Abstract
Background
Biting midges of the genus Culicoides Latreille (Diptera: Ceratopogonidae) are involved in the transmission of several viruses affecting humans and livestock, particularly bluetongue (BTV). Over the last decade, Culicoides surveillance has been conducted discontinuously and at various temporal and spatial scales in mainland France following the BTV epizootics in 2008–2009 and its reemergence and continuous circulation since 2015. The ability to predict seasonal dynamics and spatial abundance of Culicoides spp. is a key element in identifying periods and areas at high risk of transmission in order to strengthen surveillance for early detection and to establish seasonally disease-free zones. The objective of this study was to model the abundance of Culicoides spp. using surveillance data.
Methods
A mixed-effect Poisson model, adjusted for overdispersion and taking into account temperature data at each trap location, was used to model the weekly relative abundance of Culicoides spp. over a year in 24 vector zones, based on surveillance data collected during 2009–2012. Vector zones are the spatial units used for Culicoides surveillance since 2016 in mainland France.
Results
The curves of the predicted annual abundance of Culicoides spp. in vector zones showed three different shapes: unimodal, bimodal or plateau, reflecting the temporal variability of the observed counts between zones. For each vector zone, the model enabled to identify periods of vector activity ranging from 25 to 51 weeks.
Conclusions
Although the data were collected for surveillance purposes, our modeling approach integrating vector data with daily temperatures, which are known to be major drivers of Culicoides spp. activity, provided areas-specific predictions of Culicoides spp. abundance. Our findings provide decisions makers with essential information to identify risk periods in each vector zone and guide the allocation of resources for surveillance and control. Knowledge of Culicoides spp. dynamics is also of primary importance for modeling the risk of establishment and spread of midge-borne diseases in mainland France.
Similar content being viewed by others
Background
Biting midges of the genus Culicoides Latreille (Diptera: Ceratopogonidae) are involved in the transmission of several viruses affecting both animals [e.g. bluetongue (BTV), Schmallenberg (SBV), Akabane, African horse sickness and epizootic hemorrhagic disease viruses] and humans (e.g. Oropouche fever virus). In Europe, the incursion of BTV and SBV in the last decade has caused substantial economic losses to farmers [1,2,3]. Since the large scale SBV epidemic that affected 29 European countries in 2011–2013, this disease appears to have settled to a low-level endemic circulation [4, 5] and is now recognized as a farm disease. In contrast, several European countries have been repeatedly affected by the circulation of both established and newly introduced BTV strains [6, 7]. Bluetongue (BT) is a disease regulated at the European level and, since 2000, the European Commission has established a series of regulations for control and surveillance in infected countries [8]. Control measures include vector control, restriction to movements of live ruminants from infected to non-infected regions and vaccination. Movement restrictions, which impose major technical and economic constraints to farmers, may be lifted in areas where evidence shows no virus circulation in livestock during vector-free periods. This decision requires a good knowledge of the temporal and spatial phenology of vector species.
In France, the main Mediterranean BTV vector, Culicoides imicola Keiffer, was detected for the first time on the island of Corsica in October 2000, just before the occurrence of important outbreaks of BTV serotype 2 (BTV-2) in the autumns of 2000 and 2001 [9, 10]. As a consequence, Culicoides surveillance was first implemented in Corsica and along the Mediterranean coast of the French mainland. This entomological surveillance was extended to the whole French mainland in 2009 to monitor vector activity following the introduction and spread of BTV-8 throughout the country in 2007–2008 [11]. The national-scale surveillance program ceased in 2012 and was implemented again in 2016–2018 following the re-emergence of BTV-8 in France in 2015 [12]. Currently, two BTV strains circulate in the French mainland (serotypes 4 and 8) and Corsica has a regulated status against several BTV strains (serotypes 1, 2, 4, 8 and 16) [13].
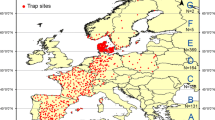
Entomological surveillance has been conducted by the French Agricultural Research Centre for International Development (CIRAD), mandated by The French Ministry of Agriculture and Food. During 2009–2012, Culicoides spp. surveillance covered the whole of mainland France with over 200 traps operating weekly or monthly depending on the season [11, 14]. During 2016–2018, the Culicoides spp. surveillance network has been optimized and operated in 24 zones, with one night of trapping per week at one site per zone from autumn to spring. These zones, recently named vector zones, were defined by an analysis (ascending hierarchical classification) of catch data collected during 2009–2012 to be homogeneous in terms of Culicoides species diversity and phenology (start and end of activity period). This entomological surveillance (which was active from November to next April) enabled the determination of periods without Culicoides vectors in each zone. The information provided by this network, coupled with the surveillance of viral circulation in livestock, allowed several French departments to be reported as BTV seasonally-free zones during the winters of 2016–2017 and 2017–2018, and thus restrictions on movements could be lifted for susceptible livestock from those zones. This status is critically important for livestock stakeholders to access the trade market (with no additional cost of serological and virological testing before the movement).
Our ability to predict the seasonal dynamics and spatial abundance of Culicoides spp. is a key element in determining high-risk transmission periods and areas to reinforce surveillance for early detection and to establish seasonally disease-free zones [8, 15]. This knowledge is also essential for modeling the transmission and spread of Culicoides-borne diseases and for identifying the most effective control measures [16, 17]. The objective of our study was to model the seasonal dynamics of Culicoides spp. in France using a combination of temperature and catch data collected during 2009–2012 for each vector zone. The results were compared with those predicted for two alternative spatial units: mainland France and iso-hygro-thermal zones, to demonstrate the relevance of vector zones as a spatial reference unit for surveillance and modeling of diseases transmitted by Culicoides spp.
Methods
Data
We used Culicoides spp. catch data obtained from 203 capture sites throughout mainland France between the second week of 2009 and the last week of 2012. This dataset includes information on the location of capture sites (latitude and longitude), the week of capture (trapping systematically occurs on Monday or Tuesday nights) and the number of specimens collected from each trap. Culicoides midges were collected with suction light traps (12 V, 8 W; manufactured by Onderstepoort Veterinary Institute, Pretoria, South Africa) installed from sunset to sunrise outside at 1.5–2.0 m above ground level immediately next to the stable or on a tree within < 30 m of the stable in close proximity to livestock. Traps were placed outdoors at exactly the same trapping location at the different sites throughout the entire study and operated one night on a monthly basis in winter and summer and on a weekly basis in spring and autumn. The samples were sent to CIRAD, the Interdepartmental Public Agency for Mosquito Control on the Mediterranean coast (EID-Med) or the Institute of Parasitology and Tropical Diseases of Strasbourg (IPPTS) for Culicoides species identification at the species level using relevant morphological identification keys [18, 19] and individual counting. We used data at the genus level, i.e. Culicoides spp. The catch data corresponds to the relative abundance (hereafter referred to as abundance) because only a fraction of the vector population is captured by the traps. Since the collections were performed in a standard manner, the numbers can be used to compare data between locations or sampling dates [20].
Weekly minimum and maximum air temperatures at an altitude of 2 m (in °C) were obtained for 2009–2012 from Meteo-France (available at https://donneespubliques.meteofrance.fr/). It provided data on an 8 km square lattice and we assigned the closest meteorological data to each capture site.
Model
Culicoides and temperature data for each capture site were associated with the corresponding zone. The catch data consisted of the total number of captured Culicoides \(Y_{ijl}\) from the capture site \(i\) on year \(j\) in week \(l\). We modelled Culicoides counts for each vector zone with a Poisson model, adjusted for overdispersion, which included a spline [21] on the week number to account for seasonal variation in count, minimum air temperatures and difference between maximum and minimum air temperatures (which were centered and reduced), and random effects on both year and capture sites:
where \(Y_{ijl}\) is the number of Culicoides at site \(i\) on year \(j\) in week \(l\); \(\lambda_{ijl}\) is the rate parameter at site \(i\) on year \(j\) in week \(l\); \(X_{l}\) is the natural spline value for the week \(l\); \(\theta min_{ijl}\) is the minimum air temperature at site \(i\) on year \(j\) in week \(l\); \(\theta delta_{ijl}\) is the difference between maximum and minimum air temperature at site \(i\) on year \(j\) in week \(l\); \(\beta_{0}\) is the global intercept; \(\beta_{1}\) is the slope for variable \(X_{l}\); \(\beta_{2}\) is the slope for variable \(\theta min\); \(\beta_{3}\) is the slope for variable \(\theta delta\); and \(u_{oi}\), \(u_{oj}\) is the random effects of the site and the year on the intercept.
We used a natural spline with five degrees of freedom (df), which allowed one or two peaks in the Culicoides seasonal dynamics. In spatial units where the model did not converge, we reduced the df by a decrement of 1 df until the model finally converged.
The ability of the model to predict Culicoides abundance was estimated using the mean absolute error (MAE) and the root-mean-square error (RMSE). We calculated both indicators on the direct predictions to estimate the explanatory ability of the model variables and then by a cross-validation procedure to test the predictive ability of the model. For the cross-validation, we randomly partitioned the data into two sets of 90% for training and 10% for testing and calculated RMSE and MAE on the testing data. This process was performed 1000 times for each vector zone. Statistical analyses and graphical representations were performed using R with packages splines and maptools [22].
In order to evaluate the relevance of the vector zones as the reference partitioning for Culicoides surveillance, we tested the model introduced above on two alternative partitionings: no partitioning (i.e. mainland France considered as a unique spatial zone), and an iso-hygro-thermal partitioning. The comparison of model predictions among partitionings was based on two criteria. The first was the ability of the model to correctly predict the presence or absence of Culicoides for each week (estimated using a receiver operating characteristic (ROC) curve approach [23,24,25]). The second was the ability of the model to provide a realistic estimate of Culicoides abundance (estimated by the proportion of observed data within the confidence interval predicted by each model). The methods describing the development of the iso-hygro-thermal partitioning and the results of the comparison of model predictions among partitionings are described in Additional file 1: Text S1.
We produced annual curves of abundance for each vector zone using weekly averaged temperatures over the four studied years (2009–2012). For each vector zone, the beginning and the end of the seasonally Culicoides-free period were defined assuming a threshold of an estimated abundance of ten Culicoides, which indicates significant activity [26]. The cumulated abundance of Culicoides over one year was obtained by calculating the area under the predicted abundance curve, with the R package pROC [27]. For the ease of understanding, the cumulated abundance was then transformed to a mean weekly abundance.
Statistical analyses and graphical representations were performed using R [28] with the R package tis [29].
Results
Each vector zone had on average 8.3 capture sites (median: 7.0; interquartile range: 5.0–11.0) during 2009–2012.
Model goodness-of-fit values and cross-validation results for each vector zone are provided in Additional file 2: Table S1. We note that the predicted values for the Culicoides abundance are very close to the observed values, except in four zones (1-3, 3-1, 3-3, 3-6) where extreme observed abundance resulted in large residuals and mathematically increased the MAE and RMSE values.
The mean effects and 95% confidence interval (CI) of the temperature variables (minimum temperature and temperature delta) estimated by the Poisson model for each zone are provided in Table 1. For five zones (in north-western France: 4-3, 4-5, 4-6; and eastern France: 1-2, 3-2), the overall effect of temperature was positive; for ten zones spread in the southern two-thirds of France (1-1, 1-4, 1-6, 1-7, 2-2, 2-3, 3-1, 3-3, 3-5, 5-5) the overall effect was negative; and in nine zones (1-3, 1-5, 1-8, 2-8, 3-4, 3-6, 3-8, 4-4, 6-8) the two temperature variables (minimum and delta) were found to have non-significant effects; by overall effect, we mean that effects are either both significant or one significant and the other non-significant.
The curves of predicted annual Culicoides abundance in vector zones showed three alternative shapes (Fig 1): unimodal (e.g. zone 4-3), bimodal (e.g. zone 3-6) or plateau-like (e.g. zone 3-4), reflecting the temporal variability in observed counts among zones. Predicted maximum abundance varied also strongly among vector zones from about 200 Culicoides (zones 2-8 and 6-8) to over 4000 Culicoides at peak (zones 4-3, 4-4 and 4-6). The cumulative Culicoides abundance varied strongly among vector zones from about 80 to 1310 Culicoides on average per week (median: 344; interquartile range: 215–624; Table 2, Fig 2). Overall, the vector period lasted between 25 and 51 weeks, starting between weeks 1 (early January) and 15 (mid-April) and ending between weeks 43 (end of October) and 51 (mid-December) (Table 2).
Discussion
In the present study, we modeled and quantified the weekly relative abundance of Culicoides spp. over a year in mainland France, using partitioning of the territory in vector zones and taking into account temporal and spatial variations in temperatures within those zones. Several studies have described the diversity and distribution of species in mainland France from surveillance data, yet, to our knowledge, our study is the first to provide zone-specific predictions of Culicoides abundance, which is critical for modeling the risk of establishment and spread of midge-borne diseases [30, 31] and implement risk-based surveillance and control measures.
The predicted curves of Culicoides abundance showed a strong seasonal pattern, reflecting the dependence of the Culicoides life-cycle on climatic conditions [32, 33], with poor tolerance of midges to low temperatures [34]. Indeed, during the cold season under temperate climates, most adult Culicoides disappear and the species survive as larvae (either due to true larval diapausing or to the prolonged duration of larval development at lower temperatures) [35]. Then, when temperatures start increasing, adults emerge and populations grow progressively to reach a peak of abundance in spring or summer depending on locations, as a function of spring temperatures and summer dryness. Indeed, temperature decreases the larval development time, the time between two blood meals, and therefore increases the laying frequency, which leads to a positive effect on the population dynamics (and its growth), and therefore we expected the temperature to have a positive effect on abundance [36]. Conversely, temperature is negatively correlated with survival [36]. Thus, there are temperature ranges for which the impact on abundance is positive, and others for which the impact on abundance is negative. It results in positive correlations in regions where temperatures do not reach high values, negative correlations in regions with high summer temperatures, or even non-linear effects. Overall, our results underlined marked differences in the shape and level of the abundance curves (with bimodal, unimodal or plateau-like patterns) among vector zones. These temporal and spatial differences reflect the large diversity of Culicoides species in mainland France, which is caused by the variety of climatic conditions, edaphic factors and farming practices. The subgenus Avaritia Fox (composed primarily of the Culicoides obsoletus (Meigen)/Culicoides scoticus Downes & Kettle complex, C. imicola, Culicoides dewulfi Goetghebuer and Culicoides chiopterus (Meigen)) is the most prevalent, representing more than 80% of captures, followed by the subgenus Culicoides (primarily, Culicoides newsteadi Austen and Culicoides pulicaris (Linnaeus)) . While C. imicola and C. newsteadi are common along the Mediterranean coast and in Corsica, other species are more widespread in temperate areas, with variation in abundance between oceanic, continental or mountain regions [14]. The dominant C. obsoletus/C. scoticus exhibits bimodal patterns of abundance in southern regions of France (with peaks in late spring and fall as populations decrease during summer due to dryness), while unimodal patterns (with a peak in summer) are more frequent the north of the country [11, 14]. Indeed, although temperatures are known as a major driver of Culicoides larvae development and adult activity, other variables (including rainfall, humidity, soil texture, normalized difference vegetation index, elevation, farming systems, densities of wild vertebrate hosts and land cover) may influence the phenology, distribution and abundance of midge species.
In order to simplify and reduce the cost of the monitoring of midge populations, entomological surveillance in France has relied, over the last years, on a spatial partitioning of the territory, defined from an ascending hierarchical classification of historical (2009–2011) Culicoides records. The comparison of model predictions based on this vector-based partitioning to those obtained with no partitioning (Additional file 1: Text S1, Figures S3, S5, S6) underlined the importance of modeling Culicoides abundance at a local scale to account for the spatial variation in both the distribution of species and the seasonal dynamics. Furthermore, our study showed that the vector-based partitioning provided a similar or better fit to catch data than an iso-hygro-thermal partitioning (Additional file 1: Text S1, Figures S1, S2, S4–S6), underlining the adequacy of the vector partitioning for planning surveillance and disease control activities.
The model included all available data on Culicoides collected during a four-year period (2009–2012), which allowed smoothing the effect of rare extreme or mild climatic events. However, we stress that the predicted vector abundance may be misjudged to some extent for different reasons. First, the data included zero counts. While some nil values may reflect the absence of vector, in other cases, zero counts may have resulted from adverse weather conditions on the day of trapping or technical problems with the trap. We decided to include all data in the model to capture the maximum variability even if zero counts were observed during the vector activity period. The use of a Poisson model adjusted for overdispersion allowed us to reduce the influence of the excess of zero counts on the estimation of abundance during the vector period. Secondly, among all Culicoides species recorded in France, only some have been connected with BTV transmission. Culicoides imicola and, to a lower extent, C. newsteadi are considered the main BTV vectors in the Mediterranean area, while C. obsoletus, C. scoticus, C. dewulfi, C. chiopterus and C. pulicaris (which are the most abundant and widely distributed species in mainland France) are involved as BTV vectors in other parts of Europe [37,38,39,40,41,42,43,44,45,46]. Virus isolations from field-collected C. imicola [47] and the reproduction of the transmission cycle in this species in experimental conditions [48] have proven this species to be a BTV vector. Likewise, C. newsteadi, C. obsoletus, C. scoticus, C. dewulfi, C. chiopterus and C. pulicaris (which are the most abundant and widely distributed species in mainland France) have either been found positive in field-collected samples [37,38,39,40,41,42,43,44,45, 49] or in experimentally-infected individuals [46] which suggests that they might act as vector species. These assertions are generally scientifically accepted [50] even if the vector competence of these species has not been comprehensively assessed in the laboratory due to technical issues, in particular the difficulties in feeding and maintaining Culicoides. As the species involved in the transmission of diseases are not exhaustively identified [37, 40, 43, 44, 46, 48, 51,52,53,54,55,56,57], we decided to use total Culicoides counts without distinction of species, which means that predicted weekly abundances may slightly overestimate the number of BTV vectors; however, the fact that species specified above represent almost 90% of all collected Culicoides in France makes us confident that the use of all Culicoides abundance data for risk assessments are valid. On the other hand, aggregating species might represent a problem for identifying accurate temporal and spatial patterns, as different species might exhibit different seasonal trends even in the same environment [58].
The spatial variation in abundance justifies the use of a regional policy for Culicoides surveillance and disease control. Culicoides-borne viruses like BTV and SBV cannot be transmitted to the susceptible host species in absence of adult vectors. Therefore, the European Union alleviates restriction measures during periods of vector inactivity, assuming that under the commonly used threshold of five parous females per trap per night, Culicoides populations are considered as inactive [8]. Our models did not include information about sex or age status of captured Culicoides; therefore, we decided to use a threshold of ten Culicoides per trap per night as a limit for declaring freedom of adult activity.
The fact that less than 5% of the total Culicoides collected using suction light traps are males suggests that not considering sex in our catch data does not affect the quality of our conclusions. Yet, the proportion of parous females in the Culicoides population may vary seasonally [59, 60]. These limits may alter the predictions of weekly abundance of Culicoides vectors and potentially overestimate the length of the activity period. We stress that the threshold of five parous females is conservative: it is likely that an abundance of more than five parous females/trap/night is required for BTV transmission to begin, but the exact threshold is not known [61, 62]. This evidence calls for more studies to refine this threshold, adjusted for the factors that may alter BTV transmission, such as vector longevity, biting rate and viral replication rate (which are highly dependent on the temperature) and disease prevalence in hosts.
Given the continuing need for optimizing the cost-effectiveness of animal disease surveillance, the knowledge of weekly Culicoides abundance in each zone creates new opportunities for a more efficient organization of field actors and allocation of resources for surveillance. Indeed, our study provides key input to conduct both serological and entomological surveillance during limited time windows before the predicted start and end of the vector in each zone. It could also be used to facilitate the planning of vector control strategies and increase their efficiency.
Conclusions
Our study provides estimates of weekly abundance of Culicoides for 24 zones, defined to be homogenous in terms of vector diversity, inactivity period and species phenology, in mainland France. This study showed the relevance of the vector partitioning (based on 24 traps versus about 160 traps previously). Beyond the value of these results for allocating efficiently the surveillance effort and resources, the knowledge of local Culicoides abundance is an essential component of epidemiological models to simulate the risk of exposure of susceptible hosts to midge-borne diseases (e.g. [17]) and to identify the most appropriate measures for control.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. Capture data are available upon request from the French Ministry of Agriculture and Food. The outputs of the climate model can be found (for non-commercial usage) on the dedicated website: https://donneespubliques.meteofrance.fr/?fond=produit&id_produit=230&id_rubrique=40.
Abbreviations
- BTV:
-
bluetongue virus
- SBV:
-
Schmallenberg virus
- CIRAD:
-
French Agricultural Research Centre for International Development
- ANSES:
-
French Agency for Food, Environmental and Occupational Health & Safety
- EID-Med:
-
Interdepartmental Public Agency for Mosquito Control on the Mediterranean coast
- IPPTS:
-
Institute of Parasitology and Tropical Diseases of Strasbourg
- df :
-
degrees of freedom
- MAE:
-
mean absolute error
- RMSE:
-
root-mean-square error
- CI:
-
confidence interval
References
Hasler B, Howe KS, Di Labio E, Schwermer H, Stark KD. Economic evaluation of the surveillance and intervention programme for bluetongue virus serotype 8 in Switzerland. Prev Vet Med. 2012;103:93–111.
Tago D, Hammitt JK, Thomas A, Raboisson D. Cost assessment of the movement restriction policy in France during the 2006 bluetongue virus episode (BTV-8). Prev Vet Med. 2014;117:577–89.
Waret-Szkuta A, Alarcon P, Hasler B, Rushton J, Corbiere F, Raboisson D. Economic assessment of an emerging disease: the case of Schmallenberg virus in France. Rev Sci Tech. 2017;36:265–77.
Delooz L, Saegerman C, Quinet C, Petitjean T, De Regge N, Cay B. Resurgence of Schmallenberg virus in Belgium after 3 years of epidemiological silence. Transbound Emerg Dis. 2017;64:1641–2.
Kerstin W, Bernd H, Franz JC, Martin B. Schmallenberg virus recurrence, Germany, 2014. Emerg Infect Dis. 2015;21:1202.
Sailleau C, Bréard E, Viarouge C, Vitour D, Romey A, Garnier A, et al. Re-emergence of bluetongue virus serotype 8 in France, 2015. Transbound Emerg Dis. 2017;64:998–1000.
Breard E, Bronner A, Calavas D, Cauchard J, Falala S, Grandcollot-Chabot M, et al. Situation de la fièvre catarrhale ovine en Europe – point de situation au 15 janvier 2018. Plateforme Epidémiosurveillance santé animale - Veille Sanitaire Internationale. 2018. https://www.plateforme-esa.fr/sites/default/files/2018-01-16_Note_FCO_Europe.pdf. Accessed 31 Aug 2018.
Official Journal of the European Union. COMMISSION REGULATION (EC) No 1266/2007 of 26 October 2007 on implementing rules for Council Directive 2000/75/EC as regards the control, monitoring, surveillance and restrictions on movements of certain animals of susceptible species in relation to bluetongue. Luxembourg: The Publications Office of the European Union; 2007. p. 37–52.
Zientara S, Rocque S, Gourreau J-M, Gregory M, Diallo A, Hendrikx P, et al. La fièvre catarrhale ovine en Corse en 2000. Epidémiol et Santé Anim. 2000;38:133–44.
Zientara S, Sailleau C, Dauphin G, Roquier C, Rémond EM, Lebreton F, et al. Identification of bluetongue virus serotype 2 (Corsican strain) by reverse-transcriptase PCR reaction analysis of segment 2 of the genome. Vet Rec. 2002;150:598–601.
Balenghien T, Garros C, Mathieu B, Setier-Rio M-L, Allène X, Gardès L, et al. La surveillance des Culicoides en France. Bull Epid Santé Anim Alim. 2018;35:8–9.
French Ministry of Agriculture, Food and Forestry, Direction générale de l’alimentation/Sous-direction de la santé et de protection animale. FCO: surveillance entomologique de novembre 2016 à mai 2017. Instruction technique DGAL/SDSPA/2016-890. 2016. https://info.agriculture.gouv.fr/gedei/site/bo-agri/instruction-2016-890. Accessed 17 Jan 2018.
European Commission. Control measures in bluetongue restricted zones. Table SANCO/10428/2007, Directorate-General for Health and Consumers. 2019. https://ec.europa.eu/food/sites/food/files/animals/docs/ad_control-measures_bt_restrictedzones.pdf. Accessed 7 Mar 2019.
Balenghien T, Delecolle JC, Setier-Rio M-L, Rakotoarivony I, Allene X, Venail R, et al. Vecteurs du virus de la fièvre catarrhale ovine: suivi des populations de Culicoides en 2011 en France. Bull Epid Santé Anim Alim. 2012;54:35–40.
French Ministry of Agriculture, Food and Forestry, Direction générale de l’alimentation/Sous-direction de la santé et de protection animale. FCO: surveillance entomologique. Instruction technique DGAL/SDSPA/2015-916. 2015. https://info.agriculture.gouv.fr/gedei/site/bo-agri/instruction-2015-916. Accessed 17 Jan 2018.
Gubbins S, Richardson J, Baylis M, Wilson AJ, Abrahantes JC. Modelling the continental-scale spread of Schmallenberg virus in Europe: approaches and challenges. Prev Vet Med. 2014;116:404–11.
Turner J, Bowers RG, Baylis M. Modelling bluetongue virus transmission between farms using animal and vector movements. Sci Rep. 2012;2:319.
Delécolle J-C. Nouvelle contribution à lʼétude systématique et iconographique des espèces du genre Culicoides, (Diptéra: Cératopogonidae) du Nord-Est de la France. Ph.D Thesis, Université Louis Pasteur de Strasbourg I, Strasbourg. 1985.
Mathieu B, Cêtre-Sossah C, Garros C, Chavernac D, Balenghien T, Carpenter S, et al. Development and validation of IIKC: an interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasit Vectors. 2012;5:137.
European Centre for Disease Prevention and Control and European Food Safety Authority. The importance of vector abundance and seasonality—results from an expert consultation. Stockholm: European Food Safety Authority; 2018.
Crambes C, Kneip A, Sarda P. Smoothing splines estimators for functional linear regression. Ann Stat. 2009;37:35–72.
Roger B, Nicholas L-K. maptools: tools for reading and handling spatial objects. 2017. https://CRAN.R-project.org/package=maptools. Accessed 20 Mar 2019.
Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94:557–92.
Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561.
Kaufmann C, Steinmann IC, Hegglin D, Schaffner F, Mathis A. Spatio-temporal occurrence of Culicoides biting midges in the climatic regions of Switzerland, along with large scale species identification by MALDI-TOF mass spectrometry. Parasit Vectors. 2012;5:246.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015.
Hallman J. tis: Time indexes and time indexed series. 2017. https://CRAN.R-project.org/package=tis. Accessed 20 Mar 2019.
Charron MVP, Kluiters G, Langlais M, Seegers H, Baylis M, Ezanno P. Seasonal and spatial heterogeneities in host and vector abundances impact the spatiotemporal spread of bluetongue. Vet Res. 2013;44:44.
Charron MVP, Seegers H, Langlais M, Ezanno P. Seasonal spread and control of Bluetongue in cattle. J Theor Biol. 2011;291:1–9.
Sanders CJ, Shortall CR, Gubbins S, Burgin L, Gloster J, Harrington R, et al. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J Appl Ecol. 2011;48:1355–64.
White SM, Sanders CJ, Shortall CR, Purse BV. Mechanistic model for predicting the seasonal abundance of Culicoides biting midges and the impacts of insecticide control. Parasit Vectors. 2017;10:162.
Verhoef FA, Venter GJ, Weldon CW. Thermal limits of two biting midges, Culicoides imicola Kieffer and C. bolitinos Meiswinkel (Diptera: Ceratopogonidae). Parasit Vectors. 2014;7:384.
Lühken R, Steinke S, Hoppe N, Kiel E. Effects of temperature and photoperiod on the development of overwintering immature Culicoides chiopterus and C. dewulfi. Vet Parasitol. 2015;214:195–9.
Purse BV, Carpenter S, Venter GJ, Bellis G, Mullens BA. Bionomics of temperate and tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu Rev Entomol. 2015;60:373–92.
Caracappa S, Torina A, Guercio A, Vitale F, Calabrò A, Purpari G, et al. Identification of a novel bluetongue virus vector species of Culicoides in Sicily. Vet Rec. 2003;153:71–4.
De Liberato C, Scavia G, Lorenzetti R, Scaramozzino P, Amaddeo D, Cardeti G, et al. Identification of Culicoides obsoletus (Diptera: Ceratopogonidae) as a vector of bluetongue virus in central Italy. Vet Rec. 2005;156:301–4.
Mellor PS, Pitzolis G. Observations on breeding sites and light-trap collections of Culicoides during an outbreak of bluetongue in Cyprus. Bull Entomol Res. 1979;69:229–34.
Romon P, Higuera M, Delecolle JC, Baldet T, Aduriz G, Goldarazena A. Phenology and attraction of potential Culicoides vectors of bluetongue virus in Basque Country (northern Spain). Vet Parasitol. 2012;186:415–24.
Savini G, Goffredo M, Monaco F, Di Gennaro A, de Santis P, Meiswinkel R, et al. The isolation of bluetongue virus from field populations of the Obsoletus Complex in central Italy. Vet Ital. 2004;40:286–91.
Torina A, Caracappa S, Mellor PS, Baylis M, Purse BV. Spatial distribution of bluetongue virus and its Culicoides vectors in Sicily. Med Vet Entomol. 2004;18:81–9.
Dijkstra E, Ven I, Meiswinkel R, Hölzel DR, Rijn PA. Culicoides chiopterus as a potential vector of bluetongue virus in Europe. Vet Rec. 2008;162:422.
Meiswinkel R, Rijn PA, Leijs P, Goffredo M. Potential new Culicoides vector of bluetongue virus in northern Europe. Vet Rec. 2007;161:564–5.
Venail R, Balenghien T, Guis H, Tran A, Setier-Rio M-L, Delecolle JC, et al. Assessing diversity and abundance of vector populations at a national scale: example of Culicoides surveillance in France after bluetongue virus emergence. In: Mehlhorn H, editor. Arthropods as vectors of emerging diseases. Heidelberg: Springer; 2012. p. 77–102.
Carpenter S, McArthur C, Selby R, Ward R, Nolan DV, Luntz AJM, et al. Experimental infection studies of UK Culicoides species midges with bluetongue virus serotypes 8 and 9. Vet Rec. 2008;163:589–92.
Du Toit RM. The transmission of blue-tongue and horse-sickness by Culicoides. Onderstepoort J Vet Res. 1944;19:7–16.
Mellor PS, Boorman J, Baylis M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol. 2000;45:307–40.
Goffredo M, Catalani M, Federici V, Portanti O, Marini V, Mancini G, et al. Vector species of Culicoides midges implicated in the 2012–2014 bluetongue epidemics in Italy. Vet Ital. 2015;51:131–8.
Carpenter S, Veronesi E, Mullens B, Venter G. Vector competence of Culicoides for arboviruses: three major periods of research, their influence on current studies and future directions. Rev Sci Tech. 2015;34:97–112.
Balenghien T, Cêtre-Sossah C, Delécolle J-C, Mathieu B, Thomas B, et al. Culicoides chiopterus: confirmation of its status as potential vector of bluetongue virus in Europe. 2008. http://www.promedmail.org/direct.php?id=20080403.1222. Accessed 2 Feb 2019.
Hoffmann B, Bauer B, Bauer C, Joachim Bätza H, Beer M, Clausen P-H, et al. Monitoring of putative vectors of bluetongue virus serotype 8, Germany. Emerg Infect Dis. 2009;15:1481–4.
Meiswinkel R, Gomulski L, Delécolle JC, Goffredo M, Gasperi G. Taxonomy of Culicoides vector complexes—unfinished business. Vet Ital. 2003;40:151–9.
Mellor PS. The replication of bluetongue virus in Culicoides vectors. Curr Top Microbiol Immunol. 1990;162:143–61.
Savini G, Goffredo M, Monaco F, Di Gennaro A, Cafiero MA, Baldi L, et al. Bluetongue virus isolations from midges belonging to the obsoletus complex (Culicoides, Diptera: Ceratopogonidae) in Italy. Vet Rec. 2005;157:133–9.
Mehlhorn H, Walldorf V, Klimpel S, Jahn B, Jaeger F, Eschweiler J, et al. First occurrence of Culicoides obsoletus-transmitted bluetongue virus epidemic in central Europe. Parasitol Res. 2007;101:219–28.
Vanbinst T, Vandenbussche F, Vandemeulebroucke E, De Leeuw I, Deblauwe I, De Deken G, et al. Bluetongue virus detection by real-time rt-pcr in Culicoides captured during the 2006 epizootic in Belgium and development of an internal control. Transbound Emerg Dis. 2009;56:170–7.
Narladkar BW, Shivpuje PR. Prevalence, population dynamics and host preferences of Culicoides spp. (Diptera: Ceratopogonidae) of livestock in Marathwada region of Maharashtra State. Vet World. 2014;7:18.
Braverman Y, Linley JR. Fecundity and proportions of gravid females in populations of the bluetongue vector Culicoides imicola (Diptera: Ceratopogonidae) and several other species in Israel. J Med Entomol. 1994;31:838–43.
Venter GJ, Nevill EM, Van Der Linde TC. Seasonal abundance and parity of stock-associated Culicoides species (Diptera: Ceratopogonidae) in different climatic regions in southern Africa in relation to their viral vector potential. Onderstepoort J Vet Res. 1997;64:259–71.
Searle KR, Barber J, Stubbins F, Labuschagne K, Carpenter S, Butler A, et al. Environmental drivers of Culicoides phenology: how important is species-specific variation when determining disease policy? PLoS ONE. 2014;9:e111876.
EFSA Panel on Animal Health and Welfare. Bluetongue: control, surveillance and safe movement of animals. EFSA J. 2017;15:4698.
Acknowledgements
The authors thank the National Entomological Surveillance System for collecting the entomological data (2009–2012), the French Ministry of Agriculture and Food for funding this collection and providing the data, and CIRAD, EID-Med and IPPTS with the support of the Directions Départementales de la Protection des Populations for implementing this collection network. The authors thank all persons involved in this network and especially those in charge of the insect identification: Claire Garros, Xavier Allène, Ignace Rakotarivony, Jonathan Lhoir (CIRAD), Jean-Claude Delécolle, Delphine Delécolle, Bruno Mathieu (IPPTS), Marie-Laure Setier-Rio, Bethsabée Scheid and Roger Venail (EID-Med).
Funding
This study was funded by the French Agricultural Research and International Cooperation Organization (CIRAD) as well as the French Agency for Food, Environmental and Occupational Health & Safety (ANSES). Facundo Muñoz’s research is partially supported by research grant MTM2016-77501-P from the State Research Agency of the spanish Ministry of Science, Innovation and Universities jointly with the European Regional Development Fund, FEDER.
Author information
Authors and Affiliations
Contributions
PV and VH conceived the study. TBald, TBale and RL provided expertise about the entomological surveillance system and about Culicoides data. PV conducted statistical analyses and FM, VH and TBale provided statistical support. All authors contributed to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Text S1.
Culicoides abundance using spatial units alternative to vector zones. Figure S1. Iso-hygro-thermal partitioning of mainland France. Figure S2. Distribution of minimum, maximum and average fortnight temperature and average specific humidity in each iso-hygro-thermal zone in mainland France. For each zone (cluster), the solid line represents the median value and dashed-lines the first and third quartiles of the distribution. Figure S3. Predicted Culicoides abundance in mainland France with no partitioning from the model. Figure S4. Predicted Culicoides abundance for each iso-hygro-thermal zone from the model in mainland France. Figure S5. ROC curves for the three spatial scales. Figure S6. Boxplot and distribution of the proportion of observed values within the predicted confidence interval for the three spatial scales.
Additional file 2: Table S1.
Values (median and interquartile range) of the mean absolute error (MAE) and root mean square error (RMSE) for each vector zone.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Villard, P., Muñoz, F., Balenghien, T. et al. Modeling Culicoides abundance in mainland France: implications for surveillance. Parasites Vectors 12, 391 (2019). https://doi.org/10.1186/s13071-019-3642-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3642-1