8 mmol/L in 50 mmol/L HEPES, pH 8.0) were added to a 96-well plate and the absorption at 490 nm was recorded every 10 s for 20 min on a microplate reader (RNE90002; Reagen, China). One unit of PO activity is defined as the amount of enzyme yielding PO that produces an increase of 0.001 absorbance units/min [40]. Each treatment was performed as three independent biological replications.
Statistical analyses
All the statistical analyses were performed using GraphPad Prism version 6.02 (GraphPad Software). A t-test was used to determine significant differences (P < 0.05) in the levels of mRNA and PO between the control and treatment groups. GraphPad Prism software was used to analyze the mosquito survival curves.
Results
Multiple amino acid sequence alignment
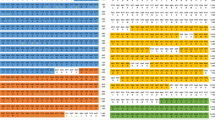
Multiple amino acid sequence alignment showed that Ae. aegypti CLIPB15 is similar to An. gambiae CLIPB15 (percent identity 49.16%), Bombyx mori PPAE (percent identity 37.10%) and Drosophila melanogaster GRASS (percent identity 34.20%); and that Ae-CLIPB22 is similar to Bombyx mori PPAE (percent identity 34.53%) and An. gambiae CLIPB15 (percent identity 34.33%) (Fig. 1).
Multiple amino acid sequence alignment of Aedes aegypti (Ae) clip domain serine proteases (CLIPs) (Ae-CLIPB15 and Ae-CLIPB22) with CLIPs from other insect species: Anopheles gambiae CLIPB15 (Ag-CLIPB15, AGAP009844-PA); Drosophila melanogaster GRASS (Dm-GRASS, Dmel_CG5896); Manduca sexta HP21 (Ms-HP21, AAV91019.1); Bombyx mori prophenoloxidase-activating enzyme (Bm-PPAE, NP_001036832.1); Aedes albopictus prophenoloxidase activating factor (Aa-PPAF1, XP_029722923.1). These sequences were aligned by the Clustal Omega program with default settings. Asterisks indicate 100% homology, colons indicate 90% homology, points indicate 80% homology
Spatiotemporal expression profiles of Ae-CLIPB15 and Ae-CLIPB22
Spatiotemporal expression profiles of Ae-CLIPB15 and Ae-CLIPB22 were analyzed by qPCR. The transcript expression levels of these genes were investigated in 11 different developmental stages including the egg, larval, pupal and adult stages, and in tissues of six different parts of the adult mosquito (legs, thorax, fat body, midgut, ovary, and Malpighian tubule). The Ae-CLIPB15 transcript expression levels in the fourth-instar larvae were significantly higher than in the other larval stages [e.g. first-instar vs fourth-instar, t-test, t(22) = 11.20, P < 0.0001], and those in adult male mosquitoes were significantly higher than in first-instar larvae [first-instar vs male adult, t-test, t(22) = 20.00, P < 0.0001] (Fig. 2a) and in adult females. The transcript expression levels of Ae-CLIPB22 had significantly increased by the fourth-instar larval stage compared to the first-instar [first-instar vs fourth-instar, t-test, t(22) = 17.67, P < 0.0001] and the higher level was maintained in the pupal and adult female mosquito stages. However, the transcript expression level of this gene decreased significantly in adult male mosquitoes [first-instar vs male adult, t-test, t(22) = 1.986, P = 0.3120] (Fig. 2b). The expression of both genes was lowest in the eggs.
Ae-CLIPB15 and Ae-CLIPB22 were expressed in different tissues of adult female mosquitoes. The transcript expression levels of Ae-CLIPB15 in the thorax [legs vs thorax, t-test, t(12) = 11.34, P < 0.0001] and fat body [legs vs fat body, t-test, t(12) = 16.80, P < 0.0001] were significantly higher than in the other tissues (Fig. 2c). Transcripts of Ae-CLIPB22 were most abundant in the midgut [legs vs midgut, t-test, t(12) = 8.583, P < 0.0001], compared to the other types of tissues (Fig. 2d).
Spatiotemporal expression profiles of Ae-CLIPB15 and Ae-CLIPB22. Relative expression levels of Ae-CLIPB15 (a) and Ae-CLIPB22 (b) at 11 developmental stages from egg to adult. Relative expression levels of Ae-CLIPB15 (c) and Ae-CLIPB22 (d) in six different types of tissues (thorax, legs, fat body, midgut, ovary, and Malpighian tubule). The statistical analyses were performed using two-way ANOVA in GraphPad software; data are presented as means ± SD (n = 3). **** P < 0.001; ns not significantly different. For abbreviations, see Fig. 1
Microbial infection and tissue injury can induce the expression of Ae-CLIPB15 and Ae-CLIPB22
To determine whether Ae-CLIPB15 and Ae-CLIPB22 respond to microbial challenge in Ae. aegypti, we first measured their expression levels after bacterial and fungal infection. Infection with the Gram-positive bacterium S. aureus and with the Gram-negative bacterium E. coli induced up-regulation of Ae-CLIPB15 [DEPC vs E. coli, t-test, t(4) = 28.66, P < 0.0001; DEPC vs S. aureus, t-test, t(4) = 15.71, P < 0.0001] and Ae-CLIPB22 (DEPC vs E. coli, t-test, t(4) = 14.36, P = 0.0002; DEPC vs S. aureus, t-test, t(4) = 26.55, P < 0.0001] (Fig. 3a, b). Infection with the fungus B. bassiana could induce up-regulation of Ae-CLIPB15 [DEPC vs B. bassiana, t-test, t(4) = 26.99, P < 0.0001] and Ae-CLIPB22 [DEPC vs B. bassiana, t-test, t(4) = 6.109, P = 0.0036] (Fig. 3c). Similarly, the expression of Ae-CLIPB15 [control vs 6 h, t-test, t(6) = 37.29, P < 0.0001] and Ae-CLIPB22 [control vs 6 h, t-test, t(6) = 13.87, P = 0.0002] was up-regulated 6 h after Ae. aegypti mosquitoes were exposed to superficial physical damage (Fig. 3d). Ae-CLIPB15 and Ae-CLIPB22 showed different responses to bacterial and fungal infection, and played a specific role in regulating wound healing in the mosquitoes.
Relative expression levels of Ae-CLIPB15 and Ae-CLIPB22 in adult mosquitoes following infection with Escherichia coli (a), Staphylococcus aureus (b) and Beauveria bassiana (c), and after physical injury (d). The control groups were injected with diethyl pyrocarbonate (Depc)-treated water. The abundances of messenger RNA (mRNA) of Ae-CLIPB15 and Ae-CLIPB22 were determined by quantitative real-time polymerase chain reaction (qPCR). The expression of these genes in the infection group was compared to that in the control group at 24 h post-infection. In the physical damage experiment, the control group was not injured. The mRNA abundance for Ae-CLIPB15 and Ae-CLIPB22 was determined by qPCR, and the mRNA expression levels of Ae-CLIPB15 and Ae-CLIPB22 in the injured groups were compared with those in the control group at 6 h and 12 h post-injury. The expression profiles were compared using two-way ANOVA in GraphPad software; data are presented as means ± SD, n = 3. ** P < 0.01, *** P < 0.001, **** P < 0.001. For other abbreviations, see Fig. 1
Knockdown of Ae-CLIPB15 and Ae-CLIPB22 resulted in higher susceptibility to bacterial and fungal infections
To confirm whether Ae-CLIPB15 and Ae-CLIPB22 contribute to the defense of Ae. aegypti mosquitoes against bacterial and fungal infection, we injected dsRNA into the mosquito thorax and monitored the survival of the female adults after infection. At 24 h post-infection, the relative mRNA expression levels of Ae-CLIPB15 [GUS vs dsAe-CLIPB15, t-test, t(6) = 12.76, P < 0.0001] and Ae-CLIPB22 [GUS vs dsAe-CLIPB22, t-test, t(6) = 14.80, P < 0.0001] were significantly decreased, indicating successful knockdown of these two genes (Figs. 4a, 5a). The female adults were also infected with bacteria and fungi after successful Ae-CLIPB15 and Ae-CLIPB22 knockdown. Knockdown of Ae-CLIPB15 [dsGUS + E. coli vs dsAe-CLIPB15 + E. coli, t-test, t(12) = 3.251, P = 0.0314; dsGUS + S. aureus vs dsAe-CLIPB15 + S. aureus, t-test, t(12) = 5.547, P = 0.0149] and Ae-CLIPB22 [dsGUS + E. coli vs dsAe-CLIPB22 + E. coli, t-test, t(12) = 3.333, P = 0.0207; dsGUS + S. aureus vs dsAe-CLIPB22 + S. aureus, t-test, t(12) = 4.803, P = 0.0187] resulted in a significant decrease in the survival rate of mosquitoes infected with E. coli (Figs. 4b, 5b) and S. aureus (Figs. 4c, 5c) compared with the control groups. The survival rate of mosquitoes infected with B. bassiana [dsGUS + B. bassiana vs dsAe-CLIPB15 + B. bassiana, t-test, t(12) = 8.71, P = 0.0074; dsGUS + B. bassiana vs dsAe-CLIPB22 + B. bassiana, t-test, t(12) = 7.251, P = 0.0102] was also significantly lower than that of the control group (Figs. 4d, 5d). In summary, these results show that both of these genes are implicated in immune reactions triggered in response to bacterial and fungal infection.
Knockdown of Ae-CLIPB15 by RNA interference (RNAi) (a) and its effect on the survival of adult mosquitoes after Escherichia coli (b), Staphylococcus aureus (c) and Beauveria bassiana (d) infection. The mRNA expression levels of Ae-CLIPB15 were examined by qPCR after double-stranded (ds) RNA injection (dsAe-CLIPB15). The expression profiles were compared using two-way ANOVA in GraphPad software; data are presented as means ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001. GUS β-Glucuronidase; for other abbreviations, see Figs. 1 and 3
Knockdown of Ae-CLIPB22 by RNAi (a) and its effect on the survival of adult mosquitoes after Escherichia coli (b), Staphylococcus aureus (c) and Beauveria bassiana (d) infection. The mRNA expression levels of Ae-CLIPB22 were examined by qPCR after dsRNA injection (dsAe-CLIPB22). The expression profiles were compared using two-way ANOVA in GraphPad software; data are presented as means ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001. For abbreviations, see Figs. 1, 3 and 4
Knockdown of Ae-CLIPB15 and Ae-CLIPB22 resulted in different regulation of transcription factors
To confirm whether Ae-CLIPB15 and Ae-CLIPB22 can regulate the Toll pathway and IMD pathway of the innate immune response of Ae. aegypti, the expression levels of NF-κB transcription factors REL1 and REL2 in the Toll pathway and IMD pathway were investigated in mosquitoes infected with Gram-negative E. coli. Ae-CLIPB15 and Ae-CLIPB22 were knocked down by injected dsRNA. The mosquitoes were infected with E. coli and S. aureus 24 h after dsRNA injection. At 12 h post-infection, total RNA was extracted and qPCR performed. The knockdown of Ae-CLIPB15 [dsGUS + E. coli vs dsAe-CLIPB15 + E. coli, t-test, t(8) = 10.81, P < 0.0001] and Ae-CLIPB22 [dsGUS + E. coli vs dsAe-CLIPB22 + E. coli, t-test, t(8) = 12.19, P < 0.0001] resulted in a significant decrease in the transcript expression levels of NF-κB transcription factor REL2 in the IMD pathway after the female adults were infected by the Gram-negative bacteria E. coli. In contrast, the expression of NF-κB transcription factor REL1 in the Toll pathway was not significantly different from that in the control group (Fig. 6a). Interestingly, when the female adults were infected with the Gram-positive bacteria S. aureus, the expression of NF-κB transcription factor REL1 in the Toll pathway was significantly decreased in the Ae-CLIPB15 knockdown adults [dsGUS + S. aureus vs dsAe-CLIPB15 + S. aureus, t-test, t(8) = 3.233, P = 0.0298] but significantly increased in Ae-CLIPB22 knockdown adults [dsGUS + S. aureus vs dsAe-CLIPB22 + S. aureus, t-test, t(8) = 12.36, P < 0.0005]. The expression of NF-κB transcription factor REL2 in the IMD pathway was significantly decreased in Ae-CLIPB15 knockdown adults [dsGUS + S. aureus vs dsAe-CLIPB15 + S. aureus, t-test, t(8) = 6.411, P = 0.0005] and Ae-CLIPB22 knockdown adults [dsGUS + S. aureus vs dsAe-CLIPB22 + S. aureus, t-test, t(8) = 15.83, P < 0.0001] (Fig. 6b). Although the expression of REL1 was not affected by the knockdown of either Ae-CLIPB15 or Ae-CLIPB22 in mosquitoes infected with E. coli, the knockdown of these genes did affect its expression in the mosquitoes infected with S. aureus. In short, these results show that both Ae-CLIPB15 and Ae-CLIPB22 are involved in the regulation of the expression of REL1 and REL2 in the Toll pathway and the IMD pathway in the innate immune response of Ae. aegypti, and suggest that these genes play different roles in the regulation of innate immune pathways. However, the differences in the expression level of REL2 do not necessarily mean that Ae-CLIPB15 and Ae-CLIPB22 directly regulate the IMD pathway, as innate immune pathways may interact with each other. Thus, the change in the expression of REL2 may have been caused by an indirect effect. In summary, further experiments are needed to examine this.
Knockdown of Ae-CLIPB15 and Ae-CLIPB22 by RNAi and the effect on the expression levels of NF-κB transcription factors REL1 (a) and REL2 (b) in the Toll pathway and IMD pathway of mosquitoes after bacterial infection. The relative expression levels of REL1 and REL2 were examined by qPCR after bacterial infection. The expression profiles were compared using two-way ANOVA in GraphPad software; data are presented as means ± SD, n = 3. * P < 0.05, *** P < 0.001, **** P < 0.001. For other abbreviations, see Figs. 1, 3, 4 and 5
Knockdown of Ae-CLIPB15 decreased proPO activation in Ae. aegypti
The change in PO activity in the Ae-CLIPB15 and Ae-CLIPB22 knockdown adults was examined in the hemolymph at 24 h post-injection. Compared with the control group, the knockdown of Ae-CLIPB15 [dsGUS vs dsAe-CLIPB15, t-test, t(12) = 3.765, P = 0.0056] resulted in a significant decrease in PO activity. However, there was no significant difference in PO activity following the knockdown of Ae-CLIPB22 (dsGUS vs dsAe-CLIPB22, t-test, t(12) = 0.7704, P = 0.7839] (Fig. 7). In general, the PO activity data showed that Ae-CLIPB15 is involved in the activation of proPO in Ae. aegypti, while Ae-CLIPB22 is not.
Phenoloxidase (PO) activity of knockdown Ae-CLIPB15 and Ae-CLIPB22 mosquitoes. Adult mosquitoes were injected with dsRNA and the hemolymph of 10 mosquitoes was collected 24 h later for PO activity assays on a microplate reader. PO activities were compared using two-way ANOVA in GraphPad software; data are presented as means ± SD, n = 3. ** P < 0.01. For other abbreviations, see Figs. 1, 3, 4 and 5
Discussion
Insects rely on their innate immune system to fight invading bacteria, fungi and parasites [16, 41,42,43,44]. The innate immune system plays an important role in limiting pathogen infection through phagocytosis, entrapment, secretion of physical barriers and melanization. These innate immune responses are mainly studied using insect models. CLIPs are essential components of the insect immune response. The CLIP catalytic domain is characterized by a conserved Tryp_SPc domain with a catalytic ternary domain, which is composed of the amino acid residues His, Asp and Ser [45, 46]. When the catalytic triad of SP mutates, the enzyme loses its catalytic activity and becomes a CSPH. The functions of CLIPs in innate immune responses include the proteolytic activation of the cytokine Spätzle to form active Toll ligands for the synthesis of AMPs and the specific activation of proPO required for melanization. In Ae. aegypti, the CLIP family is classified into five subfamilies—CLIPA, CLIPB, CLIPC, CLIPD, CLIPE—each of which comprises polygenes. In this study, we were able to confirm the roles of Ae-CLIPB15 and Ae-CLIPB22, two members of the CLIPB subfamily of Ae. aegypti, in the innate immune response of adult Ae. aegypti mosquitoes.
The qPCR results showed that the kinetics of the induction of Ae-CLIPB15 and Ae-CLIPB22 differ following infection by Gram-negative and Gram-positive bacteria and fungi, but that these genes are not co-regulated. Interestingly, Ae-CLIPB15 and Ae-CLIPB22 are also induced by aseptic injury. Our results suggest that Ae-CLIPB15 and Ae-CLIPB22 may play a role in microbial defense and potentially a regulatory role in wound healing.
The immune response of arthropods is mainly regulated by two pathways, the Toll pathway and the IMD pathway [15]. In Ae. aegypti, NF-κB transcription factors REL1 and REL2 of the Toll pathway and IMD pathway are the main participants in the activation of genes coding for AMPs and other immune effector factors. Spz1C, Toll5A, CLIPB5 and CLIPB29 of Ae. aegypti have been shown to mediate the response of the Toll pathway to fungal infection [3, 47]. In An. gambiae, the IMD pathway is activated by peptidoglycan recognition protein or indirectly activated by a serine protease cascade [48]. However, according to current knowledge of insect immunity, CLIPs are not involved in the IMD pathway. Here, we used RNAi to knock down the dsRNA expression of Ae-CLIPB15 and Ae-CLIPB22 to study their role in the innate immunity of Ae. aegypti. After Ae-CLIPB15 and Ae-CLIPB22 were knocked down, the survival rate of Ae. aegypti was reduced after infection with pathogenic bacteria compared with that of wild type mosquitoes, and their resistance to bacterial infection decreased by 50%.
The transcription of REL1 and REL2 was affected to varying degrees when Ae-CLIPB15 and Ae-CLIPB22 were knocked down. However, as the expression of REL1 and REL2 was mostly inhibited following Ae-CLIPB15 and Ae-CLIPB22 knockdown, we suggest that these latter genes are involved in the regulation of the Toll pathway and have an indirect effect on the activation of the IMD pathway. This may also explain why the survival rate of the adult mosquitoes that had been infected with bacteria and fungi after Ae-CLIPB15 and Ae-CLIPB22 knockdown was lower than that of the wild type mosquitoes. However, further biochemical study is definitely needed to examine whether Ae-CLIPB15 and Ae-CLIPB22 are involved in the regulation of the Toll pathway or the IMD pathway. In arthropods, the central component of the extracellular enzyme cascade is a CLIP which regulates various innate immune responses such as the Toll pathway and the IMD pathway [42]. Our results confirm that Ae-CLIPB15 and Ae-CLIPB22 participate in the regulation of innate immune responses in Ae. aegypti; however, their role in the regulation of a serine protease cascade remains to be clarified.
Mosquitoes that are capable of melanization initially received a great deal of attention as specific phenotypes that are resistant to parasites of public health importance, such as those that cause malaria and filariasis [49,50,51]. Melanization is a powerful defense response in Ae. aegypti. In this process, the PO activation system is triggered by soluble receptor molecules that recognize molecular patterns associated with pathogens or abnormal cells, resulting in the activation of serine proteases. These then activate a CLIPC, which in turn activates the terminal CLIPB protease in the cascade, also known as proPO activating protease (PAP) or proPO activating enzyme (PPAE). The activated PAP then cleaves the proPO to give PO. PO then acts as a catalyst for the formation of active intermediates of a quinone for the synthesis of melanin [52], which has a variety of protective functions. In addition, the formation of an active PO complex on the surface of the foreign body is mediated by one or more proteolytic inactivated CLIPAs, which need to be activated through proteolysis to play a role in the process. However, not all the CLIPs involved in the regulation of the proPO cascade activate proPO. Some CLIPs can inhibit melanization in An. gambiae [53]. Under normal physiological conditions, the proPO activation cascade is turned off, mainly by a single highly conserved serine protease inhibitor: Spn27A in Drosophila melanogaster [54, 55], Serpin-3 in Manduca sexta [56], and SRPN2 in mosquitoes [3, 17, 18, 57]. In this study, we knocked down Ae-CLIPB15 and Ae-CLIPB22 and explored their role in the proPO activation system. The knockdown of Ae-CLIPB15 led to a significant decrease in PO activity in the mosquitoes. However, there was no significant difference in PO activity in Ae-CLIPB22 knockdown mosquitoes. Ae-CLIPB15 was involved in the regulation of PO activity in Ae. aegypti, while Ae-CLIPB22 was not. This suggests that the CLIP family comprises serine proteases that are involved in a complex network of regulatory cascades that are independent of each other yet show some similarities. Further research is needed on this.
In summary, CLIPs are essential regulators of immune responses in many insects. All presently identified proteases that directly cleave insect proPO belong to the CLIPB subfamily. However, it is not clear how a single melanization cascade is regulated in the context of numerous physiological functions. Deciphering the regulatory mechanisms that lead to melanization in different defense responses, such as wound healing and pathogen isolation, is of particular interest. CLIPs may also have non-immune functions during insect development, e.g. embryonic dorsal pattern formation in Drosophila, and play a role in other physiological systems, all of which remain to be discovered [58]. To further improve our understanding of CLIP cascades and their role in a variety of immune responses, we also need to study other organisms, especially arthropod vectors, which show complex interactions between their immune systems and the pathogens that they transmit.
Conclusions
This study provides evidence for the role of Ae-CLIPB15 and Ae-CLIPB22 in the innate immune regulation of mosquitoes. Genetic interference of Ae-CLIPB15 and Ae-CLIPB22 could affect the resistance of adult Ae. aegypti to pathogens. However, further biochemical study is definitely needed to explore the regulatory mechanisms of innate immunity in Ae. aegypti. Our results suggest that Ae-CLIPB15 and Ae-CLIPB22 are potential targets for vector transmission control using RNAi technology.
Availability of data and materials
The data supporting the findings of this article are included in the article and its additional files.
Abbreviations
- AMP:
-
Antimicrobial peptide
- cDNA:
-
Complementary DNA
- CLIP:
-
Clip domain serine protease
- CSPH:
-
Clip domain serine protease homolog
- DEPC:
-
Diethyl pyrocarbonate
- dsRNA:
-
Double-stranded RNA
- GUS:
-
β-Glucuronidase
- IMD:
-
Immunodeficiency
- mRNA:
-
messenger RNA
- PAP:
-
Prophenoloxidase activating enzyme
- PO:
-
Phenoloxidase
- proPO:
-
Prophenoloxidase
- qPCR:
-
Quantitative real-time polymerase chain reaction
- RNAi:
-
RNA interference
- SPH:
-
Serine protease homolog
References
Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol. 2005;357:661–75.
Rajah MM, Pardy RD, Condotta SA, Richer MJ, Sagan SM. Zika virus: emergence, phylogenetics, challenges, and opportunities. ACS Infect Dis. 2016;211:763–72.
Zou Z, Shin SW, Alvarez KS, Kokoza V, Raikhell AS. Distinct melanization pathways in the mosquito Aedes aegypti. Immunity. 2010;32:41–53.
Wang YH, Chang MM, Wang XL, Zheng AH, Zou Z. The immune strategies of mosquito Aedes aegypti against microbial infection. Dev Comp Immunol. 2018;83:12–21.
Dimopoulos G. Insect immunity and its implication in mosquito-malaria interactions. Cell Microbiol. 2003;51:3–14.
Rodriguez-Andres J, Rani S, Varjak M, Chase-Topping ME, Beck MH, Ferguson MC, et al. Phenoloxidase activity acts as a mosquito innate immune response against infection with Semliki Forest virus. Plos Pathog. 2012;8(11):e1002977.
Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003;313:117–27.
Yassine H, Kamareddine L, Chamat S, Christophides GK, Osta MA. A serine orotease homolog negatively regulates TEP1 consumption in systemic infections of the malaria vector Anopheles gambiae. J Innate Immun. 2014;66:806–18.
Yassine H, Kamareddine L, Osta MA. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. Plos Pathog. 2012;8(11):e1003029.
Brey PT, Lebrun RA, Papierok B, Ohayon H, Vennavalli S, Hafez J. Defense reactions by larvae of Aedes aegypti during infection by the aquatic fungus Lagenidium giganteum (Oomycete). Cell Tissue Res. 1988;2531:245–50.
Christensen BM. Observations on the immune response of Aedes trivittatus against Dirofilaria immitis. Trans R Soc Trop Med Hyg. 1981;75(3):439–43.
Michel K, Budd A, Pinto S, Gibson TJ, Kafatos FC. Anopheles gambiae SRPN2 facilitates midgut invasion by the malaria parasite Plasmodium berghei. EMBO Rep. 2005;69:891–7.
Habtewold T, Povelones M, Blagborough AM, Christophides GK. Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. Plos Pathog. 2008;45:e1000070-e.
Zhang X, An C, Sprigg K, Michel K. CLIPB8 is part of the prophenoloxidase activation system in Anopheles gambiae mosquitoes. Insect Biochem Mol Biol. 2016;71:106–15.
Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti Toll pathway controls dengue virus infection. Plos Pathog. 2008;47:e1000098.
Hoffmann JA, Reichhart J-M. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;32:121–6.
Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science (New York, NY). 2007;3165832:1738–43.
Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, et al. Immunity-related genes and gene families in Anopheles gambiae. Science (New York, NY). 2002;2985591:159–65.
Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–31.
Zou Z, Evans JD, Lu Z, Zhao P, Williams M, Sumathipala N, et al. Comparative genomic analysis of the Tribolium immune system. Genome Biol. 2007;88:R177.
Zou Z, Lopez DL, Kanost MR, Evans JD, Jiang H. Comparative analysis of serine protease-related genes in the honey bee genome: possible involvement in embryonic development and innate immunity. Insect Mol Biol. 2006;155:603–14.
Cao X, He Y, Hu Y, Zhang X, Wang Y, Zou Z, et al. Sequence conservation, phylogenetic relationships, and expression profiles of nondigestive serine proteases and serine protease homologs in Manduca sexta. Insect Biochem Mol Biol. 2015;62:51–63.
Gulley MM, Zhang X, Michel K. The roles of serpins in mosquito immunology and physiology. J Insect Physiol. 2013;592:138–47.
Gubb D, Sanz-Parra A, Barcena L, Troxler L, Fullaondo A. Protease inhibitors and proteolytic signalling cascades in insects. Biochimie. 2010;9212:1749–59.
Park JW, Kim CH, Rui J, Park KH, Ryu KH, Chai JH, et al. Beetle immunity. Adv Exp Med Biol. 2010;708:163–80.
Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204.
Kwon TH, Kim MS, Choi HW, Joo CH, Cho MY, Lee BL. A masquerade-like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect Holotrichia diomphalia larvae. Eur J Biochem. 2000;26720:6188–96.
Lee KY, Zhang R, Kim MS, Park JW, Park HY, Kawabata S-I, et al. A zymogen form of masquerade-like serine proteinase homologue is cleaved during pro-phenoloxidase activation by Ca2+ in coleopteran and Tenebrio molitor larvae. Eur J Biochem. 2002;26917:4375–83.
Yu X-Q, Jiang H, Wang Y, Kanost MR. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2003;332:197–208.
Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol. 2005;353:241–8.
Wang Y, Lu ZQ, Jiang HB. Manduca sexta proprophenoloxidase activating proteinase-3 (PAP3) stimulates melanization by activating proPAP3, proSPHs, and proPOs. Insect Biochem Mol Biol. 2014;50:82–91.
Lu Z, Jiang H. Expression of Manduca sexta serine proteinase homolog precursors in insect cells and their proteolytic activation. Insect Biochem Mol Biol. 2008;38(1):89–98.
Wang Y, Jiang H. A positive feedback mechanism in the Manduca sexta prophenoloxidase activation system. Insect Biochem Mol Biol. 2008;388:763–9.
Kim MS, Baek MJ, Lee MH, Park JW, Lee SY, Soderhall K, et al. A new easter-type serine protease cleaves a masquerade-like protein during prophenoloxidase activation in Holotrichia diomphalia larvae. J Biol Chem. 2002;27742:39999–40004.
Wang R, Lee SY, Cerenius L, Soderhall K. Properties of the prophenoloxidase activating enzyme of the freshwater crayfish Pacifastacus leniusculus. Eur J Biochem. 2001;2684:895–902.
Hillyer JF, Schmidt SL, Fuchs JF, Boyle JP, Christensen BM. Age-associated mortality in immune-challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell Microbiol. 2005;71:39–51.
Guan H, Wang M, Liao C, Liang J, Mehere P, Tian M, et al. Identification of aaNAT5b as a spermine N-acetyltransferase in the mosquito, Aedes aegypti. PLoS ONE. 2018;13(3):e0194499.
Ballester M, Cordon R, Folch JM. DAG expression: high-throughput gene expression analysis of real-time PCR data using standard curves for relative quantification. PLoS ONE. 2013;8(11):e80385.
Whyard S, Erdelyan CNG, Partridge AL, Singh AD, Beebe NW, Capina R. Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasite Vector. 2015;8:96.
Jiang H, Wang Y, Yu X-Q, Kanost MR. Prophenoloxidase-activating proteinase-2 from hemolymph of Manduca sexta. A bacteria-inducible serine proteinase containing two clip domains. J Biol Chem. 2003;2786:3552–61.
Cirimotich CM, Dong YM, Garver LS, Sim SZ, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;344:387–95.
Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743.
Xing LS, Yuan CF, Wang ML, Lin Z, Shen BC, Hu ZH, et al. Dynamics of the interaction between cotton bollworm Helicoverpa armigera and nucleopolyhedrovirus as revealed by integrated transcriptomic and proteomic analyses. Mol Cell Proteomics. 2017;166:1009–28.
Xiong G-H, Xing L-S, Lin Z, Saha TT, Wang C, Jiang H, et al. High throughput profiling of the cotton bollworm Helicoverpa armigera immunotranscriptome during fungal and bacterial infections. BMC Genomics. 2015. https://doi.org/10.1186/s12864-015-1509-1.
Di Cera E. Serine proteases. IUBMB Life. 2009;615:510–5.
Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;43:337–60.
Shin SW, Kokoza V, Bian G, Cheon H-M, Kim YJ, Raikhel AS. REL1, a homologue of Drosophila dorsal, regulates Toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem. 2005;28016:16499–507.
Blumberg BJ, Trop S, Das S, Dimopoulos G. Bacteria- and IMD pathway-independent immune defenses against Plasmodium falciparum in Anopheles gambiae. PLoS ONE. 2013;8–9:e72130.
Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science (New York, NY). 1986;2344776:607–10.
Chen CC, Laurence BR. Selection of a strain of Anopheles quadrimaculatus with high filaria encapsulation rate. J Parasitol. 1987;732:418–9.
Hurd H, Taylor PJ, Adams D, Underhill A, Eggleston P. Evaluating the costs of mosquito resistance to malaria parasites. Evol Int J Organ Evol. 2005;59(12):2560–72.
Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;198:116–26.
Barillas-Mury C. CLIP proteases and Plasmodium melanization in Anopheles gambiae. Trends Parasitol. 2007;237:297–9.
Ligoxygakis P, Pelte N, Ji C, Leclerc V, Duvic B, Belvin M, et al. A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 2002;21(23):6330–7.
De Gregorio E, Han S-J, Lee W-J, Baek M-J, Osaki T, Kawabata S-I, et al. An immune-responsive serpin regulates the melanization cascade in Drosophila. Dev Cell. 2002;3(4):581–92.
Zhu Y, Wang Y, Gorman MJ, Jiang H, Kanost MR. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J Biol Chem. 2003;278(47):46556–64.
Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;3306000:88–90.
Castillejo-Lopez C, Hacker U. The serine protease Sp7 is expressed in blood cells and regulates the melanization reaction in Drosophila. Biochem Biophys Res Commun. 2005;3382:1075–82.
Acknowledgements
We are grateful to Prof. Tong-Yan Zhao of the Beijing Institute of Microbiology and Epidemiology for kindly providing the Ae. aegypti strain.
Funding
This work was supported by the National Natural Science Foundation of China (31860702; 31960703).
Author information
Authors and Affiliations
Contributions
QH conceived and designed the study and critically revised the manuscript. HCW and QHW performed the experiments and analyzed the data; HCW also drafted the manuscript. BB commented on the experimental design and methodology and substantially revised the manuscript. YXL helped to analyze the data. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, HC., Wang, QH., Bhowmick, B. et al. Functional characterization of two clip domain serine proteases in innate immune responses of Aedes aegypti. Parasites Vectors 14, 584 (2021). https://doi.org/10.1186/s13071-021-05091-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-05091-9