Abstract
Background
The worldwide genus Anopheles Meigen, 1918 is the only genus containing species evolved as vectors of human and simian malaria. Morbidity and mortality caused by Plasmodium Marchiafava & Celli, 1885 is tremendous, which has made these parasites and their vectors the objects of intense research aimed at mosquito identification, malaria control and elimination. DNA tools make the identification of Anopheles species both easier and more difficult. Easier in that putative species can nearly always be separated based on DNA data; more difficult in that attaching a scientific name to a species is often problematic because morphological characters are often difficult to interpret or even see; and DNA technology might not be available and affordable. Added to this are the many species that are either not yet recognized or are similar to, or identical with, named species. The first step in solving Anopheles identification problem is to attach a morphology-based formal or informal name to a specimen. These names are hypotheses to be tested with further morphological observations and/or DNA evidence. The overarching objective is to be able to communicate about a given species under study. In South America, morphological identification which is the first step in the above process is often difficult because of lack of taxonomic expertise and/or inadequate identification keys, written for local fauna, containing the most consequential species, or obviously, do not include species described subsequent to key publication.
Methods
Holotypes and paratypes and other specimens deposited in the Coleção Entomológica de Referência, Faculdade de Saúde Pública (FSP-USP), Museo de Entomología, Universidad del Valle (MUSENUV) and the US National Mosquito Collection, Smithsonian Institution (USNMC) were examined and employed to illustrate the identification keys for female, male and fourth-instar larvae of Anopheles.
Results
We presented, in four concurrent parts, introduction and three keys to aid the identification of South American Anopheles based on the morphology of the larvae, male genitalia and adult females, with the former two keys fully illustrated.
Conclusions
Taxonomic information and identification keys for species of the genus Anopheles are updated. The need for further morphology-based studies and description of new species are reinforced.

Similar content being viewed by others
Background
Malaria continues to be a serious public health problem. In 2016, there were an estimated 216 million cases of malaria in 91 countries, an increase of 5 million cases over 2015. Malaria deaths reached 445,000 in 2016, a similar number (446,000) to 2015 (http://www.who.int/news-room/fact-sheets/detail/malaria) [1]. In 2017, there were an estimated 219 million cases of malaria worldwide, mostly in developing countries, resulting in an estimated 435,000 deaths of which 266,000 deaths were children under 5 years of age [2].
In Central and South America, malaria transmission occurs primarily at altitudes of less than 1000 meters above sea level. It is not surprising therefore that the incidence of malaria is highest in lowland countries, such as those situated in the Amazon basin: Brazil, Colombia, Venezuela, Ecuador, Peru, and the Guianas. Brazil, Colombia, Peru, and Venezuela contributed approximately 82% of the 875,000 malaria cases reported in the region in 2016: Brazil (18.0%); Colombia (15.3%); Peru (14.3%) and Venezuela (34.4%) [1]. In 2017, an increase in malaria cases was reported in Brazil, Ecuador, Mexico, Nicaragua, and Venezuela [2].
Variability in the epidemiological components of malaria transmission (host(s), parasites, environment) varies widely by locality, with differences in physiography, regional and local ecological characteristics, vector competence and vectorial capacity of individual species of Anopheles Meigen, 1818. Mosquito vectors can differ in physiology, behavior, and ecology, as well as in morphological characters used for their identification. These differences taken together can facilitate the definition of species and are the source of characteristics for species identification. The most accessible method of identification is by morphological characteristics. However, if basic (alpha) morphological taxonomy is incomplete, it is possible to make incorrect identifications of morphologically similar species of very different public health importance. This is especially the case for species complexes that include both vector and non-vector species. The definition and delineation of morphologically similar species often requires auxiliary tools, for example molecular markers, which include DNA sequences. DNA not only has given us evolutionary insights but also many new characters for the recognition and identification of species. However, molecular methods are not available in many malaria-endemic areas in South America. Recently, multiple studies (e.g. [3,4,5,6,7,8,9]) have reported satisfactory resolution of taxonomic problems using a combination of molecular markers, and/or morphological characters from all developmental stages. These studies have led to description or re-description of multiple species, and the recognition of complexes of morphologically similar species, which are summarized here.
In spite of technological and analytical advances, the identification of mosquito species using external morphological characters is still a preferred method since microscopes are easy to use, relatively inexpensive and can also be employed to study live specimens for ecological studies, such as capture-mark-release-recapture. Even though there are many keys addressing morphological identification of Neotropical Anopheles, they are limited in geographical or taxonomic scope and/or are outdated. For this reason, we present comprehensive keys (based on the morphology of male genitalia, females, and fourth-instar larvae) to all Anopheles species recorded in South America.
Mosquitoes (family Culicidae Meigen, 1818), belong to order Diptera (true flies, i.e. insects with two wings), and like many other insect orders (Coleoptera, Hymenoptera, etc.) they are holometabolous, meaning they have distinctly different egg, larval, pupal and adult stages. The culicids are recognized as a monophyletic group (with a single common ancestor) [10,11,12], that diverged from its nearest relative, family Chaoboridae Edwards, 1912, about 255 million years ago (mya) [13]. The two culicid subfamilies, Anophelinae Grassi, 1900 and Culicinae Meigen, 1818, diverged 229–192 mya [14].
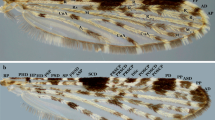
Adult mosquitoes (Fig. 1) can be distinguished [15] by: scales on wing veins and usually also on the head, legs, thorax and abdomen; proboscis long, extending well beyond the clypeus; characteristic wing venation (also found, however, in families Dixidae Schiffner, 1868 and Chaoboridae) [16] (i.e. subcostal (Sc) vein ending near or beyond midpoint of the costal vein (C), Sc and vein R1 both reach C in the apical half of the wing in front of R2 and R3, and vein M three-branched); antennal pedicel prominent and nearly always larger than the scape. Mosquito (and chaoborid) larvae [16, 17] have the three thoracic segments fused into a rounded composite structure that is wider than the head or abdomen; abdominal segment X with a fan-like ventral brush; lateral tufts of long setae on most thoracic and abdominal segments; labrum with a distinctive brush of long setae on either side (Culicidae only); and antenna moderately long, usually with a number of apical setae (Culicidae only).
Female anopheline mosquito, lateral view. Abbreviations: Ap, antepronotum; C-I, forecoxa; C-II, midcoxa; C-III, hindcoxa; Fe-I, forefemur; Fe-II, midfemur; Fe-III, hindfemur; Hl, halter; La, labellum; Mks, mesokatepisternum; Mm, mesepimeron; MPlp1–5, maxillary palpus, segments 1–5; Mpn, mesopostnotum; MS, mesothoracic spiracle; Mts, metepisternum; P, proboscis; Pa, paratergite; PA, postspiracular area; Ppn, postpronotum; Ps, proepisternum; S-I-VIII, sterna I-VIII; Scu, scutum; Stm, scutellum; Ta-III1–5, hindtarsomeres 1–5; Te-I-VIII, terga I-VIII; Ti-III, hindtibia; Tr-I, foretrochanter; Tr-II, midtrochanter; Tr-III, hindtrochanter
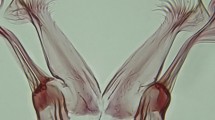
While the above characters serve for identification, another overlapping set of characters further characterize the family and can be used for phylogenetic analyses [18]. According to [10], the synapomorphies of Culicidae are internal premandibles in the larvae, apparently without residual external sclerites, and a long proboscis, with stilettes corresponding to the maxillae, mandibles and the labrum-hypopharynx, all encased in the labium [16]. In a study using characters of the adults and fourth-instar larvae, Harbach et al. [12] defined three synapomorphies for adult Culicidae: presence of erect scales on the head, dorsally; mouthparts forming a long proboscis; and the presence of prealar setae. In the larval stage, mosquitoes can be identified by: a well-sclerotized head that is clearly separated from the thorax; legless thoracic segments that are little differentiated from each other; and an abdomen made up of 10 segments, but with only nine apparent. The larvae are metapneustic (single spiracle in the post-abdominal region) with a spiracular plate that opens at the end of a siphon or on a small dorsal lobe that has a narrow, sclerotized posterior band in the dorsal region of abdominal segment VIII [12], and frequently with two pairs of anal papillae inserted at the end of segment X.
The Culicidae, and related families (Dixidae, Chaoboridae and Corethrellidae Edwards, 1932), pass through four larval instars (first-, second-, third- and fourth-instar) before developing into the pupal stage. The pupal and larval stages have serially (segmentally) homologous setae that have also been shown to be homologous between the larval and pupal stages (e.g. [19,20,21]). A common numbering system for these setae makes comparison of taxa straightforward.
Currently, the family Culicidae includes nearly 3600 valid species and a number of subspecies, which would add up well over 3600 [22] within two subfamilies (Anophelinae and Culicinae). Subfamily Anophelinae (genera Anopheles, Chagasia Cruz, 1906 and Bironella Theobald, 1905) contains about 500 described species. Most species of the Anophelinae can be distinguished in the adult stage from those of the subfamily Culicinae by the female maxillary palpus about as long as the proboscis, the male palpus also long and usually clubbed apically, a rounded (not tri-lobed) scutellum (except genus Chagasia), and the characteristic elevated angle of the abdomen at rest or when feeding. The larvae have no respiratory siphon and characteristically lie parallel to the water surface, although some other mosquitoes, such as genus Uranotaenia Lynch Arribálzaga, 1891, have a very short siphon and can be mistaken for Anopheles at first glance. The larvae of most species of Anopheles have at least some abdominal seta 1 broadened and leaf-like, which allows the larva to take advantage of surface tension to remain parallel to the surface.
The internal classification and phylogeny of the genus Anopheles has been reported by Harbach & Kitching [23], Harbach [24], Sallum et al. [25, 26], Foster et al. (2017) [27]. Worldwide there are three genera in the subfamily Anophelinae; Anopheles, Bironella and Chagasia (but see [27]). Most species belong to genus Anopheles while Bironella (Australasian) and Chagasia (Neotropical) have eight and five species respectively, which are not of medical importance. Genus Anopheles has seven subgenera (but see [27] and [28]): Anopheles (183 species); Baimaia Harbach, Rattanarithikul & Harrison, 2005 (1 species); Cellia Theobald, 1902 (224 species); Christya Theobald, 1903 (2 species); Kerteszia Theobald, 1905 (12 species); Lophopodomyia Antunes, 1937 (6 species); Nyssorhynchus Blanchard, 1902 (40 species); Stethomyia Theobald, 1902 (5 species) (Table 1), and approximately 13 recently discovered new species of the subgenera Anopheles and Nyssorhynchus [29]. Species of all subgenera except for Baimaia and Cellia occur in South America, and species of the subgenera Kerteszia, Lophopodomyia, Nyssorhynchus and Stethomyia are only found in the Neotropics. The largest cosmopolitan genera are Anopheles and Cellia. From a malaria transmission standpoint, a relatively small number of species of subgenus Cellia (i.e. the Gambiae Complex) are responsible for much of world’s malaria transmission [8]. The subgenera are further subdivided into informal morphologically similar groupings, usually for convenience, but which often lack phylogenetic significance [24].
Recently, Foster et al. [27] proposed that the Neotropical subgenera of Anopheles should be elevated to genus rank, see [28] for discussion of these changes, which were proposed subsequent to the writing of our keys. If these subgenera are considered by future authors to be genera, agreement of genus-species gender and inclusion or deletion of parentheses around author names as appropriate will be needed. For example, An. (Ker.) boliviensis (Theobald, 1905) would be Kerteszia boliviensis Theobald since it was originally described in genus Kerteszia.
In South America, the genus Anopheles has approximately 86 formally named species, with many yet to be named. Some Anopheles are associated with undisturbed forested areas while others are more abundant in areas that have been severely altered ecologically as a result of human activities, such as farming and logging. In the Neotropics, the genus Anopheles consists mostly of subgenera not found elsewhere in the world (see Table 1 for species, authors, and publication dates). Even the widespread subgenus Anopheles is comprised mostly of a group of species unique to the region (Series Arribalzagia). Phylogenetic studies have shown that the genus Chagasia, the earliest extant branch in the subfamily Anophelinae, is found only in Latin America. This has led to the quite plausible assertion that genus Anopheles originally evolved in this region of the world [24].
Subgenus Anopheles
Subgenus Anopheles (with 183 species) is represented by 29 species in the Neotropics and an additional number of putative species, which were newly discovered by Bourke et al. [29]. Most belong to Arribalzagia Series (23 species), a group only found in Central and South America. Most of the species of the series are forest mosquitoes, found in swamps or slow-moving streams. Anopheles (Anopheles) pseudopunctipennis Theobald, 1901 (Anopheles Series, Pseudopunctipennis Group) is widespread, found in freshwater sometimes at higher elevations, usually in association with the green alga Spyrogyra Nees, 1820. Some species in this subgenus are important in malaria transmission, e.g. An. (Anopheles) calderoni Wilkerson, 1991, An. (Anopheles) fluminensis Root, 1927, An. (Anopheles) pseudopunctipennis, An. (Anopheles) punctimacula Dyar & Knab, 1906, and An. (Anopheles) vestitipennis Dyar & Knab, 1906.
Subgenus Kerteszia
Larvae of the subgenus Kerteszia (12 species) almost exclusively utilize water collections in species of the mostly Neotropical plant family Bromeliaceae (bromeliads). They are most similar to larvae of the subgenus Nyssorhynchus but can be distinguished by a number of morphological characters [30], including differing setae on the gonocoxites [24]. Some species of Kerteszia have historically been quite important malaria vectors (e.g. An. bellator Dyar & Knab, 1906, An. cruzii Dyar & Knab, 1908, An. homunculus Komp, 1937, An. neivai Howard, Dyar & Knab, 1913, and An. pholidotus Zavortink, 1973). Logging activities diminish larval habitats, as many bromeliads are only found in trees. Anopheles bambusicolus Komp, 1937, as its name might imply, are the only Kerteszia spp. not to occupy bromeliads, but instead are found in unbroken bamboo internodes. One of us (RW) also found larvae in Amazonian nut pods in Peru (unpublished observation), and in a discarded tire in Brazil [31].
Subgenus Lophopodomyia
Larvae of the subgenus Lophopodomyia (six species) are found in forested, shaded habitats in small slow-moving streams. They are similar to larvae of the subgenus Anopheles, but can be distinguished by characteristics of the male genitalia [24], long setae on the prothoracic and mesothoracic pleural groups (P,M-9-12), well-developed palmate setae, and fringed pupal paddles. Females are not known to be of medical importance but can blood-feed on humans when they enter the forest environment [32].
Subgenus Nyssorhynchus
Larvae of the subgenus Nyssorhynchus (40 species with numerous cryptic unnamed species) are found in a large variety of open or partly shady areas [32]. Species included in the Nyssorhynchus are most closely related to species of the subgenus Kerteszia but can be distinguished by several morphological characters [30]. The subgenus contains many important malaria vectors including An. albimanus Wiedemann, 1820 (malaria vector in Central America and northern South America), species of the An. albitarsis Lynch Arribálzaga, 1878 complex (9 species), An. aquasalis Curry, 1932 (found in brackish waters), An. darlingi Root, 1926 (commonly associated with rivers and streams), An. nuneztovari Gabaldon, 1940, An. triannulatus (Neiva & Pinto, 1922), and many comprise species complexes.
Subgenus Stethomyia
Larvae of the subgenus Stethomyia (5 species) are, as in Lophopodomyia, forest species found in well-shaded small streams and swampy areas. Adults are mostly dark-colored but have a characteristic silvery stripe on the scutum. The setae of the gonocoxite are distinctive [24]. In the larva, head seta 2-C are widely separated, reminiscent of subgenus Cellia, thoracic setae P,M,T-9–12 have thorn-like branches, and the abdomen lacks distinct palmate setae. The species are not known to be of medical importance, but females will bite humans [32].
Distribution of Neotropical Anopheles spp.
The distributions of the species treated here are summarized in Table 2. Location should be considered as one of the most important “characters” used for identification. For example, if a species is only known from east of the Andes mountains but is reported from the Pacific side of the cordillera, it should be flagged as a possible misidentification or perhaps evidence of cryptic diversity. Likewise, species reported well outside known recorded ranges should be carefully evaluated. The combination of species found in a given country is usually unique since ecologies are heterogeneous among countries (or states, provinces, etc.). In fact, most identifiers will first ask the question “Where was it collected?” before selecting a key.
Methods
Illustrations for keys are photomicrographs or drawings. Photomicrographs were taken using a digital Canon Eos T3i, attached to a light Diaplan Leitz microscope, using the program Helicon Focus, which was used to build single in-focus images by stacking multiple images of the same structure. Photomicrographs of the male genitalia were taken from specimens from FSP-USP and MUSENUV. For few species, illustrations were reproduced from published literature. Keys are presented in Part II for the fourth-instar larvae [33], Part III for the male genitalia [34], and Part IV for the adult females [35].
Results and discussion
As a basis for these keys, the primary types (holotypes and paratypes) and other specimens deposited in the Coleção Entomológica de Referência, Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo, Brazil (FSP-USP) [36,37,38], Museo de Entomología, Universidad del Valle, Santiago de Cali, Colombia (MUSENUV) and the US National Mosquito Collection, Smithsonian Institution, Washington, DC, USA (USNMC), and original descriptions, keys, summaries and revisions from the published literature were examined. Primary publications for identification of Neotropical Anopheles include: key to genus Anopheles based on the morphology of male genitalia (Amazonian Brazil) [39]; keys to genus Anopheles based on the morphology of male genitalia, females and larvae (Venezuela) [40,41,42,43,44]; keys to Anopheles females and larvae (Brazil and other countries of South America) [45, 46]; key to Anopheles subgenus Nyssorhynchus Blanchard, 1902 females (western Venezuela) [47]; keys to Anopheles subgenus Nyssorhynchus based on the morphology of male genitalia, females and larvae (Amazonian Region) [48]; revision of all stages of Anopheles subgenus Nyssorhynchus, Albimanus Section [49]; genus Anopheles, comprehensive (North and South America) [32, 50]; keys to genus Anopheles females and larvae (Colombia) [51]; keys to genus Anopheles females and larvae (Venezuela, eastern and western South America, Central America and Panama) [52,53,54,55]; key to Anopheles subgenus Kerteszia Theobald, 1905 females [56]; keys to genus Anopheles male genitalia, larvae and eggs (Neotropics) [57]; revision of all stages of Anopheles subgenus Nyssorhynchus, Argyritarsis Section [58]; key to larvae (Venezuela) [59]; summary of Anopheles subgenus Nyssorhynchus, with definition of the Myzorhynchella Series [30]; key to females of Anopheles subgenus Nyssorhynchus (Venezuela) [60]; key to Anopheles females (Central America) [61]; key to Anopheles females (in Spanish) (Central America) [62]; and, revision of Anopheles subgenus Kerteszia [63]. From these available published literature records, we initiated the keys using [32, 49, 50, 56, 63] and [61].
Based on the published literature and specimens of additional species deposited in the previously mentioned collections [58,59,60], we present a list of species included in the identification keys. Table 1 shows the traditional classification of the genus Anopheles, including formal and informal groups, species authorship, and the date of publication.
The subgenus Nyssorhynchus includes the Albimanus [49], Argyritarsis [58], and Myzorhynchella Sections [30, 64]. The Albimanus and Argyritarsis Sections further include series, groups, subgroups, and species complexes [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. The classification into section categories does not indicate monophyletic groups as shown in phylogenetic studies using morphology [25] and sequence data [26, 27]. Currently, the internal classification of Nyssorhynchus is primarily based on morphological similarities of the adults, males, and females, and it is herein adopted for convenience (Table 3). Species complex, such as An. arthuri Unti, 1941, includes additional three phylogenetic taxa, which were found in studies using mitochondrial and nuclear genes [69, 81]. Despite these taxa have not been formally described, they are included in Table 2, and details for species identification are provided in Sallum et al. [33, 34].
Conclusion
Taxonomic information and identification keys for species of South American Anopheles were updated and revealed the need for further morphology-based studies and descriptions of species of several complexes, species which have been defined on the basis of DNA sequence data but have not been formally named.
Availability of data and materials
Not applicable.
References
WHO. World malaria report 2017. Geneva: World Health Organization; 2017.
WHO. World malaria report 2018. Geneva: World Health Organization; 2018.
Emerson KJ, Conn JE, Bergo ES, Randel MA, Sallum MAM. Brazilian Anopheles darlingi Root (Diptera: Culicidae) clusters by major biogeographical region. PLoS One. 2015;10:e0130773.
Sant’Ana DC, Sallum MAM. Anopheles (Nyssorhynchus) striatus, a new species of the Strodei Subgroup (Diptera, Culicidae). Rev Bras Entomol. 2017;2016:136–45.
Scarpassa VM, Cunha-Machado AS, Saraiva JF. Evidence of new species for malaria vector Anopheles nuneztovari sensu lato in the Brazilian Amazon region. Malar J. 2016;15:205.
Gómez GF, Bickersmith SA, González R, Conn JE, Correa MM. Molecular taxonomy provides new insights into Anopheles species of the Neotropical Arribalzagia Series. PLoS One. 2015;10:e0119488.
Rahola N, Makanga B, Yangari P, Jiolle D, Fontenille D, Renaud F, et al. Description of Anopheles gabonensis, a new species potentially involved in rodent malaria transmission in Gabon, Central Africa. Infect Genet Evol. 2014;28:628–34.
Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–74.
Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. Rijeka, Croatia: InTech; 2013.
Wood DM, Borkent A. Phylogeny and classification of the Nematocera. In: McAlpine JF, Wood DM, editors. Manual of Nearctic Diptera, vol. 3. Hull: Research Branch Agriculture Canada Monograph No. 32, Canadian Government Publishing Centre; 1989.
Miller BR, Crabtree MB, Savage HM. Phylogenetic relationships of the Culicomorpha inferred from 18S and 5.8S ribosomal DNA sequences (Diptera: Nematocera). Insect Mol Biol. 1997;6:105–14.
Harbach RE, Kitching IJ. Phylogeny and classification of the Culicidae (Diptera). Syst Entomol. 1998;23:237–370.
Yeates DK, Wiegmann BM. Phylogeny and evolution of Diptera: recent insights and new perspectives. In: Yeates DK, Wiegmann BM, editors. The evolutionary biology of flies. Columbia: Columbia University Press; 2005.
Reidenbach KR, Cook S, Bertone MA, Harbach RE, Wiegmann BM, Besansky NJ. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol Biol. 2009;9:298.
McAlpine JF. Key to families - adults. In: McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM, editors. Manual of Nearctic Diptera, vol. 1. Ottawa: Biosystematics Research Insitute; 1981.
McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM. Manual of Nearctic Diptera, vol. 1. Ottawa: Biosystematics Research Institute; 1981.
Teskey HJ. Key to families-larvae. In: McAlpine JF, Peterson BV, Shewell GE, Teskey HJ, Vockeroth JR, Wood DM, editors. Manual of Nearctic Diptera, vol. 1. Ottawa: Biosystematics Research Institute; 1981.
Harbach RE. The Culicidae (Diptera): a review of taxonomy, classification and phylogeny. Zootaxa. 2007;1668:591–638.
Belkin JN. A revised nomenclature for the chaetotaxy of the mosquito larva. Am Midl Nat. 1950;44:678–98.
Barr AR, Myers CM. Pupae of the genus Culiseta Felt. I. The homology of larval and pupal setae (Diptera: Culicidae). Ann Entomol Soc Am. 1962;55:94–8.
Knight KL, Chamberlain RW. A new nomenclature for the chaetotaxy of the mosquito pupa, based on a comparative study of the genera (Diptera: Culicidae). Proc Helminthol Soc Wash. 1948;15:1–18.
Mosquito Taxonomic Inventory. http://mosquito-taxonomic-inventory.info/simpletaxonomy/term/6045/n. Accessed 14 Jan 2019.
Harbach RE, Kitching IJ. The phylogeny of Anophelinae revisited: inferences about the origin and classification of Anopheles (Diptera: Culicidae). Zool Scripta. 2015;45:499–514.
Harbach RE. The phylogeny and classification of Anopheles. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. Rijeka, Croatia: InTech; 2013.
Sallum MAM, Schultz T, Wilkerson RC. Phylogeny of Anophelinae (Diptera, Culicidae) based on morphological characters. Ann Entomol Soc Am. 2000;93:745–75.
Sallum MAM, Schultz TR, Foster PG, Aronstein K, Wirtz RA, Wilkerson RC. Phylogeny of Anophelinae (Diptera: Culicidae) based on nuclear ribosomal and mitochondrial DNA sequences. Syst Entomol. 2002;27:361–82.
Foster PG, Oliveira TMP, Bergo ES, Conn JE, Sant’Ana DC, Nagaki SS, et al. Phylogeny of Anophelinae using mitochondrial protein coding genes. R Soc Open Sci. 2017;4:170758.
Harbach RE. An Anopheles by any other name …? J Med Entomol. 2018;55:1069–70.
Bourke BP, Conn JE, de Oliveira TMP, Chaves LSM, Bergo ES, Laporta GZ, et al. Exploring malaria vector diversity on the Amazon Frontier. Malar J. 2018;17:342.
Peyton EL, Wilkerson RC, Harbach RE. Comparative analysis of the subgenera Kerteszia and Nyssorhynchus of Anopheles (Diptera: Culicidae). Mosq Syst. 1992;24:51–69.
Luz E, Consolim J, Barbosa OC, Torres PB. Larvas de Anopheles (subgênero Kerteszia) Theobald 1905 encontradas em criadouros artificiais, no Estado do Paraná, Brasil. Rev Saude Publica. 1987;21:466–8.
Forattini OP. Entomologia Médica. Parte Geral, Diptera, Anophelini, vol. I. São Paulo: Editora da Universidade de Sao Paulo; 1962.
Sallum MAM, González Obando R, Carrejo N, Wilkerson RC. Identification keys to the Anopheles mosquitoes of South America (Diptera: Culicidae). II. Fourth instar larvae. Parasit Vectors. 2020. https://doi.org/10.1186/s13071-020-04299-5.
Sallum MAM, González Obando R, Carrejo N, Wilkerson RC. Identification keys to the Anopheles mosquitoes of South America (Diptera: Culicidae). III. Male genitalia. Parasit Vectors. 2020. https://doi.org/10.1186/s13071-020-04300-1.
Sallum MAM, González Obando R, Carrejo N, Wilkerson RC. Identification keys to the Anopheles mosquitoes of South America (Diptera: Culicidae). IV. Adult females. Parasit Vectors. 2020. https://doi.org/10.1186/s13071-020-04301-0.
Forattini OP, Rabello EX, Cotrim MD. Catálogo das coleções entomológicas da Faculdade de Saúde Pública da Universidade de São Paulo (1a Série) Culicidae, vol. 4. São Paulo: Universidade de São Paulo, Faculdade de Saúde Pública; 1970.
Forattini OP, Rabello EX, Cotrim MD. Catálogo das coleções entomológicas da Faculdade de Saúde Pública da Universidade de São Paulo (1a Série). Ceratopogonidae, Psychodidae, Simuliidae. Rev Saude Publica. 1971;5:301–66.
Forattini OP, Sallum MAM, Kakitani I. Catálogo das coleções entomológicas da Faculdade de Saúde Pública da Universidade de São Paulo (2a serie). Rev Saude Publica. 1988;22:519–47.
Causey OR, Deane LM, Deane MP. II. An illustrated key by male genitalic characteristics for the identification of thirty-four species of Anophelini, with a note on dissection technique. Studies on Brazilian Anophelines from the Northeast and Amazon Regions. Baltimore: The Johns Hopkins Press; 1946. p. 21–31.
Cova Garcia P, Sutil OE. Clave para adultos hembras de anofelinos de Venezuela. Bol Dir Mal San Amb. 1975;15:201–16.
Cova Garcia P, Sutil OE. Clave para larvas de anofelinos de Venezuela. Bol Dir Mal San Amb. 1975;15:6–24.
Cova Garcia P, Sutil OE. Clave para la identificación de los anofelinos de Venezuela por las terminalia del macho. Bol Dir Mal San Amb. 1976;16:13–32.
Cova Garcia P, Sutil OE, Rausseo JA. Mosquitos (Culicinos) de Venezuela, vol. I. Caracas: Ministerio de Sanidad y Asistencia Social; 1966.
Cova Garcia P, Sutil OE, Rausseo JA. Mosquitos (Culicinos) de Venezuela, vol. II. Caracas: Ministerio de Sanidad y Asistencia Social; 1966.
Deane LM, Causey OR, Deane MP. I. An illustrated key by adult female characteristics for the identification of thirty-five species of Anophelini, with notes on the malaria vectors (Diptera, Culicidae). Studies on Brazilian anophelines from the Northeast and Amazon regions. Baltimore: The Johns Hopkins Press; 1946.
Deane MP, Causey OR, Deane LM III. An illustrated key by laral characteristics for the identification of thirty-two species of Anophelini, with descriptions of two larvae. Studies on Brazilian anophelines from the Northeast and Amazon regions. Baltimore: The Johns Hopkins Press; 1946.
Delgado N, Rubio-Palis Y. Identification of Anopheles (Nyssorhynchus) (Diptera: Culicidae) occurring in western Venezuela. Mosq Syst. 1993;25:222–30.
Faran ME, Linthicum KJ. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq Syst. 1981;13:1–81.
Faran ME. Mosquito studies (Diptera: Culicidae) XXXIV. A revision of the Albimanus Section of the subgenus Nyssorhynchus of Anopheles. Contr Amer Entomol Inst. 1980;15:1–215.
Forattini OP. Culicidologia Medica. Identificacão, Biologia, Epidemiologia, vol. II. São Paulo: Editora da Universidade de Sao Paulo; 2002.
González R, Carrejo NS. Introducción al estudio taxonómico de Anopheles de Colombia. Claves y notas de distribución. Secunda Edición. Cali, Colombia: Programa Editorial Universidade del Valle, Colombia; 2009.
Stojanovich DJ, Gorham JR, Scott HG. Clave illustrada para los mosquitos anofelinos de América Central y Panamá. Atlanta: United States Department of Health, Education, and Welfare; 1966.
Stojanovich DJ, Gorham JR, Scott HG. Clave illustrada para los mosquitos anofelinos de Venezuela. Atlanta: United States Department of Health, Education, and Welfare; 1966.
Gorham JR, Stojanovich CJ, Scott HG. Clave ilustrada para los mosquitos anofelinos de Sudamérica oriental. Illustrated key to the anopheline mosquitoes of Eastern South America. Atlanta: United States Department of Health Education and Welfare; 1967.
Gorham JR, Stojanovich CJ, Scott HG. Clave ilustrada para los mosquitos anofelinos de Sudamérica occidental. Mosq Syst. 1973;5:97–156.
Harrison BA, Ruiz-López F, Calderon Falero G, Savage HM, Pecor JE, Wilkerson RC. Anopheles (Kerteszia) lepidotus (Diptera: Culicidae), not the malaria vector we thought it was: Revised male and female morphology; larva, pupa, and male genitalia characters; and molecular verification. Zootaxa. 2012;3218:1–17.
Lane J. Neotropical Culicidae, vol. I. São Paulo: University of São Paulo; 1953.
Linthicum KJ. A revision of the Argyritarsis Section of the subgenus Nyssorhynchus of Anopheles (Diptera: Culicidae). Mosq Syst. 1988;20:98–271.
Navarro JC. Actualización taxonómica de la tribu Anophelini de Venezuela, con nueva clave para la identificación de larvas de 4to estadio. Bol Dir Mal San Amb. 1996;36:25–43.
Rubio-Palis Y. Anopheles (Nyssorhynchus) de Venezuela taxonomía, bionomía, ecología e importancia médica. Maracay, Venezuela: Escuela de Malaríología y Saneamiento Ambiental “Dr. Amoldo Gabaldon” y el Proyecto Control de Enfermedades Endémicas; 2000.
Wilkerson RC, Strickman D. Illustrated key to the female anopheline mosquitoes of Central America and Mexico. J Am Mosq Control Assoc. 1990;6:7–34.
Wilkerson RC, Strickman D, Fernández-Salas I, Ibáñez-Bernal S. Clave ilustrada para la identificación de las hembras de mosquitos de México y Centroamérica: Secretaria de Salud. Mexico: Subsecretaria de Coordinacion y Desarrollo; 1993.
Zavortink TJ. Mosquito studies (Diptera, Culicidae) XXIX. A review of the subgenus Kerteszia of Anopheles. Contr Amer Entomol Inst. 1973;9:1–54.
Theobald FV. A monograph of the Culicidae of the world, vol. IV. London: Trustees of the British Museum; 1907.
Conn J, Rangel Puertas Y, Seawright JA. A new cytotype of Anopheles nuneztovari from western Venezuela and Colombia. J Am Mosq Control Assoc. 1993;9:294–301.
Sierra DM, Velez ID, Linton YM. Malaria vector Anopheles (Nyssorhynchus) nuneztovari comprises one genetic species in Colombia based on homogeneity of nuclear ITS2 rDNA. J Med Entomol. 2004;41:302–7.
Ruiz F, Quiñones ML, Erazo HF, Calle DA, Alzate JF, Linton YM. Molecular differentiation of Anopheles (Nyssorhynchus) benarrochi and An. (N.) oswaldoi from southern Colombia. Mem Inst Oswaldo Cruz. 2005;100:155–60.
Wilkerson RC, Parsons TJ, Klein TA, Gaffigan TV, Bergo E, Consolim J. Diagnosis by random amplified ploymorphic DNA polymerase chain reaction of four cryptic species related to Anopheles (Nyssorhynchus) albitarsis (Diptera: Culicidae) from Paraguay, Argentina, and Brazil. J Med Entomol. 1995;32:697–704.
Greni SE, Demari-Silva B, de Oliveira TMP, Suesdek L, Laporta GZ, Sallum MAM. A multi-gene analysis and potential spatial distribution of species of the Strodei Subgroup of the genus Nyssorhynchus (Diptera: Culicidae). J Med Entomol. 2018;55:1486–95.
Calado DC, Foster PG, Bergo ES, dos Santos CLS, Galardo AKR, Sallum MAM. Resurrection of Anopheles goeldii from synonymy with Anopheles nuneztovari (Diptera, Culicidae) and a new record for Anopheles dunhami in the Brazilian Amazon. Mem Inst Oswaldo Cruz. 2008;103:791–9.
González CR, Sallum MAM. Anopheles (Nyssorhynchus) atacamensis (Diptera: Culicidae), a new species from northern Chile. Mem Inst Oswaldo Cruz. 2010;105:13–24.
Guimaraes JH. Systematic database of Diptera of the Americas south of the United States. Family Culicidae. Sao Paulo: Plêiade/Fapesp: Sociedade Brasileira de Entomologia; 1997.
Juri MJD, Stein M, Sallum MAM. Occurrence of Anopheles (Anopheles) neomaculipalpus Curry in northwestern Argentina. J Vector Borne Dis. 2011;48:64–6.
Montoya-Lerma J, Solarte YA, Giraldo-Calderon GI, Quinones ML, Ruiz-Lopez F, Wilkerson RC, et al. Malaria vector species in Colombia—a review. Mem Inst Oswaldo Cruz. 2011;106(Suppl. 1):223–38.
Motoki MT, Wilkerson RC, Sallum MAM. The Anopheles albitarsis complex with the recognition of Anopheles oryzalimnetes Wilkerson and Motoki, n. sp. and Anopheles janconnae Wilkerson and Sallum, n. sp. (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 2009;104:823–50.
Nagaki SS, de Albuquerque Motta M, Sallum MAM. Redescription of Anopheles (Nyssorhynchus) antunesi Galvao & Amaral and description of a new species of the Myzorhynchella Section (Diptera: Culicidae) from Serra da Mantiqueira, Brazil. Mem Inst Oswaldo Cruz. 2010;105:278–85.
Sallum MA, Foster PG, Santos CLSD, Flores DC, Motoki MT, Bergo ES. Resurrection of two species from synonymy of Anopheles (Nyssorhynchus) strodei Root, and characterization of a distinct morphological form from the Strodei Complex (Diptera: Culicidae). J Med Entomol. 2010;47:504–26.
Sallum MAM, Wilkerson RC, Forattini OP. Taxonomic study of species formerly identified as Anopheles mediopunctatus and resurrection of An. costai (Diptera: Culicidae). J Med Entomol. 1999;36:282–300.
Wilkerson RC, Sallum MAM. Anopheles (Anopheles) forattinii: a new species in the series Arribalzagia (Diptera: Culicidae). J Med Entomol. 1999;36:345–54.
Wilkerson RC, Linton YM, Strickman D. Mosquitoes of the world. Vol. 2. Baltimore: Johns Hopkins University Press (In Press).
Bourke BP, Oliveira TP, Suesdek L, Bergo ES, Sallum MA. A multi-locus approach to barcoding in the Anopheles strodei subgroup (Diptera: Culicidae). Parasit Vectors. 2013;6:111.
Acknowledgements
This work would not have been possible without the examination and permission for photographs of specimens from one of the major collections of mosquitoes in South America, repository of valuable reference specimens of multiple species of Anopheles of the Neotropical Region, including primary and secondary type specimens, at the Coleção Entomológica de Referência da Faculdade de Saúde Pública, Universidade de São Paulo, Brazil (FSP-USP). We also thank the Museum of Entomology at the Universidad del Valle, Colombia, for allowing the use of photographic resources and preparing digital images of various parts of its entomological collection. MAMS extends her thanks to the Saúde Pública, Universidade de São Paulo, for their continued support for research projects and the logistics facility for the preparation and maintenance of thousands of specimens of the mosquito collection; and the Fundação de Amparo à Pesquisa do Estado São Paulo for continuous financial support that allowed the execution of hundreds of field sampling efforts for research in the systematics and ecology of mosquitoes (FAPESP Grants #2014/26229-7; #2011/20397-7; #2005/53973-0; CNPq # 301877/2016 to MAMS). RGO and NSC give special thanks to the Facultad de Ciencias Naturales y Exactas de la Universidad del Valle, Colombia, for continuous support and the logistics facility. Project Amazon Malaria Initiative (AMI) - Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA) provided partial financial support with assistance from USAID and coordination with PAHO/WHO. We are in debt to Yvonne-Marie Linton (Walter Reed Army Institute of Research) and Bruce Harrison (in memoriam) for their thoughtful review of the first version of the identification keys for females, males, and larvae, Caio Cesar Moreira, Faculdade de Saúde Pública, Universidade de São Paulo, for final editing of all illustrations, Ralph E. Harbach (Natural History Museum, London, UK) for his thoughtful revision and valuable contribution, and Aneta Kostadinova for her suggestions and editing the last version of the manuscript. The activities undertaken at WRBU were performed in part under a Memorandum of Understanding between the Walter Reed Army Institute of Research (WRAIR) and the Smithsonian Institution, with institutional support provided by both organizations. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Army, the Department of Defense, or the U.S. Government.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP grant no. 2014/26229-7, CNPq grant no. 301877/2016-5 to MAMS; the Armed Forces Health Surveillance Board – Global Emerging Infectious Disease Surveillance (AFHSB-GEIS) [P0116_19_WR_05 and P0140_20_WR_05].
Author information
Authors and Affiliations
Contributions
MAMS and RCW conceived the study. MAMS, RGO and RCW constructed the identification keys. MAMS, RGO and NC prepared all illustrations. MAMS, RCW and RGO wrote the manuscript. All authors revised successive drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sallum, M.A.M., Obando, R.G., Carrejo, N. et al. Identification keys to the Anopheles mosquitoes of South America (Diptera: Culicidae). I. Introduction. Parasites Vectors 13, 583 (2020). https://doi.org/10.1186/s13071-020-04298-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04298-6