Abstract
Background
Since their detection in 2013, Aedes aegypti has become a widespread urban pest in California. The availability of cryptic larval breeding sites in residential areas and resistance to insecticides pose significant challenges to control efforts. Resistance to pyrethroids is largely attributed to mutations in the voltage gated sodium channels (VGSC), the pyrethroid site of action. However, past studies have indicated that VGSC mutations may not be entirely predictive of the observed resistance phenotype.
Methods
To investigate the frequencies of VGSC mutations and the relationship with pyrethroid insecticide resistance in California, we sampled Ae. aegypti from four locations in the Central Valley, and the Greater Los Angeles area. Mosquitoes from each location were subjected to an individual pyrethrum bottle bioassay to determine knockdown times. A subset of assayed mosquitoes from each location was then analyzed to determine the composition of 5 single nucleotide polymorphism (SNP) loci within the VGSC gene.
Results
The distribution of knockdown times for each of the five Californian populations sampled was non-parametric with potentially bimodal distributions. One group succumbs to insecticidal effects around 35–45 min and the second group lasts up to and beyond the termination of the assay (120+ min). We detected 5 polymorphic VGSC SNPs within the sampled California populations. One is potentially new and alternatively spliced (I915K), and four are documented and associated with resistance: F1534C, V1016I, V410L and S723T. The Central Valley populations (Clovis, Dinuba, Sanger and Kingsburg) are fairly homogenous with only 5% of the mosquitoes showing heterozygosity at any given position. In the Greater LA mosquitoes, 55% had at least one susceptible allele at any of the five SNP loci. The known resistance allele F1534C was detected in almost all sampled mosquitoes (99.4%). We also observe significant heterogeneity in the knockdown phenotypes of individuals with the identical VGSC haplotypes suggesting the presence of additional undefined resistance mechanisms.
Conclusions
Resistance associated VGSC SNPs are prevalent, particularly in the Central Valley. Interestingly, among mosquitoes carrying all 4 resistance associated SNPs, we observe significant heterogeneity in bottle bioassay profiles suggesting that other mechanisms are important to the individual resistance of Ae. aegypti in California. Keywords: Aedes aegypti, Resistance, Pyrethroid, IPLEX genotyping, Voltage gated sodium channel, California.
Graphical Abstract

Similar content being viewed by others
Background
The yellow fever mosquito, Aedes aegypti (Linnaeus 1762), is a major vector of arboviruses such as dengue, Zika, chikungunya, and yellow fever viruses. This medically important vector was detected in California in 2013 in response to a residential service request [1]. It has now been detected in 17 counties throughout the state despite aggressive surveillance and treatment efforts, presenting a significant challenge to local control agencies [1,2,3].
Questions remain about the timing of Aedes aegypti’s arrival to California, as well as their origins [4, 5]. Multiple lines of evidence point to multiple potential introductions into the state. Populations in Southern California are thought to have arrived from Mexico, while populations in the Central Valley may have been introduced, in part, from the Southeastern United States. Upon detection in 2013, the Consolidated Mosquito Abatement District (CMAD) implemented an integrated vector control management strategy which involved extensive public education, thorough property inspections, sanitation, insecticide treatment at larval sources and residual barrier spraying with pyrethroids. Despite these efforts, Ae. aegypti successfully overwintered and continued to spread. The peridomestic habits of this mosquito make its reduction and eradication difficult. It is likely that the invasive populations of Ae. aegypti arrived in California with genetic mutations conferring resistance to the type I pyrethroid insecticides applied for vector control in California [6, 7].
Pyrethroid based compounds are used as adulticides and are favored for their efficacy and low mammalian toxicity [8]. Pyrethroids act on insect voltage gated sodium channels (VGSC) by binding to open channels and blocking the channel in the open conformation. This results in prolonged depolarization of the membrane and failure of neuronal function [9]. This class of insecticides is partitioned into two types (I and II) based on the presence or absence of a cyano moiety at the alpha carbon [10]. Point mutations within the channel domain of the protein can confer type-specific resistance by causing structural changes that reduce or eliminate the insecticides ability to bind the channel [11]. These mutations change the amino acid composition of the protein at specific locations that result in changes in charge and steric hindrance of the ion channel. These changes allow the VGSC to maintain normal function in the presence of pyrethroids.
Phenotypic analysis by CDC bottle bio-assay revealed that California Ae. aegypti were resistant to type I pyrethroids. This prompted researchers to determine the genomic sequence of the VGSC gene from representatives of populations from the Central Valley of California [6]. This sequencing revealed mutations known to be prevalent in Ae. aegypti from the Americas. Public health agencies began to screen for known pyrethroid resistance associated single nucleotide polymorphisms (SNPs) in the VGSC gene: F1534C, V1016I, and V410L [7]. These mutations are annotated based on their orthologous position in the Musca domestica VGSC protein (Genbank accession number: ANW06229) [12, 13]. V410L is located in the sixth transmembrane region of the first domain, V1016I is located in the sixth transmembrane region of the second domain, and F1534C is located in the sixth transmembrane region of the third domain (Fig. 1) [11, 14]. Recently, another mutation (S723T) was linked to Deltamethrin resistance, though its impact on the resistance phenotype remains unknown [15]. This SNP is localized in the intracellular region of the second transmembrane repeat domain. The F1534C mutation confers a low level of resistance on its own to type I pyrethroids. The V410L SNP, first described in 2017 [14], confers resistance to both type I and type II pyrethroids. The V1016I mutation on its own does not confer resistance, however in conjunction with F1534C it provides elevated insensitivity to type I and type II pyrethroids [9, 16, 17]. Mosquitoes homozygous for V1016I, F1534C, and V410L mutations exhibit a high level of resistance to both type I and type II pyrethroids [18]. As each SNP provides differing levels of protection against different classes of pyrethroids, testing for multiple SNPs in the field is relevant to screening for pyrethroid resistance [14].
Topology of the mosquito sodium channel. The Ae. aegypti reference sequence was translated in CLC Main Workbench, Version 7. The resulting amino acid sequence was aligned to the Musca domestica (ANW06229) and Drosophila melanogaster (AAB59195) reference protein sequences. SNP annotations were transferred to Musca to determine the Musca protein position, and structural annotations were transferred from Drosophila to Aedes. The topology of the sodium channel was illustrated using Protter version 1 [49]. The sodium channel protein contains four homologous repeat domains (I–IV). Each repeat domain has six α-helical transmembrane segments (1–6, 7–12, 13–18, 19–24). Filled circles represent the five SNPs assayed in this study. Ref. = Reference. Alt. = alternate. aa = amino acid
Detecting and quantifying insecticide resistance in mosquitoes provides mosquito abatement groups with the tools to tailor their control strategies. The two primary methods of measuring resistance in the field are PCR testing for specific resistance associated alleles, and observing phenotypic resistance through the CDC Bottle Bioassay [19]. The CDC Bottle bioassay involves placing 10-25 mosquitoes in an insecticide coated Wheaton bottle followed by observation of the time and proportion of knockdown for 2 h after exposure. This procedure allows districts to investigate and quantify population level resistance. However, a direct analysis of the relationship between VGSC genotypes of individual mosquitoes and their associated bottle bioassay phenotypes (knockdown time) has not been completed with California Ae. aegypti populations. To measure the relationship between genotype and phenotype, individual mosquitoes were subjected to phenotypic assays followed by VGSC SNP genotyping of individuals at the upper and lower ends of the phenotypic spectrum. The Ae. aegypti samples were derived from multiple locations in California to explore population-level differences in response to insecticide exposure and VGSC genotype. We hypothesized that individuals carrying more resistance associated alleles will exhibit longer knockdown times when exposed to a modified bottle bioassay designed for individual mosquitoes.
Materials and methods
Mosquito collection and colony maintenance
Adult mosquitoes were collected from four towns in 2018 in the Central Valley of California: Dinuba, Clovis, Sanger and Kingsburg (Fig. 2) as well as from the Greater LA area. These sites were chosen because they have high prevalence of Ae. aegypti, and the inclusion of mosquitoes from both Southern California and the Central Valley encompass two genetically distinct groups. Eggs from these adults were collected, allowed to develop for at least 5 days, then flooded in trays of 1 l of water and reared according to standard protocols [20]. Mosquitoes were reared on a diet of ground rodent chow at 27 °C under 14:10 h (light:dark) photoperiod and adults were held at 70% relative humidity. Adults 1–3 days post-eclosion were then collected and individually exposed to a bottle bioassay to record individual knockdown time. Adult mosquitoes were fed on 10% sucrose solution ad libitum and did not receive a blood meal prior to insecticide exposure.
Locations from which Ae. aegypti were analyzed. Cities where founders for each lab strain were collected. Individuals were collected from the 5 cities we labeled on the map. Each lab strain was reared from individuals collected at various sites in these cities and reared together to increase specimen numbers.
Individual adult bottle bioassay
To determine time to knockdown for individual mosquitoes, a modified bottle bioassay was developed based on the CDC protocol [19]. 250 ml Wheaton bottles (Fisher #06-404B) were coated with technical grade pyrethrum purchased from Chem Service (West Chester, PA). Pyrethrum was chosen because it is the natural derivative of the pyrethroid class of insecticides and is used in California areas as adulticide. The pyrethrum (Lot #7581300) was diluted in acetone to a concentration of 15.6 μg/ml, the diagnostic dose recommended by the CDC for Aedes mosquitoes and bottles were coated with the insecticide following the procedure described in [21]. Individual female mosquitoes were then aspirated into each bottle and observed for knockdown for up to 2 h. Individuals were determined as knocked down when the bottle was rotated, and the mosquito could not reorient itself upright. Bottles were monitored continuously and exact time to knockdown was recorded. A susceptible lab colony, Rockefeller, was assayed as a reference for knockdown behavior in the bottle assay. For each population 80–95 adult female mosquitoes were assayed. Following knockdown, individuals were placed in the Lysis Buffer provided with the Zymo Quick DNA/RNA Miniprep kit (Cat #: D7001) and homogenized using Axygen mortar and pestles (Product PES-15-B-S1). Samples were stored at 4 °C.
DNA extraction
DNA was extracted using the Zymo Quick-DNA/RNA Miniprep kit (Product D7001) using the suggested protocol for Solid Tissue Samples. DNA concentration for each sample was tested with a Qubit instrument (Life Technologies). Approximately 4 ng/µl in a total volume of 30 µl was extracted from each whole individual.
SNP genotyping
A portion of bottle-assayed mosquitoes were sent for genotype analysis. Samples for genetic analysis were selected primarily from the upper and lower tertiles of knockdown times for each population (Table 1, Fig. 3). Our SNPs were identified from published whole genome sequences of Ae. aegypti. Sequences from the VGSC genomic locus of California mosquitoes were generated using primers reported in [22] (Genbank ID: KU728155-6). These sequences were aligned to the VGSC locus [AAEL023266 (3: 315,926,360–316,405,639)] in the Ae. aegypti AaegL5 reference genome. SNPs were identified within the coding regions of VGSC by comparison of aligned sequences against the reference sequence in VCF files using the Integrative Genomics Viewer [23]. Analysis of the data revealed 5 non-synonymous SNPs within the coding sequence. One additional mutation, previously reported from other studies [24], was included in case a limited sample size of genome data missed low frequency variations within the Californian derived sequences. The final 5 SNPs screened included 3:315939224 (F1534C), 3:315983763 (V1016I), 3:315999297 (T915K), 3:316014588 (S723T), and 3:316080722 (V410L). DNA was sent to the UC Davis Veterinary Genetics Laboratory for iPLEX assay using the MassARRAY System (Agena Biosciences, San Diego, CA) [25]. Detailed information on the 5 SNPs are provided in Additional file 1: Table S1.
Distribution of knockdown times for each population. Each sample is represented by a circle. Black filled circles indicate genotyped samples, while the white circles represented samples that were not genotyped. Differences between group medians were determined by using the Mood’s median test followed by fdr correction. Letters indicate statistically significant differences.
Data analysis
Statistical analysis was performed in R Version 3.5.2 [26]. Survival analysis was performed using the survival package [27]. The data was right-censored at 120 min, the end of the assay period. Pairwise comparisons of survival curves were performed through a log-rank analysis. Knockdown distribution normality was tested using the Shapiro–Wilk test in Base R. The box plot and histograms were created with ggplot2 [28]. P-value thresholds were adjusted for multiple comparison correction using the Benjamini and Hotchberg method [29] (Additional file 2: Table S2).
Results
Individual adult bottle bioassay
The knockdown times of all assayed mosquitoes are displayed in Fig. 3 and Additional file 3: Figure S1. Mosquitoes that did not knockdown during the assay period were coded as such and considered using the Kaplan–Meier analysis which accounts for right-censored data. Aside from the susceptible laboratory strain, Rockefeller, the Sanger and Kingsburg populations had the lowest median knockdown times and are significantly lower than that of Clovis or Greater LA (Fig. 3, Log-rank test < 0.05). The distribution of knockdown times for each of the five Californian populations sampled was non-parametric (Fig. 3, Additional file 3: Figure S1) and appear to potentially have bimodal distributions, though an assay with a greater dose of insecticide would be needed to confirm the bimodal nature of the distribution. The distribution reveals the presence of two subsets of mosquitoes in each population with one group succumbing to insecticidal effects around 35–45 min and the second group lasting up to and beyond the termination of the assay. However, the relative proportions of these distributions differ between populations (Additional file 3: Figure S1).
SNP genotype by population
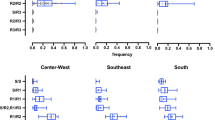
Samples for genetic analysis were selected primarily from the upper and lower tertiles of knockdown times for each population. We detected 5 SNPs within the sampled California populations (Table 1). All, except I915K, were reported previously [13, 14, 31]. The I915K SNP is in a region which is alternatively spliced and its presence in the resulting transcripts has not been determined conclusively. The assayed mosquitoes from the Central Valley populations (Clovis, Dinuba, Sanger and Kingsburg) are fairly homogenous with only 5% (12/242) of the mosquitoes tested showing heterozygosity at any of the 5 positions assayed (Fig. 4a). Alternatively, analysis of the Greater LA mosquitoes revealed that 71% had at least one susceptible allele at any of the five assayed SNPs (Table 1, 48/67). The F1534C mutation is nearly fixed across the genotyped subset of mosquitoes from all sampled populations. Only two mosquitoes were heterozygous (R/S) at position 1534. One individual from Clovis was heterozygotic at the amino acid positions F1534C, V1016I, V410L and S723T and one from LA was heterozygous at F1534C, and homozygous susceptible at V1016, V410 and S723 (Fig. 4a, Table 1). The I915K SNP was only found in a heterozygous conformation in the genotyped samples from the Dinuba population [5% (3/60)]. These mosquitoes were also heterozygous at the V1016I, V410L and S723T loci. In the Greater LA population 22% (15/67) were heterozygous at I915K. Of these 47% (7/15) were also homozygous susceptible at V1016, V410 and S723. The other 53% (8/15) were heterozygous at these positions (Fig. 4). The sites V1016I, V410L and S723T, are observed together frequently; an individual heterozygous or homozygous at one site was correspondingly heterozygous or homozygous across all three with exceptions in the Clovis population. In the Clovis population 13% (8/60) mosquitoes were heterozygous only at sites S723T and V410L (Fig. 4a).
Median knockdown time for each present genotype. a Frequency of the 8 observed genotypes by population and their respective median knockdown times. b Kaplan-Meier analysis of observed knockdown between the 8 genotypes within all populations. C. P-values for log-rank comparisons between each genotype.
VGSC SNP genotype and knockdown time
The majority of the genotyped individuals were homozygous for the resistance associated alleles at all five loci tested. However, 0.8% (2/246) individuals with this genotype, both from Sanger, demonstrated knock down in as little as 6 min after exposure to pyrethrum, overlapping with the susceptible Rock strain. Of the individuals with all 5 alternate resistance associated alleles 9% (23/246) knocked down in 20 min or less. A higher level of diversity of VGSC genotypes (6) was observed in the Greater LA mosquitoes relative to the Central Valley populations (4) (Fig. 4a). This is consistent with previous reports showing little to no polymorphism in VGSC SNPs in central California populations [5, 7]. The increased genetic variation observed in the Greater LA population relative to the Central Valley population is also consistent with a previous study monitoring both Central Valley and southern California locations [7]. It is noteworthy that independent studies utilizing whole genome sequence [5], SNP chip [4], and microsatellite [4] indicated different genetic makeup separating Southern CA and Central CA. Of the mosquitoes tested from populations in the Central Valley, only 5% (3/60) and 15% (9/60) samples from Dinuba and Clovis respectively carried “susceptible” alleles (Fig. 4a). Of note, <1% (2/309) of the mosquitoes tested from all populations were heterozygous at position F1534C, and those mosquitoes knocked down at 5 (Greater LA) and 18 (Clovis) minutes, though it is difficult to ascertain the significance of this result with such a low frequency of this genotype.
Survival analysis
To compare the relationship between genotype and phenotype, Kaplan-Meier survival analysis was utilized to account for individuals that may not have experienced the “event” (in this case, knockdown) during the study period [30]. Survival curve analysis of mosquitoes with equivalent genotypes reveals a range of median knockdown times that roughly correlate with the relative proportions of susceptible versus resistant VGSC alleles. In cases where only a single individual represented a genotype, P-values were not reported (Fig. 4c). In general, individuals containing susceptible VGSC alleles did not last as long as those carrying the fully resistant genotype. However, a small proportion of individuals carrying the homozygous resistant genotype at all alleles have knockdown times equivalent to individuals with homozygous susceptible genotypes (Fig. 4a, b). We reason this is unlikely due to methodological error, as these low knockdown times were observed across multiple populations. When controlling for genotype, there were still significant differences between the strains (Fig. 5). All the Sanger and Kingsburg mosquitoes were homozygous at all 5 resistance loci and had median knockdown times of 38 min (N = 58) and 42 min (N = 64) respectively (Fig. 5). This observation contrasts with median knockdown times observed in mosquitoes from the Clovis (106 min) and Greater LA (101 min) populations with the identical VGSC genotype. Statistical comparison of the survival curves of these groups demonstrates significant differences in knockdown times between populations even though the individuals tested carry identical VGSC resistance loci.
Discussion
Using the individual bottle bioassay in this study allowed us to explore the phenotypic variability of resistance in Ae. aegypti, and pair that phenotypic information with genotype, a previously unexplored relationship in Ae. aegypti. This individual bottle bioassay method yielded a non-normal, potentially bimodal distribution of knockdown times. In general, individuals with susceptible alleles succumbed to knockdown earlier, however, there was some overlap in the knockdown times of individuals with fully resistant genotypes and those with susceptible alleles.
Target site mutations in the VGSC gene were prevalent in our sample populations and play an important role in resistance. Yet, significant variability in average knockdown times was observed between populations when controlling for genotype. This observation suggests the presence of other mechanisms of resistance that vary in frequency between populations. Another documented mechanism of pyrethroid resistance in Ae. aegypti is metabolic detoxification by enzymes such as Cytochrome P450s and GSTs [31,32,33].
The observed variability between populations indicates that other factors are at play in mediating pyrethroid resistance in these mosquitoes and that these factors are not universally distributed within and between populations. The establishment of Ae. aegypti in California has raised concerns that they could facilitate local transmission of arboviruses such as dengue and Zika as seen in Florida, Texas and Puerto Rico [34,35,36]. While pyrethroids are favored as a chemical method for their control, resistance is widespread in California and globally [6, 7, 9, 37]. Despite significant work on pyrethroid resistance in Ae. aegypti, questions remain concerning the identification and relative compounding roles that VGSC mutations, metabolic detoxification mechanisms, other resistance mechanisms, and environmental factors have on conferring insecticide resistance. Additional resistance mechanisms such as reduced cuticular permeability and behavioral resistance have been shown to be significant in insects [38, 39].
The VGSC mutations V1016I, F1534C and V410L are prevalent in Ae. aegypti in the Americas and have been closely monitored by the California Department of Public Health (CDPH) [7, 14, 16, 18]. These surveillance efforts found that between 2015 and 2017 resistant alleles were largely fixed in the Central Valley population, and less abundant (> 80% for F1534C and > 61% for V1016I from 2015–2017) but increasing in prevalence in the Southern population of Ae. aegypti [7]. Studies in Mexico have identified similar trends [18]. Our results were similar to the CDPH findings (Table 1). In our samples F1534C was nearly fixed, with only two heterozygous individuals (one from Clovis and one from Greater LA). The detection of a heterozygote from Clovis was surprising, given our relatively small sample size compared to the 1200 mosquitoes tested by Liebmen et al. between 2015 and 2017. Our sample mosquitoes were captured in late 2018, and follow the general trend found in [7]. In California, Ae. aegypti appear to have dispersed along the major Interstate 5 route so it is possible that mosquitoes from the Southern Population and Central Valley population are moved between regions [3]. Recent whole genome sequencing data as well as SNP chip data support multiple introductions of Ae. aegypti into CA and found evidence of distinct genetic clusters converging in the Central Valley [4, 5].
The majority of the tested individuals from our Central Valley samples carried all 5 VGSC mutations (Figs. 4, 5). Resistance alleles were likely prevalent in the founder populations in California [6]. Resistance is widespread in populations in the United States, though the susceptible F1534 allele is still found in some areas [40, 41]. Interestingly, bottle bioassay results reported by local control agencies had not always followed the patterns that would be expected given the relative proportions of resistance associated SNPs, which was also in line with our results using the individual bottle bioassay test (Figs. 3, 4, 5). It will be an important next step to test the association between the SNPs identified in this study and Deltamethrin, a type II pyrethroid, as this ingredient is used more widely throughout the state.
The Xenopus oocyte expression system for Ae. aegypti sodium channels has facilitated investigation into the role of individual mutations assayed in pyrethroid resistance [14, 42,43,44]. The mutations V410L + F1534C and V1016I + F1534C have even been studied in combination [14, 17, 44]. However, little is known about the S723T mutation [45] though with the high frequency of this mutation in all populations tested (59% in Greater LA and > 92% in all Central Valley populations, Table 1), future functional analyses would be important to understanding the role this mutation plays in resistance. These mutations act in combination with metabolic mechanisms of pyrethroid resistance mediated by the upregulation, overexpression or duplication of cytochrome P450 enzymes encoding genes [15, 46,47,48]. Bottle bioassays with cytochrome P450 inhibitor, piperonyl butoxide (PBO), have implicated that cytochrome P450s play a synergistic role in combination with VGSC mutations to confer resistance in mosquitoes from Clovis [6]. The significant differences in knockdown time found in our samples with the resistant genotypes (Fig. 5) supports the hypothesis that other mechanisms are important and variable within California populations and these other mechanisms should be explored in future studies. This indicates that running bottle bioassays that include the cytochrome P450 inhibitor PBO will provide local control agencies important information when evaluating resistance. Given the functional evidence in the literature, and our assay results (Figs. 4, 5), the increasing prevalence of the F1534C mutation, particularly in combination with V410L and V1016I reliably indicates an elevated level of resistance to type I pyrethroids [7, 14, 42, 44]. However, the individual bottle assays employed here prompt us to question that, assuming good conditions, the presence of resistance associated alleles in the VGSC gene may not guarantee phenotypic resistance. With this in mind, it is important to carefully explore the role of specific alleles on resistance to further inform control efforts. For this reason, it is important to pursue detailed investigations in the biology underlying the resistance phenotype. Additional knowledge on this subject would facilitate identification of additional genetic and/or biochemical markers. The ideal goal would be to identify markers that provide quantitative measures of the resistance phenotype in field caught mosquitoes which would boost the predictive power of these assays. In addition, identification of new targets underlying the phenotype opens the door for development of alternative strategies and new synergists to augment existing insecticidal compounds.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Metzger ME, Yoshimizu MH, Padgett KA, Hu R, Kramer VL. Review detection and establishment of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes in California, 2011–2015. J Med Entomol. 2017;54:533–43.
Gloria-Soria A, Brown JE, Kramer V, Yoshimizu MH, Powell JR. Origin of the dengue fever mosquito, Aedes aegypti, in California. PLoSNegl Trop Dis. 2014;8:e3029.
California Department of Public Health. Aedes aegypti and Aedes albopictus Mosquitoes in California Detection Sites by County/City.
Pless E, Gloria-Soria A, Evans BR, Kramer V, Bolling BG, Tabachnick WJ, et al. Multiple introductions of the dengue vector, Aedes aegypti, into California. PLoSNegl Trop Dis. 2017;11:1–17.
Lee Y, Schmidt H, Collier TC, Conner WR, Hanemaaijer MJ, Slatkin M, et al. Genome-wide divergence among invasive populations of Aedes aegypti in California. BMC Genomics. 2019;20:1–10.
Cornel AJ, Holeman J, Nieman CC, Lee Y, Smith C, Amorino M, et al. Surveillance , insecticide resistance and control of an invasive Aedes aegypti (Diptera: Culicidae) population in California; 2016. p. 1–16.
Liebman KA, Billeter SA, Yoshimizu MH, Yang F, Metzger ME, Schildhauer S, et al. Identification of molecular determinants of resistance to pyrethroid insecticides in Aedes aegypti (Diptera: Culicidae) populations in California, USA. J Med Entomol. 2019;56:1353–8.
Housset P, Dickmann R. A promise fulfilled—pyrethroid development and the benefits for agriculture and human health. Bayer Crop Sci J. 2009;62:135–44.
Chen M, Du Y, Nomura Y, Zhorov BS, Dong K. Chronology of sodium channel mutations associated with pyrethroid resistance in Aedes aegypti. Arch Insect Biochem Physiol. 2020;104:e21686.
Vijverberg HPM, vandenBercken J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol. 1990;21:105–26.
Du Y, Nomura Y, Zhorov BS, Dong K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects. 2016;7:60.
Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol Gen Genet. 1996;252:51–60.
Sun H, Tong KP, Kasai S, Scott JG. Overcoming super-knock down resistance (super-kdr) mediated resistance: multi-halogenated benzyl pyrethroids are more toxic to super-kdr than kdr house flies. Insect Mol Biol. 2016;25:126–37.
Haddi K, Tomé HVV, Du Y, Valbon WR, Nomura Y, Martins GF, et al. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci Rep. 2017;7:1–9.
Saavedra-Rodriguez K, Campbell CL, Lenhart A, Penilla P, Lozano-Fuentes S, Black WC. Exome-wide association of deltamethrin resistance in Aedes aegypti from Mexico. Insect Mol Biol. 2019;28:591–604.
Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black WC IV. Coevolution of the Ile 1,016 and Cys1,534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoSNegl Trop Dis. 2015;9:e0004263.
Chen M, Du Y, Wu S, Nomura Y, Zhu G, Zhorov BS, et al. Molecular evidence of sequential evolution of DDT- and pyrethroid-resistant sodium channel in Aedes aegypti. PLoSNegl Trop Dis. 2019;13:e0007432.
Saavedra-Rodriguez K, Maloof FV, Campbell CL, Garcia-Rejon J, Lenhart A, Penilla P, et al. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Sci Rep. 2018;8:1–9.
Brogdon WG, Chan A. Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. CDC Methods. 2012. p. 1–28.
Clemons A, Mori A, Haugen M, Severson DW, Duman-Scheel M. Culturing and egg collection of Aedes aegypti. Cold Spring HarbProtoc. 2010;5:1–6.
Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc. 1998;14:159–64.
Martins AJ, de Lins RMMA, Linss JGB, Peixoto AA, Valle D. Voltage-gated sodium channel polymorphism and metabolic resistance in pyrethroid-resistant Aedes aegypti from Brazil. Am J Trop Med Hyg. 2009;81:108–15.
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6.
Kawada H, Higa Y, Komagata O, Kasai S, Tomita T, Thi Yen N, et al. Widespread distribution of a newly found point mutation in voltage-gated sodium channel in pyrethroid-resistant Aedes aegypti populations in Vietnam. PLoSNegl Trop Dis. 2009;3:e527.
Lee Y, Weakley AM, Nieman CC, Malvick J, Lanzaro GC. A multi-detection assay for malaria transmitting mosquitoes. J Vis Exp. 2015;96:e52385.
R Development Core Team R et al. R: a language and environment for statistical computing; 2020.
Therneau TM, Lumley T. Package ‘survival.’ R Top Doc. 2014;2:3.
Villanueva RAM, Chen ZJ. ggplot2: elegant graphics for data analysis (2nd ed.). Measurement. 2019;17:160–7.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Faucon F, Gaude T, Dusfour I, Navratil V, Corbel V, Juntarajumnong W, et al. In the hunt for genomic markers of metabolic resistance to pyrethroids in the mosquito Aedes aegypti: an integrated next-generation sequencing approach. PLoSNegl Trop Dis. 2017;11:1–20.
Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoSNegl Trop Dis. 2017;11:e0005625.
Yang F, Schildhauer S, Billeter SA, HardstoneYoshimizu M, Payne R, Pakingan MJ, et al. Insecticide resistance status of Aedes aegypti (Diptera: Culicidae) in California by biochemical assays. J Med Entomol. 2020;57:1176–83.
Grubaugh ND, Ladner JT, Kraemer MUG, Dudas G, Tan AL, Gangavarapu K, et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546:401–5.
Teets FD, Ramgopal MN, Sweeney KD, Graham AS, Michael SF, Isern S. Origin of the dengue virus outbreak in Martin County, Florida, USA 2013. Virol Rep. 2014;1–2:2–8.
Murray KO, Rodriguez LF, Herrington E, Kharat V, Vasilakis N, Walker C, et al. Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector Borne Zoonotic Dis. 2013;13:835–45.
Smith LB, Kasai S, Scott JG. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. YPEST. 2016;133:1–12.
Zalucki MP, Furlong MJ. Behavior as a mechanism of insecticide resistance: evaluation of the evidence. CurrOpin Insect Sci. 2017;21:19–25.
Balabanidou V, Grigoraki L, Vontas J. Insect cuticle: a critical determinant of insecticide resistance. CurrOpin Insect Sci. 2018;27:68–74.
Kandel Y, Vulcan J, Rodriguez SD, Moore E, Chung H-N, Mitra S, et al. Widespread insecticide resistance in Aedes aegypti L. from New Mexico, U.S.A. PLoS ONE. 2019;14:e0212693.
Mundis SJ, Estep AS, Waits CM, Ryan SJ. Spatial variation in the frequency of knockdown resistance genotypes in Florida Aedes aegypti populations. Parasit Vectors. 2020;13:241.
Hu Z, Du Y, Nomura Y, Dong K. A sodium channel mutation identified in Aedes aegypti selectively reduces cockroach sodium channel sensitivity to type I, but not type II pyrethroids. Insect Biochem Mol Biol. 2011;41:9–13.
Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, et al. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci. 2013;110:11785–90.
Fan Y, Scott JG. TheF1534C voltage-sensitive sodium channel mutation confers 7- to 16-fold resistance to pyrethroid insecticides in Aedes aegypti. Pest Manag Sci. 2020;76:2251–9.
Itokawa K, Sekizuka T, Maekawa Y, Yatsu K, Komagata O, Sugiura M, et al. High-throughput genotyping of a full voltage gated sodium channel gene via genomic DNA using target capture sequencing and analytical pipeline MoNaS to discover novel insecticide resistance mutations. PLoSNegl Trop Dis. 2019;13:1–17.
Bariami V, Jones CM, Poupardin R, Vontas J, Ranson H. Gene amplification, abc transporters and cytochrome p450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoSNegl Trop Dis. 2012;6:e1692.
Smith LB, Tyagi R, Kasai S, Scott JG. CYP-mediated permethrin resistance in Aedes aegypti and evidence for trans-regulation. PLoSNegl Trop Dis. 2018;12:e0006933.
Smith LB, Sears C, Sun H, Mertz RW, Kasai S, Scott JG. CYP-mediated resistance and cross-resistance to pyrethroids and organophosphates in Aedes aegypti in the presence and absence of kdr. Pestic Biochem Physiol. 2019;160:119–26.
Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–6.
Acknowledgements
We would like to thank the California Department of Public Health, Vector-borne Disease Section for their consultation. This work was supported, in part, by the USDA National Institute of Food and Agriculture (NIFA) grant numbers (Hatch projects #1025565 + #1017520).
Funding
AC acknowledges funding support from the Pacific Southwest Regional Center of Excellence for Vector-Borne Diseases funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000516) pacvec.us. The funding body played no role in the design of this experiment.
GA and ETK acknowledge funding support from the Pacific Southwest Regional Center of Excellence for Vector-Borne Diseases funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000516).
Author information
Authors and Affiliations
Contributions
ETK, LKM, KB, AJC, YL, and GMA conceived and designed the study. ETK, LKM, KB, KVS, AZ, and TVS collected data. ETK, LKM, and GMA analyzed the data. ETK and LKM prepared the manuscript. ETK, LKM, AJC, YL, and GMA revised and edited the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
iPLEX MassARRAY primers for 5 SNPs used in this study.
Additional file 2: Table S2.
Population name and generation for bottle assay testing.
Additional file 3: Figure S1.
Histogram of knockdown times for assayed mosquitoes with kernel density plot. Knockdown time distribution was non-normal (Shapiro-Wilk test, p <0.00005 for each population).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mack, L.K., Kelly, E.T., Lee, Y. et al. Frequency of sodium channel genotypes and association with pyrethrum knockdown time in populations of Californian Aedes aegypti. Parasites Vectors 14, 141 (2021). https://doi.org/10.1186/s13071-021-04627-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04627-3