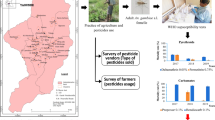
Abstract
Background
Malaria control in Kenya is based on case management and vector control using long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS). However, the development of insecticide resistance compromises the effectiveness of insecticide-based vector control programs. The use of pesticides for agricultural purposes has been implicated as one of the sources driving the selection of resistance. The current study was undertaken to assess the status and mechanism of insecticide resistance in malaria vectors in irrigated and non-irrigated areas with varying agrochemical use in western Kenya.
Methods
The study was carried out in 2018–2019 in Homa Bay County, western Kenya. The bioassay was performed on adults reared from larvae collected from irrigated and non-irrigated fields in order to assess the susceptibility of malaria vectors to different classes of insecticides following the standard WHO guidelines. Characterization of knockdown resistance (kdr) and acetylcholinesterase-inhibiting enzyme/angiotensin-converting enzyme (Ace-1) mutations within Anopheles gambiae s.l. species was performed using the polymerase chain reaction (PCR) method. To determine the agricultural and public health insecticide usage pattern, a questionnaire was administered to farmers, households, and veterinary officers in the study area.
Results
Anopheles arabiensis was the predominant species in the irrigated (100%, n = 154) area and the dominant species in the non-irrigated areas (97.5%, n = 162), the rest being An. gambiae sensu stricto. In 2018, Anopheles arabiensis in the irrigated region were susceptible to all insecticides tested, while in the non-irrigated region reduced mortality was observed (84%) against deltamethrin. In 2019, phenotypic mortality was decreased (97.8–84% to 83.3–78.2%). In contrast, high mortality from malathion (100%), DDT (98.98%), and piperonyl butoxide (PBO)-deltamethrin (100%) was observed. Molecular analysis of the vectors from the irrigated and non-irrigated areas revealed low levels of leucine-serine/phenylalanine substitution at position 1014 (L1014S/L1014F), with mutation frequencies of 1–16%, and low-frequency mutation in the Ace-1R gene (0.7%). In addition to very high coverage of LLINs impregnated with pyrethroids and IRS with organophosphate insecticides, pyrethroids were the predominant chemical class of pesticides used for crop and animal protection.
Conclusion
Anopheles arabiensis from irrigated areas showed increased phenotypic resistance, and the intensive use of pesticides for crop protection in this region may have contributed to the selection of resistance genes observed. The susceptibility of these malaria vectors to organophosphates and PBO synergists in pyrethroids offers a promising future for IRS and insecticide-treated net-based vector control interventions. These findings emphasize the need for integrated vector control strategies, with particular attention to agricultural practices to mitigate mosquito resistance to insecticides.
Graphic abstract

Similar content being viewed by others
Background
Frequent and prolonged droughts have resulted in the need to increase food production by irrigation in Kenya. This has led to altered ecosystems, with adverse consequences on human health due to water-related diseases [1, 2]. The ongoing deforestation, reclamation of marshlands, and establishment of irrigation systems for food production has resulted in increased vector populations and hence potential malaria transmission risk [3,4,5,6,7,8]. Agricultural activities have been intensified in many regions in Africa, with increasing interest in household-owned irrigation [9, 10] practices. These agricultural practices have contributed to major health problems such as increased malaria and schistosomiasis transmission [11,12,13,14,15,16,17]. Some of the agrochemicals used for crop protection contain the same active chemical ingredients as the insecticides used for public health purposes [18]. One of the unintended consequences of the use of the pesticides for agricultural purposes is the increasing selection for resistance on malaria vectors, as some pesticides used could contaminate nearby mosquito breeding habitats [19,20,21].
In Kenya, 80% of the land surface area is classified as arid and semi-arid [22, 23], thus necessitating irrigation for food production to sustain the ever-growing population. This has led to the introduction of several irrigation systems across the country, including the Ahero area in western Kenya [24,25,26] and the Mwea area in central Kenya [14,15,16,17], predominantly for rice plantations. Anopheles arabiensis has been observed as a predominant malaria vector in arid and semi-arid areas in Kenya [27, 28], and in high-density irrigated areas [14, 29, 30]. This species is an important vector in rice irrigation schemes [31, 32], and exhibits exophilic and significant exophagic behavior [33], increasing its transmission potential, especially outdoors [30, 34, 35]. Although this species shows strong zoophilic behavior, it is partly anthropophilic, depending on the availability of other animal blood meal sources [30, 36, 37]. This vector usually has low sporozoite rates [30, 37]; nevertheless, its high density in irrigated areas makes it an important and probably the major malaria vector in these irrigated areas [14, 30] in the absence of Anopheles funestus.
Insecticide resistance has been observed to manifest via two major mechanisms, an increase in metabolic detoxification of insecticides and target-site resistance as a result of mutations. Metabolic detoxification is achieved through the overproduction of cytochrome P450 [38], esterase [39], and glutathione S-transferase [40] enzymes in the presence of insecticides. Target-site resistance results from point mutation of genes encoding target proteins that interact with insecticides [41]. These often affect the sodium channels (responsible for pyrethoid and DDT insensitivity), resulting in knockdown resistance (kdr), acetylcholine neurotransmitters (hydrolyzed by acetylcholinesterase [AChE] enzyme which is responsible for organophosphate and carbamate insensitivity), and γ-aminobutyric acid (GABA) receptors for cyclodiene and fipronil insecticides. This mutation has been reported in several regions in sub-Saharan Africa [42,43,44,45,46]. In Kenya, the major target-site resistance encountered is the kdr [47, 48]. The use of pesticides/insecticides in agriculture and public health has been implicated in the development of insecticide resistance in major malaria vectors [20]. The scale-up of long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) has been considered the major cause of malaria vector insecticide resistance in many malaria-endemic African countries [49,50,51,52]. Extensive use of pyrethroid-based pesticides in agriculture for crop and livestock protection may further enhance/induce resistance [53,54,55].
The current study was undertaken in an irrigated and non-irrigated areas of Homa Bay County, western Kenya, where malaria vector control with high coverage of pyrethroid-treated LLINs and IRS using pirimiphos-methyl (Actellic®300 CS), an organophosphate insecticide, is ongoing [56]. This study aimed to assess the susceptibility status of malaria vectors and the underlying mechanism of resistance in different ecosystems with various agricultural activities. This information will guide mitigation and improved insecticide resistance management strategies.
Methods
Study site and design
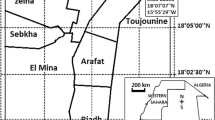
This study was conducted in Homa Bay County, western Kenya, a semi-arid malaria-endemic area situated along the southern shores of Lake Victoria’s Winam Gulf at an altitude of 1040–1330 m above sea level (Fig. 1). This region experiences average annual temperatures of 22.5 °C and rainfall of 1100 mm, with two rainy seasons. The long rains occur between March and May, and the short rains between September and November. A concrete channel irrigation system was constructed in the study area by the Ministry of Environment, Natural Resources and Regional Development Authorities of Kenya in 2007, known as the Kimira-Oluch Small-holder Farm Improvement Project (KOSFIP) (www.afdb.org). This project was undertaken to support subsistence and cash crop production such as cotton and fruits. The local community practice crop and animal farming in addition to fishing. The main malaria vectors are An. arabiensis and An. funestus sensu lato. Over time, malaria control in this area has relied on pyrethroid-based insecticide-treated nets. However, in 2018 and 2019, IRS was implemented using an organophosphate, pirimiphos-methyl (Actellic® 300 CS), resulting in a dramatic reduction of malaria vector populations [56]. A significant decrease in An. funestus s.l. population occurred, tending to near extinction levels [56].
Mosquito larval samples were collected from different village clusters in the irrigated and non-irrigated study areas (Fig. 1). The non-irrigated clusters are at least 2 km from the irrigation channels. Larvae were collected between February and July 2018 and 2019 from all the selected clusters in the irrigated and non-irrigated (10 clusters each) areas (Fig. 1). Various habitat types were sampled during each sampling season. After the long rains (May–July), more habitat types were encountered compared to the dry season (February–March). The habitat types included man-made ponds, swamps, irrigation lining, drainage ditches, natural ponds, river edges, and hoof/footprints.
Malaria vector larval sampling
Larval sampling was carried out using standard larval 350 ml dippers. Anopheline larvae were collected and transported to the International Center of Excellence for Malaria Research (ICEMR) insectary in Tom Mboya University College, for rearing to adults pending phenotypic insecticide resistance tests and molecular analyses.
Larval and adult mosquito rearing in the insectary
Field-collected mosquito larvae were placed in larval trays in the larval rearing room and fed a daily diet of Whiskas® cat food (Mars, Incorporated, McLean, VA, USA). Temperature and humidity in the larval room were maintained between 27 and 32°C and 40–60%, respectively. Pupae were collected daily and placed in holding cages covered with mosquito mesh netting where they emerged into adults. Emerged An. gambiae s.l. mosquitoes were maintained in the insectary adult rearing room with regulated temperatures (25–28 °C) and humidity (60–75%) and fed 6% glucose solution soaked in cotton wool. The females that emerged in the insectary were used for insecticide resistance bioassays and knockdown resistance (kdr) and Ace-1enzyme molecular analyses.
WHO susceptibility test
Two- to five-day-old female adult An. gambiae s.l. mosquitoes were used to determine susceptibility to diagnostic concentrations of pyrethroid (0.05% deltamethrin), organophosphate (5% malathion), and organochlorine (4% DDT) insecticides. Standard WHO tube bioassay tests were conducted as per WHO guidelines [57]. Mosquitoes used as control samples were simultaneously placed in WHO tubes lined with untreated papers. Mosquitoes were exposed to insecticide-impregnated test papers for 60 min and the number of knocked down females recorded every 10 min. Fifteen to 20 female mosquitoes were used in each test. At the end of the exposure period, mosquitoes were transferred into holding tubes, maintained on 6% glucose and observed after 24 h for mortality. The positive controls used were an insectary colony of the susceptible An. gambiae s.s. Kisumu strain. The final mortality was recorded 24 h post-exposure. Additionally, a synergist piperonyl butoxide (PBO)-deltamethrin exposure bioassay was carried out for a total of 120 min, with 60 min PBO exposure followed by 60 min deltamethrin exposure, with mortality recorded 24 h post-exposure. Knockdown was recorded every 10 min during the exposure period, and mortality counts were recorded after 24 h exposure to PBO alone and PBO-deltamethrin combined. Both live and dead mosquitoes were preserved individually at −20 °C for molecular species identification and the detection of kdr mutations and multiple copies of the AChE enzyme encoded by the Ace-1 gene.
An. gambiae s.l. DNA extraction and species identification
All field-collected specimens were morphologically identified as An. gambiae s.l. using Gillies and De Meillon taxonomic keys [58]. DNA from the whole female body was extracted from a proportion of the bioassayed adult females following the Musapa et al. protocol [59]. The molecular species identification was carried out as described by the Scott et al. [60] and Paskewitz and Collins protocols [61].
Genotyping of kdr and Ace-1 alleles
DNA samples of An. gambiae s.l. mosquitoes were assayed to detect the voltage-gated sodium channel (vgsc) L1014S (kdr-east) and L1014F (kdr-west) mutations and mutations in the Ace-1gene. Assays were performed on both live and dead mosquitoes post-bioassay tests using the published protocols [62,63,64].
Investigation of households using chemicals for public health, agriculture (farms) and veterinary (livestock) purposes
Different questionnaires were prepared and surveys were conducted with randomly selected farmers, households, and veterinary officers/agricultural extension workers to identify the chemicals used in public health, crops, and animal pest control (Additional files 1, 2, 3, 4, 5). Other methods for personal protection against malaria vector and other biting mosquitoes used by the selected households were also surveyed (Additional file 4). The surveys were carried out by trained data clerks sourced from the villages, and the questions were asked in English, Swahili, or the local language (Dholuo), depending on the respondent’s preferred language. The survey lasted between 10 and 20 min. Information recorded included the role of the respondent in the household, farm, or shop; mosquito prevention methods used in the household; crops grown and animals kept; pests that affected the crops and animals; insecticides used in crop and animal pest control; the application frequency of the identified insecticide; how long the insecticide had been used in that particular farm; excess chemical and empty container disposal; and cleaning of empty containers, in addition to other questions (Additional files 1, 2, 3, 4, 5).
Data management and analysis
During fieldwork, all larval data were entered into Open Data Kit (ODK) on tablets and then uploaded to the online database. The collection site (irrigated or non-irrigated), habitat type, habitat size, and number of larvae sampled were recorded in the field. This was done to identify the most productive habitats. In the insectary and laboratory, data were recorded on the respective laboratory data forms and later entered in Microsoft Excel spreadsheets, followed by error checks and corrections.
The WHO bioassay knockdown was recorded every 10 min for 1 h and final mortality was recorded at 24 h for all test runs with corresponding negative and positive controls. Abbot’s formula was used to correct percentage mortality in cases where the negative control mortality was between 5 and 20%; experiments where negative control mortality was above 20% were discarded and 95% confidence limits of the adjusted mortality determined. Mortality of 98–100% in the sample population indicated susceptibility to the tested insecticide. Mortality of between 90 and 98% suggested possible resistance, and less than 90% mortality indicated resistance in the tested species [57]. Probit analysis was conducted using PoloPlus version 1 software to determine the 50% knockdown time (KDT50).
The allele frequencies of kdr L1014 mutations and Ace-1 G119 mutations were determined on Microsoft Excel. Genepop Hardy Weinberg exact tests were used to determine the differences between the kdr alleles in the irrigated and non-irrigated areas of both the dead and alive mosquitoes.
Pesticide use questionnaire data were entered and analyzed in Excel. Chi-square and t tests were used to determine the significance of the statistical difference between pesticide use in the irrigated and non-irrigated areas.
Results
An. gambiae s.l. species identification
A total of 1657 female mosquitoes were tested for susceptibility to deltamethrin, malathion, and DDT in 2018 and 2019 (Table 1). Nine hundred and fifty-nine (959) females were identified to species from irrigated and 698 from non-irrigated areas, and they were all An. arabiensis.
An. arabiensis insecticide bioassays
Mortality in the positive control Kisumu strain was 100% in all tests, while the mortality in the negative control (wild-caught mosquitoes which were not exposed to insecticide) ranged between 4.7 and 15.4% in all the tests. Phenotypic resistance to deltamethrin was observed in the non-irrigated areas in 2018, with possible phenotypic resistance in the irrigated areas (Table 1). Significantly higher mortality from deltamethrin exposure was recorded in non-irrigated than in irrigated areas in 2018 (Z test; z-stat = 5.4, p < 0.00001). However, in 2019, phenotypic resistance to deltamethrin was observed in both the irrigated and non-irrigated areas, and the mortality rates were comparable between the two areas (78% in irrigated and 83% in non-irrigated area) (Table 1). Susceptibility to malathion (100%) and DDT (98.98–100%) was recorded in both zones in the study site. All the mosquitoes tested on the PBO-deltamethrin combination were susceptible (100%) in irrigated and non-irrigated areas (Table 1). The KDT50 of all the chemicals tested was less than 30 min.
Frequency of kdr and Ace-1 alleles
A total of 317 mosquitoes [both alive (n = 38) and dead (n = 279)] were tested for the presence of mutations in the vgsc gene. Generally, both kdr-east and kdr-west mutations were observed in both the irrigated and the non-irrigated areas (Table 2). The mutation frequency was low in all tests, ranging from 1 to 16%. However, regardless of study area, no mutation was detected in the Ace-1 gene in 2018, and very low mutation frequency (0.7%) was found in the non-irrigated area in 2019. The kdr allele and genotype frequencies differed significantly between irrigated and non-irrigated zones (Pearson chi-square = 17.804, df = 2, P = 0.0001 and Pearson chi-square = 14.848, df = 4, P = 0.012, respectively). The kdr genotype results show significant deviation from Hardy–Weinberg equilibrium (HWE) in non-irrigated zones due to heterozygote deficit (P < 0.01), while marginally significant heterozygote deficit was observed in the irrigated zone (P = 0.0528). Overall, kdr genotype frequencies were not consistent with HWE (chi-square > 36.7, df = 4, P < 0.0001), indicating that the kdr allele has experienced strong selective pressure.
Public health, agricultural and veterinary chemical use
Among the 200 households surveyed (98 were in the irrigated area and 102 in the non-irrigated area), the proportion of households that used LLINs, IRS and other commercial insecticides was 91.8%, 84.4% and 51% in the irrigated area and 91.2%, 91.2% and 39.2% in the non-irrigated area, respectively (Table 3). There was a higher proportion of households in the non-irrigated area (84.3%) that used pesticides in agriculture and veterinary pest control compared to those in the irrigated area (80.6%). There was however no significant difference in the use of public health (vector control) and agricultural (crops)/veterinary (animals) chemicals between the two zones (t test; df = 6, t-stat = 0.1, p = 0.9).
There was no observable difference when the irrigated (75.5%) and the non-irrigated (83.3%) areas were compared in relation to the combined use of public health and agriculture/veterinary chemicals (t test; df = 10, t-stat = 0.2, p = 0.9). However, a significantly higher use of pyrethroids was detected in the irrigated than the non-irrigated area (Z test; Z-stat = 2.7, p = 0.007). Households that confirmed the use of chemicals but did not know the chemicals used (unknown classes) were also significantly higher in the non-irrigated area (Z test; Z-stat = −3.2, p = 0.001) (Table 4). No difference in the duration of chemical use for crop protection and livestock pest control (Table 5) was reported (Additional file 6).
Discussion
This study was carried out to determine the levels of susceptibility of An. arabiensis to pyrethroid, organophosphate, and organochloride insecticides in an area where public health vector control was undertaken using pyrethroids in insecticidal nets and organophosphates in IRS. The observations herein highlight the importance of multidisciplinary coordination between relevant ministries, including agriculture, public health and environment (IRM plan Kenya, 2020–2024; unpublished). Deltamethrin resistance was observed in both the irrigated and non-irrigated areas. Both kdr-east and kdr-west mutations were also observed with no ACE-1 mutations in both irrigated and non-irrigated areas. Additionally, both areas used chemical-based public health interventions for mosquito biting prevention. However, greater use of pyrethroid agricultural pesticides was observed in the irrigated than the non-irrigated area.
With the scaled-up mass distribution of bed nets, increased insecticide resistance in malaria vectors has been reported across sub-Saharan Africa against different classes of insecticides in use (http://www.irmapper.com). The results from this study show moderate phenotypic resistance of An. arabiensis against deltamethrin, a chemical compound used in LLINs (DawaPlus), in irrigated and non-irrigated areas. This is an indication that there is increasing resistance against deltamethrin in An. Arabiensis in the area. Similar studies indicating increased resistance against pyrethroids in An. Arabiensis have been reported in Kenya [65]. This might be due to the contribution from both public health interventions (LLINs) and agricultural activity. One plausible explanation for similar levels of insecticide resistance across areas experiencing different agrochemical exposure intensity may be the wide coverage of public health vector control activities in the region. Previous studies have observed that agricultural chemicals intensify insecticide resistance in areas where public health interventions already exist, due to increased selection pressure [18, 66]. In addition, it was observed that a majority of crop pesticides were used weekly, while the animal pesticides were applied either monthly or every 3 months. This study did not detect resistance against DDT, an indication that the An. arabiensis population in this area currently has no cross-resistance between pyrethroids and organochlorides. This is consistent with other studies conducted in Africa, where no cross-resistance was observed in An. gambiae s.l. [67] or An. funestus [68] species, in contrast to where DDT was previously used for IRS.
The current study reveals higher frequencies of kdr compared to previous studies [69]. With the development of reduced nervous sensitivity at the para-type sodium channel, reduced susceptibility to pyrethroid insecticides has been observed, resulting in knockdown resistance. Kdr has evolved separately in An. gambiae s.s. and An. arabiensis [70,71,72]. In this study, the kdr allele and genotype frequencies were higher in the non-irrigated than the irrigated areas. Lower frequencies of kdr-east than kdr-west were observed in the irrigated region in the study area. This could be a result of agricultural practices, as it has been observed that the mutation might have originally arisen due to the use of agricultural pesticides [73, 74]. The kdr genotypes in non-irrigated areas deviated significantly from Hardy–Weinberg equilibrium due to heterozygote deficit, while minimal heterozygote deficit was observed in the irrigated zone. This is an indication that the kdr allele has been under strong selective pressure in this area.
Ace-1 mutation was observed at a very low frequency in the non-irrigated area. This mutation has been associated with carbamate and organophosphate resistance [75]. A recent study from western Kenya observed this mutation at a low frequency in An. arabiensis [65]. Residuals from the pesticides used for agricultural purposes could not be dissociated from the observed resistance, as the organophosphates were found to be commonly used for animal pest control. The presence of this mutation in low frequencies in the non-irrigated area is a matter that needs to be further investigated, as an increase in this mutation may impact the gains achieved so far with the ongoing IRS program in the region.
Among the insecticides widely used for agricultural purposes in Homa Bay, pyrethroids appear to be the most common, followed by organophosphates. This reinforces our assumption that the use of these chemicals could be contributing to resistance in this region. When synergist was added to deltamethrin, a pyrethroid in the bioassay, susceptibility was restored, suggesting that the resistance mechanism to this compound involved metabolic pathways, notably monooxygenase enzymes. With the observed susceptibility of the malaria vectors in Homa Bay to PBO-deltamethrin, the introduction of PBO-impregnated nets in the study area will probably be effective in malaria vector control.
Conclusion
This study revealed increased phenotypic resistance in the Anopheles arabiensis from the irrigated area, and the intensive use of pesticides for crop protection in this region may have contributed to the selection pressure resulting in the resistance genes observed. The study findings show that there is a need for continued monitoring of insecticide resistance status, as insecticide resistance poses a major challenge to malaria vector control programs. Additionally, collaboration between the agriculture, public health and environment sectors will be key to insecticide use and resistance management. However, a key limitation of this study was the absence of a comprehensive analysis to ascertain the actual contribution of agricultural/veterinary pesticides to insecticide resistance in An. arabiensis in Homa Bay, western Kenya. Further studies are required to determine the insecticide concentration levels in breeding habitats. Such insecticides would likely originate from agricultural pest control activities.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files.
Abbreviations
- Ace :
-
Angiotensin-converting enzyme
- Ach:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- AS-PCR:
-
Allele-specific polymerase chain reaction
- DDT:
-
Dichlorodiphenyltrichloroethane
- DNA:
-
Deoxyribonucleic acid
- F:
-
Phenylalanine
- G:
-
Glycine
- ICEMR:
-
International Centers of Excellence for Malaria Research
- IRS:
-
Indoor residual spraying
- Kdr :
-
Knockdown resistance
- KOSPIF:
-
Kimira-Oluch Small-holder Farm Improvement Project
- L:
-
Leucine
- LLINs:
-
Long-lasting insecticide-treated nets
- ODK:
-
Open Data Kit
- PBO:
-
Piperonyl butoxide
- S:
-
Serine
- s.l.:
-
Sensu lato
- s.s.:
-
Sensu stricto
- vgsc :
-
Voltage-gated sodium channel
- WHO:
-
World Health Organization
References
Lebel J. Health: an ecosystem approach. Ottawa: International Development Research Centre (IDRC); 2003.
Schumann B, Kinsman J, Lindvall K. ClimRef project—resilient public health in the context of large-scale, drought-related migration in East Africa: knowledge status and knowledge needs, Kenya country report. 2018.
Paul P, Kangalawe RY, Mboera LE. Land-use patterns and their implication on malaria transmission in Kilosa District, Tanzania. Trop Dis Travel Med Vaccines. 2018;4(1):6.
Carlson JC, Byrd BD, Omlin FX. Field assessments in western Kenya link malaria vectors to environmentally disturbed habitats during the dry season. BMC Public Health. 2004;4:33.
Minakawa N, Sonye G, Mogi M, Githeko A, Yan G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002;39(6):833–41.
Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, Yan G. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. Am J Trop Med Hyg. 2005;73(1):157–65.
Munga S, Yakob L, Mushinzimana E, Zhou G, Ouna T, Minakawa N, Githeko A, Yan G. Land use and land cover changes and spatiotemporal dynamics of anopheline larval habitats during a four-year period in a highland community of Africa. Am J Trop Med Hyg. 2009;81(6):1079–84.
Afrane YA, Githeko AK, Yan G. The ecology of Anopheles mosquitoes under climate change: case studies from the effects of deforestation in East African highlands. Ann N Y Acad Sci. 2012;1249:204–10.
Thakurdas V. Increasing food production in sub-Saharan Africa through farmer-managed small-scale irrigation development. Ambio. 1994;23(8):524–6.
Fanadzo M. Revitalisation of smallholder irrigation schemes for poverty alleviation and household food security in South Africa: a review. Afr J Agric Res. 2012;7(13):1956–69.
Buchner R, Nair I, Pinto B. Unintended consequences of dams and water security: an insight into women’s vulnerability and the spread of malaria in Ethiopia. In: Water, climate change and the boomerang effect. London: Routledge; 2018. p. 173–97.
Deribew A, Dejene T, Kebede B, Tessema GA, Melaku YA, Misganaw A, Gebre T, Hailu A, Biadgilign S, Amberbir A. Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: analysis of the global burden of diseases 2015. Malar J. 2017;16(1):271.
Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. The influence of dams on malaria transmission in sub-Saharan Africa. EcoHealth. 2017;14(2):408–19.
Mwangangi JM, Shililu J, Muturi EJ, Muriu S, Jacob B, Kabiru EW, Mbogo CM, Githure J, Novak RJ. Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar J. 2010;9(1):228.
Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI, Novak RJ. Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme, Kenya. Malar J. 2008;7(1):43.
Mutero CM, Kabutha C, Kimani V, Kabuage L, Gitau G, Ssennyonga J, Githure J, Muthami L, Kaida A, Musyoka L. A transdisciplinary perspective on the links between malaria and agroecosystems in Kenya. Acta Trop. 2004;89(2):171–86.
Kamau L, Vulule JM. Status of insecticide susceptibility in Anopheles arabiensis from Mwea rice irrigation scheme, Central Kenya. Malar J. 2006;5:46.
Nkya TE, Akhouayri I, Kisinza W, David J-P. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43(4):407–16.
Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107.
Antonio-Nkondjio C, Fossog BT, Ndo C, Djantio BM, Togouet SZ, Awono-Ambene P, Costantini C, Wondji CS, Ranson H. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): influence of urban agriculture and pollution. Malar J. 2011;10(1):154.
Nkya TE, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, Kisinza W, David J-P. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasites Vectors. 2014;7(1):480.
Biamah EK. Coping with drought: options for soil and water management in semi-arid Kenya. Wageningen: Wageningen University and Research Centre; 2005.
Maundu P, Kibet S, Morimoto Y, Imbumi M, Adeka R. Impact of Prosopis juliflora on Kenya’s semi-arid and arid ecosystems and local livelihoods. Biodiversity. 2009;10(2–3):33–50.
Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Plasmodium falciparum sporozoite and entomological inoculation rates at the Ahero rice irrigation scheme and the Miwani sugar-belt in western Kenya. Ann Trop Med Parasitol. 1993;87(4):379–91.
Mbogo C. Insecticide resistance, host preference and Plasmodium falciparum parasite rates in Anopheles mosquitoes in Mwea and Ahero rice schemes. J Mosq Res. 2015. https://doi.org/10.5376/jmr.2015.05.0014.
Kamau L, Agai D, Matoke D, Wachira L, Gikandi G, Vulule JM. Status of insecticide susceptibility in Anopheles gambiae sensu lato and Anopheles funestus mosquitoes from western Kenya. J Insect Sci. 2008. https://doi.org/10.1673/031.008.1101.
Bayoh MN, Akhwale W, Ombok M, Sang D, Engoki SC, Koros D, Walker ED, Williams HA, Burke H, Armstrong GL. Malaria in Kakuma refugee camp, Turkana, Kenya: facilitation of Anopheles arabiensis vector populations by installed water distribution and catchment systems. Malar J. 2011;10(1):149.
Mala AO, Irungu LW, Shililu JI, Muturi EJ, Mbogo CM, Njagi JK, Mukabana WR, Githure JI. Plasmodium falciparum transmission and aridity: a Kenyan experience from the dry lands of Baringo and its implications for Anopheles arabiensis control. Malar J. 2011;10(1):121.
Ng’ang’a PN, Shililu J, Jayasinghe G, Kimani V, Kabutha C, Kabuage L, Kabiru E, Githure J, Mutero C. Malaria vector control practices in an irrigated rice agro-ecosystem in central Kenya and implications for malaria control. Malar J. 2008;7(1):146.
Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14(1):244.
Muturi EJ, Kim C-H, Baliraine FN, Musani S, Jacob B, Githure J, Novak RJ. Population genetic structure of Anopheles arabiensis (Diptera: Culicidae) in a rice growing area of central Kenya. J Med Entomol. 2014;47(2):144–51.
Muturi EJ, Mwangangi JM, Beier JC, Blackshear M, Wauna J, Sang R, Mukabana WR. Ecology and behavior of Anopheles arabiensis in relation to agricultural practices in central Kenya. J Am Mosq Control Assoc. 2013;29(3):222–30.
Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, Yan G. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16(1):443.
Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes: new insights into malaria vectors. Croatia: InTech; 2013. p. 671–704.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13(1):330.
Githeko A, Service M, Mbogo C, Atieli F, Juma F. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994;58(3–4):307–16.
Highton R, Bryan JH, Boreham P, Chandler J. Studies on the sibling species Anopheles gambiae Giles and Anopheles arabiensis Patton (Diptera: Culicidae) in the Kisumu area, Kenya. Bull Entomol Res. 1979;69(1):43–53.
Vontas J, Katsavou E, Mavridis K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: muddying the waters. Pestic Biochem Physiol. 2020;170:104666.
Prasad KM, Raghavendra K, Verma V, Velamuri PS, Pande V. Esterases are responsible for malathion resistance in Anopheles stephensi: a proof using biochemical and insecticide inhibition studies. J Vector Borne Dis. 2017;54(3):226.
Ranson H, Hemingway J. Mosquito glutathione transferases. Methods Enzymol. 2005;401:226–41.
Casida JE, Durkin KA. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu Rev Entomol. 2013;58:99–117.
Ndiath MO, Cailleau A, Orlandi-Pradines E, Bessell P, Pagès F, Trape J-F, Rogier C. Emerging knock-down resistance in Anopheles arabiensis populations of Dakar, Senegal: first evidence of a high prevalence of kdr-e mutation in West African urban area. Malar J. 2015;14(1):1–9.
Irving H, Wondji CS. Investigating knockdown resistance (kdr) mechanism against pyrethroids/DDT in the malaria vector Anopheles funestus across Africa. BMC Genet. 2017;18(1):1–11.
Badolo A, Bando H, Traoré A, Ko-Ketsu M, Guelbeogo WM, Kanuka H, Ranson H, Fukumoto S. Detection of G119S ace-1 R mutation in field-collected Anopheles gambiae mosquitoes using allele-specific loop-mediated isothermal amplification (AS-LAMP) method. Malar J. 2015;14(1):1–8.
Elanga-Ndille E, Nouage L, Ndo C, Binyang A, Assatse T, Nguiffo-Nguete D, Djonabaye D, Irving H, Tene-Fossog B, Wondji CS. The G119S acetylcholinesterase (Ace-1) target site mutation confers carbamate resistance in the major malaria vector Anopheles gambiae from Cameroon: a challenge for the coming IRS implementation. Genes. 2019;10(10):790.
Taylor-Wells J, Brooke BD, Bermudez I, Jones AK. The neonicotinoid imidacloprid, and the pyrethroid deltamethrin, are antagonists of the insect Rdl GABA receptor. J Neurochem. 2015;135(4):705–13.
Ochomo E, Subramaniam K, Kemei B, Rippon E, Bayoh NM, Kamau L, Atieli F, Vulule JM, Ouma C, Gimnig J, et al. Presence of the knockdown resistance mutation, Vgsc-1014F in Anopheles gambiae and An. arabiensis in western Kenya. Parasites Vectors. 2015;8:616.
Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, Malima R, Kafuko J, Mohamed M, Magesa S. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health. 2014;19(3):331–41.
Oxborough RM, Seyoum A, Yihdego Y, Dabire R, Gnanguenon V, Wat’Senga F, Agossa FR, Yohannes G, Coleman S, Samdi LM. Susceptibility testing of Anopheles malaria vectors with the neonicotinoid insecticide clothianidin; results from 16 African countries, in preparation for indoor residual spraying with new insecticide formulations. Malar J. 2019;18(1):264.
Oxborough RM. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J. 2016;15:146.
Tokponnon FT, Sissinto Y, Ogouyémi AH, Adéothy AA, Adechoubou A, Houansou T, Oke M, Kinde-Gazard D, Massougbodji A, Akogbeto MC. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: evidence from health facility data from Benin. Malar J. 2019;18(1):37.
Hakizimana E, Karema C, Munyakanage D, Iranzi G, Githure J, Tongren JE, Takken W, Binagwaho A, Koenraadt CJ. Susceptibility of Anopheles gambiae to insecticides used for malaria vector control in Rwanda. Malar J. 2016;15(1):582.
Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, Atieli HE, Zhou G, Githeko AK, Yan G. Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, western Kenya. Emerg Infect Dis. 2015;21(12):2178–81.
Wanjala CL, Kweka EJ. Malaria vectors insecticides resistance in different agroecosystems in western Kenya. Front Public Health. 2018;6:55.
Hien AS, Soma DD, Hema O, Bayili B, Namountougou M, Gnankine O, Baldet T, Diabate A, Dabire KR. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso, West Africa. PLoS ONE. 2017;12(3):e0173098.
Fighting malaria and saving lives. https://www.pmi.gov/home.
Organization WH. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2016.
Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). 1968.
Musapa M, Kumwenda T, Mkulama M, Chishimba S, Norris DE, Thuma PE, Mharakurwa S. A simple Chelex protocol for DNA extraction from Anopheles spp. JoVE. 2013;71:e3281.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49(4):520–9.
Paskewitz SM, Collins F. Use of the polymerase chain reaction to identify mosquito species of the Anopheles gambiae complex. Med Vet Entomol. 1990;4(4):367–73.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, Vontas J, Field LM. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
Mathias DK, Ochomo E, Atieli F, Ombok M, Bayoh MN, Olang G, Muhia D, Kamau L, Vulule JM, Hamel MJ. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malar J. 2011;10(1):10.
Djogbenou LS, Assogba B, Essandoh J, Constant EA, Makoutode M, Akogbeto M, Donnelly MJ, Weetman D. Estimation of allele-specific Ace-1 duplication in insecticide-resistant Anopheles mosquitoes from West Africa. Malar J. 2015;14:507.
Owuor KO, Machani MG, Mukabana WR, Munga SO, Yan G, Ochomo E, Afrane YA. Insecticide resistance status of indoor and outdoor resting malaria vectors in a highland and lowland site in western Kenya. PLoS ONE. 2021;16(3):e0240771.
Chouaibou MS, Fodjo BK, Fokou G, Allassane OF, Koudou BG, David JP, Antonio-Nkondjio C, Ranson H, Bonfoh B. Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Cote d’Ivoire. Malar J. 2016;15(1):426.
Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol. 2013;27(3):276–83.
Cuamba N, Morgan JC, Irving H, Steven A, Wondji CS. High level of pyrethroid resistance in an Anopheles funestus population of the Chokwe District in Mozambique. PLoS ONE. 2010;5(6):e11010.
Hemming-Schroeder E, Strahl S, Yang E, Nguyen A, Lo E, Zhong D, Atieli H, Githeko A, Yan G. Emerging pyrethroid resistance among Anopheles arabiensis in Kenya. Am J Trop Med Hyg. 2018;98(3):704–9.
Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt curve analysis. Malar J. 2006;5:16.
Himeidan YES, Dukeen M, El Rayah EA, Adam I. Anopheles arabiensis: abundance and insecticide resistance in an irrigated area of eastern Sudan. EMHJ. 2004;10(1–2):167–74.
Diabaté A, Brengues C, Baldet T, Dabire K, Hougard J-M, Akogbeto M, Kengne P, Simard F, Guillet P, Hemingway J. The spread of the Leu-Phe kdr mutation through Anopheles gambiae complex in Burkina Faso: genetic introgression and de novo phenomena. Trop Med Int Health. 2004;9(12):1267–73.
Akogbeto M, Djouaka R, Noukpo H. Use of agricultural insecticides in Benin. Bull Soc Pathol Exot. 2005;98(5):400–5.
Akogbéto MC, Djouaka RF, Kindé-Gazard DA. Screening of pesticide residues in soil and water samples from agricultural settings. Malar J. 2006;5(1):22.
Djogbénou L, Weill M, Hougard J-M, Raymond M, Akogbeto M, Chandre F. Characterization of insensitive acetylcholinesterase (ace-1 R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J Med Entomol. 2007;44(5):805–10.
Acknowledgements
The authors wish to thank the community and the leaders of Homa Bay County for allowing us to work in the area. We acknowledge the sub-Saharan Africa ICEMR Laboratory technical staff for providing technical support.
Funding
The work was supported by grants from the National Institutes of Health (U19 AI129326, D43 TW001505 and R01 AI050243).
Author information
Authors and Affiliations
Contributions
GY, JWK, and AKG conceived and designed the research. PWO, HA, BMO, KO, and CJO participated in the fieldwork and data collection. PWO and DZ participated in laboratory analysis. PWO, AKG, DZ, and GZ did the data analysis. MCL determined the study site demarcations. PWO and AKG drafted the manuscript, JG edited the draft manuscript. The final manuscript was edited by GY and AKG. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Maseno University Ethics Review Committee (MUERC Protocol No. 00456) and the University of California, Irvine Institutional Review Board (UCI IRB) and received authorization from the Ministry of Health, Kenya. Informed consent was sought from household heads before data were collected from the households.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Questionnaire for Agro-vet shops.
Additional file 2.
Questionnaire for Farmers (Crops).
Additional file 3.
Questionnaire for Farmers (Animals).
Additional file 4.
Questionnaire for Households.
Additional file 5.
Questionnaire for Veterinary Officers/ Agricultural Extension Officers.
Additional file 6.
Table S1. Proportion of use of different chemical classes in agriculture (farms) and veterinary (animals) in households in irrigated and non-irrigated areas.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Orondo, P.W., Nyanjom, S.G., Atieli, H. et al. Insecticide resistance status of Anopheles arabiensis in irrigated and non-irrigated areas in western Kenya. Parasites Vectors 14, 335 (2021). https://doi.org/10.1186/s13071-021-04833-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04833-z