Abstract
Background
In Lithuania, the first case of canine subcutaneous dirofilariosis was recorded in 2010. Since then, an increasing number of cases of canine dirofilariosis have been documented in different veterinary clinics throughout the country. Human dirofilariosis was diagnosed in Lithuania for the first time in September 2011. However, to the authors’ knowledge, there are no published data on the presence and prevalence of autochthonous dirofilariosis in dogs and humans in the country. The present study provides information about the predominant species and prevalence of Dirofilaria in dogs and describes the cases of human dirofilariosis in Lithuania. It also outlines PCR detection of the bacterial endosymbiont Wolbachia that contributes to the inflammatory features of filarioid infection.
Results
A total of 2280 blood samples and six adult worms from pet and shelter dogs were collected in the central and eastern regions of Lithuania in 2013–2015. Based on their morphological appearance, morphometric measurements and molecular analysis, all the adult nematodes were identified as Dirofilaria repens. The diagnosis of microfilariae in blood samples was based on blood smear analysis and Knott’s test. The PCR and sequence analysis of the ribosomal DNA ITS2 region and cox1 gene confirmed the presence of D. repens. Overall, 61 (2.7%) of the 2280 blood samples were found to be positive for the presence of D. repens. The infection rate of D. repens was significantly higher in shelter dogs (19.0%; 19/100) than in pet dogs (1.9%; 42/2180) (χ2 = 100.039, df = 1, P < 0.0001). Forty-nine DNA samples of D. repens-infected dogs were tested for the presence of the bacterial endosymbiont Wolbachia and, of these, 40 samples (81.6%) were found to be positive. Three ocular and six subcutaneous cases of human dirofilariosis were diagnosed in Lithuania in the period 2011–2018.
Conclusions
To the authors’ knowledge, this is the first report of autochthonous D. repens infection in dogs and humans in Lithuania. The present data demonstrate that D. repens is the main etiological agent of dirofilariosis in Lithuania. The DNA of the filarioid endosymbiotic bacterium Wolbachia was detected in the vast majority of dogs infected with D. repens.
Similar content being viewed by others
Background
Dirofilariosis is an emerging vector-borne parasitic zoonotic infection caused by nematodes of the genus Dirofilaria. The parasites are transmitted by a large variety of mosquito species belonging to the Culicidae family, including some species of the Lithuanian mosquito fauna, i.e. Culex pipiens (s.l.), Anopheles maculipennis (s.l.) and Aedes vexans [1,2,3]. Carnivores are the definitive hosts. The majority of cases in humans and animals are caused by two Dirofilaria species, Dirofilaria repens and Dirofilaria immitis, both of which can infect numerous mammalian species [4].
Among mammalian hosts, domesticated dogs (Canis familiaris) function as reservoirs and are the most important source for human transmission of both species [4]. Dirofilariosis caused by D. repens and D. immitis has been also reported in wild carnivores, e.g. wolves (Canis lupus), red foxes (Vulpes vulpes) and golden jackals (Canis aureus) [5,6,7].
The life-cycle of D. repens comprises five larval stages. Adult D. repens worms in dogs produce the first-stage larvae (microfilariae) and release them into the bloodstream. Only mature helminths of the genus Dirofilaria can produce microfilariae [4].
Canine subcutaneous dirofilariosis (D. repens) is often considered asymptomatic, although in some cases the parasites cause subcutaneous nodules, while circulating microfilariae cause dermatological signs such as pruritus, erythema, alopecia, diffused dermatitis and itching [8].
The traditional picture of human dirofilariosis caused by D. repens is associated with subcutaneous nodules and ocular locations. However, there have been some reports of human dirofilariosis in unusual sites. Parasites have been found in the lungs, scrotum, penis, spermatic cord, epididymis and female mammary glands [9, 10]. In most human cases of ocular dirofilariosis caused by D. repens, the parasites have been found in nodules or cysts in the eye or in periocular tissues [10]. Only a few cases of humans showing circulating microfilariae have been reported. Due to incomplete development, humans are not suitable hosts for disease transmission [11,12,13].
Many filarioid nematode species harbour intracellular bacteria of the genus Wolbachia (Rickettsiaceae) [14]. Wolbachia is found in all filarioid life stages and is essential for embryogenesis, normal development, fertility and the long-term survival of the adult worm [15,16,17]. Wolbachia is released into the blood and interacts with host tissues when adult worms and microfilariae die, stimulating inflammation and inducing immune responses [16, 18].
In Lithuania, the first case of canine dirofilariosis was recorded in 2010 in the small animal clinic of the Veterinary Academy in Kaunas in central Lithuania [19]. Since then, a growing number of canine dirofilariosis cases have been documented in different veterinary clinics throughout the country. Human dirofilariosis was diagnosed in Lithuania for the first time in September 2011. However, to the authors’ knowledge, no data have been published about the presence and prevalence of autochthonous D. repens infection in dogs and humans in Lithuania. Therefore, the aim of the present study was to determine the predominant species and prevalence rate of dirofilariosis in dogs and to describe the cases of human dirofilariosis in Lithuania. A further objective of this work was to perform molecular detection of the bacterial endosymbiont Wolbachia, which plays an important role in D. repens biology and contributes to the inflammatory pathology of the infection.
Methods
Sample collection
Samples collected from dogs
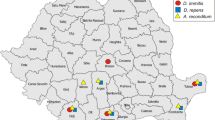
In co-operation with veterinary clinics and animal shelters in Kaunas (central Lithuania) and Vilnius (eastern Lithuania), a total of 2280 dogs (2180 pet dogs and 100 shelter dogs) were investigated for the presence of microfilariae in their blood in 2013–2015. None of the dogs examined had been imported from endemic countries or had ever travelled outside Lithuania. Dogs younger than 6 months were excluded due to the long life-cycle of Dirofilaria. The sex, age and body size of the shelter dogs were recorded.
The shelter and pet dog blood samples were taken from the cephalic vein, collected in EDTA-containing vacutainers and then stored at 4 °C or -20 °C until DNA isolation.
The microfilariae were detected based on blood smear microscopy and the modified Knott’s test. PCR and sequence analysis were applied in order to confirm diagnosis and molecularly characterise the Dirofilaria species.
In six dogs presented in small animal clinics, the adult worms were removed from skin nodules using a surgical technique. Adult nematodes were identified by morphological and molecular methods.
Samples collected from humans
Data on human dirofilariosis cases (patients’ travel history and other anamnestic data) were collected from the National Public Health Surveillance Laboratory (NVSPL), where each of the nine human dirofilariosis cases during the period 2011–2018 had been registered. Adult worms were removed from human patients during surgical procedures by ophthalmologists and surgeons in different areas of Lithuania. Helminths were subsequently sent to the NVSPL for further investigation. Species identification was undertaken using morphological and morphometric analysis. To confirm the diagnosis in the first case (Patient no. 1; Table 1), helminths were sent for investigation to the Swiss Institute of Parasitology of the University of Zurich (SCUP).
Canine blood smear
After blood collection, a thin blood smear was immediately prepared. The slides were air-dried, fixed with methanol and stained with Giemsa stain. Slides were observed for microfilaria by light microscopy at 100× and 500× (oil immersion) magnification [20].
Modified Knott’s test
Briefly, 1 ml of EDTA blood was added to 9 ml of 2% formalin, mixed by inversion and centrifuged at 3000× g for 5 min. The supernatant was discarded. The sediment was mixed with 35 μl of 0.1% methylene blue and 20 μl of this mixture was observed using a light microscope [21].
Morphological identification of adult worms collected from dogs
The collected adult nematodes were measured and morphologically studied using light microscopy. The parasites were identified by an evaluation of the macroscopic and microscopic characteristics. About 1 cm of the nematode cephalic and caudal end was prepared with 50% glycerol for transparent slides. The middle parts of the nematodes were used for molecular identification. The length and the distance between the oral opening and vulva (for females) or the length of the left and right spicule (for males) of the nematodes were measured for morphological nematode identification [22].
Molecular detection
Molecular analysis was performed to differentiate between and accurately identify the filarioid species. DNA was isolated from 200 µl aliquots of EDTA blood using a GeneJet Whole Blood Genomic DNA Purification Kit (Thermo Fisher Scientific, Vilnius, Lithuania) according to the manufacturer’s instructions. DNA from mature helminths was extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
To identify filarioid species, conventional PCR and pan-filarial primers (DIDR-F1, DIDR-R1) were used that amplify fragments of different length of the internal transcribed spacer region 2 (ITS2) of the ribosomal DNA from six different filarioid species (D. repens, D. immitis, Acanthocheilonema reconditum, Acanthocheilonema dracunculoides, Brugia pahangi and Brugia malayi) [23]. The PCR was carried out as described by Rishniw et al. [23]. The identification was performed based on 484 bp fragments for D. repens. Blood samples positive for microfilaria and adult nematode samples were then verified with a D. repens-specific primer set (DR COI-F1/DR COI-R1) based on partial (209 bp) amplification of cytochrome oxidase subunit 1 (cox1) gene, as described by Rishniw et al. [23].
Wolbachia endosymbiont
Samples positive for Dirofilaria spp. nematodes in pet and shelter dogs were further analysed by PCR (primers 16SwolbF/16SwolbR3) of the 16S rRNA gene fragment of Wolbachia endosymbiont bacteria [24, 25] in 2017. The specific products obtained of 1018 bp were considered a positive result.
Sequence analysis
PCR products of D. repens (ITS2 region and cox1 gene) and positive Wolbachia (16S rRNA) samples were extracted from gel using a GeneJet Gel Extraction Kit (Thermo Fisher Scientific) and sent off for sequencing (Macrogen, Amsterdam, Netherlands). The DNA sequences obtained were analysed using the Mega software package v.6.05, and compared with the GenBank database, searching for similar sequences using BLAST. Phylogenetic trees were constructed using the maximum-likelihood (ML) and neighbour-joining (NJ) methods.
Sequences obtained from PCR products of the partial ITS2 (n = 1) region and cox1 (n = 3) gene of D. repens (derived from the blood samples of dogs and adult nematodes) and the partial 16S rRNA gene of Wolbachia (n = 2) were deposited in GenBank under accession numbers MH469230, MH469227-MH469229 and MK050782-MK050783, respectively.
Statistical analysis
Pearson’s Chi-square analysis was performed with D. repens infection in shelter dogs (0, negative; 1, positive) against independent variables, including sex, age (0.5–3 years; > 3–6 years; and > 6 years), and body size (small, ≤ 10 kg; medium-sized, > 10–25 kg; and large, > 25 kg) groups.
Statistical analysis was carried out using the statistical software IBM SPSS Statistics software v.23 for Windows (SPSS Inc., Chicago, IL, USA); P > 0.05 was considered insignificant.
Results
Overall, 61 (2.7%, 95% CI: 2.3–3.9%) of the 2280 blood samples from pet and shelter dogs were found to be positive for the presence of microfilariae. A total of 42 (1.9%, 95% CI: 1.5–2.8%) of the 2180 pet dog blood smears (Fig. 1a) were positive for microfilariae. Using the modified Knott’s method (Fig. 1b), microfilariae were found in 19 (19%, 95% CI: 11.8–27.4%) of the 100 shelter dogs. The infection rate of D. repens parasites was significantly higher in shelter dogs (19.0%) than in pet dogs (1.9%) (χ2 = 100.039, df = 1, P < 0.0001).
The shelter dogs were 0.5 to 18 years-old, with a median age of 5 years (IQR 2.0–10.0). Infected animals were identified in all age groups: 0.5–3 years, prevalence of 18.4% (7/38); > 3–6 years, prevalence of 9.1% (2/22); and > 6 years, prevalence of 25.0% (10/40). The infection rate in males was 22.2% (10/45) and 16.4% (9/55) in females. There were no significant differences in prevalence (P > 0.05) between shelter dogs of different sexes and ages. Additionally, dogs in the medium (20.8%; 15/72) and large size (33.3%, 2/6) dog groups were more frequently infected than small dogs (3.7%, 1/22); however, body size was not a statistically significant factor (χ2 = 3.165, df = 1, P = 0.106; χ2 = 4.084, df = 1, P = 0.107, respectively).
All measurements of adult worms removed from dogs (Fig. 2) were within the known range for D. repens. One male and five females were identified.
Human cases
Overall, nine cases of human dirofilariosis infection were observed between 2011 and 2018 in Lithuania (Table 1). In three cases worms were found in the ocular localisation, and in six cases in subcutaneous tissue (Fig. 3). Seven of the nine infected humans had not travelled outside Lithuania. However, two patients had travelled to endemic countries within the two years prior to diagnosis. All nine nematodes were identified as D. repens.
Subconjuctival localisation of a D. repens adult worm (arrow) in the human eye, patient no. 7 (Table 1)
Molecular and phylogenetic analysis
PCR amplification of the partial ITS2 region and cox1 gene confirmed the D. repens species in all the positive samples (62 blood and 6 adult worm samples). No other filarioid species were found during this study.
Ten randomly selected PCR products of D. repens for the ITS2 region and cox1 gene were purified and sequenced to confirm the PCR results. The sequence analysis of the partial ITS2 region showed that Lithuanian D. repens isolates derived from the blood of dogs were 100% identical to each other and to the corresponding D. repens sequence from Tunisia deposited in GenBank (KR676387) and shared 99% similarity (with one to three nucleotides difference) with D. repens isolates from the Czech Republic and India (Fig. 4).
The phylogenetic analysis of the partial cox1 gene for D. repens isolates from mosquitoes, dogs and humans revealed four different cox1 variants. Analysed sequences included three variable sites. Lithuanian D. repens cox1 sequences derived from canine blood and adult nematode samples were identical to each other and showed 99–100% similarity to other European strains of D. repens (GenBank: MG787424, KC985240, KR780980, KY828979, KR998258, AM749230 and MF045816) (Fig. 5). Wolbachia endobacteria were found to be positive for D. repens in 40 of the 49 samples (81.6%, 95% CI: 69.4–91.3%). Sequence analysis of the partial 16S rRNA gene showed that the obtained sequences were identical, displaying homology (100%) with sequences of Wolbachia endosymbiont of D. repens in Croatia (accession number KY114937) and Italy (accession number AJ276500). The phylogenetic analyses of 16S rRNA sequences confirmed that Wolbachia endosymbionts of Dirofilaria separated according to the host species (Fig. 6).
A phylogenetic tree of filarioid nematodes based on cox1 gene sequences created using the maximum likelihood method and bootstrap analysis of 1000 replicates. The representative sequences obtained in this study are marked with a black square (MH469227: representative of sequences obtained from canine blood; MH469228, MH469229: sequences obtained from two adult nematodes)
Neighbour-joining phylogenetic tree for the partial 16S rRNA gene of the Wolbachia endosymbiont. The phylogenetic tree was created using the Kimura 2-parameter model with a bootstrap analysis of 1000 replicates. Sequences with accession numbers were taken from GenBank for comparison. The identification source (host) and country codes are provided. Samples sequenced in the present study are marked with a black rhombus
Discussion
Until 2001, canine D. repens infection had only been reported in southern European countries [26]. In recent years, however, autochthonous cases of dirofilariosis in dogs caused by D. repens have been reported in previously non-endemic countries with the following prevalences: 13.0–49.2% in Serbia [27,28,29,30]; 11.7–37.5% in Poland [31,32,33,34]; 20.0–34.5% in Slovakia [35,36,37]; 4.3–20.5% in Romania [38,39,40,41]; 15.8% in Latvia [42]; 14.0% in Hungary [43]; 9.0% in the Czech Republic [44]; and 6.8% in Germany [45]. Autochthonous D. repens cases have also been recorded in the Netherlands [46], Austria [47, 48], Belarus [49] and Ukraine [50]. New D. repens infections in northernmost Europe, which are likely to be autochthonous, have been reported in Estonia [51] and Finland [52]. Climatic changes, global warming and the movement of dogs across Europe are the main factors influencing the continuing spread of Dirofilaria in European countries [11, 53]. The geographical distribution of dirofilariosis depends on the presence of the definitive host and appropriate vectors in the area. Environmental temperature is one of the most important abiotic factors influencing the survival of insects and allowing the development of Dirofilaria into the infective stage in the mosquito. Various species of the genera Aedes, Ochlerotatus, Culex, Culiseta and Anopheles are potential vectors of D. repens [16, 54] in Europe. Currently, 37 mosquito species are known to be present in Lithuania [1, 55]. Of these, Aedes vexans, Culiseta annulata, Culex pipiens, Anopheles maculipennis, Ochlerotatus caspius and Ochlerotatus excrucians are potential vectors for D. repens in Lithuania [16, 56,57,58]. To the best of the authors’ knowledge, there is no information about the prevalence of D. repens in Lithuanian mosquitoes, therefore further investigation is needed for accurate determination of competent vectors for D. repens in Lithuania. Environmental and climatic changes are now strongly influencing the activity patterns of mosquitoes in temperate areas, allowing more generations of vectors each year [54]. Furthermore, climate change and global warming are factors driving the disease vectors’ ability to invade new areas in which they could potentially transmit pathogens.
This study reports the prevalence and molecular characterisation of D. repens in dogs in the Baltic region (Lithuania). It is worth mentioning that the examined dogs had never travelled outside Lithuania. The overall prevalence of D. repens infection in pet and shelter dogs was 2.7%, with a significantly higher prevalence detected in shelter dogs (19.0%). A similar prevalence of infection among shelter dogs has been obtained in the neighbouring countries of Latvia (15.8%; 22/139) [42] and Poland (11.3%; 14/124) [59], while a lower incidence of D. repens infection has been detected in Italy (3.4%; 4/118) [60] and Romania (2.2%; 2/92) [39]. Several factors could explain the difference in the prevalence of infection in shelter and pet dogs detected in the present study. The detection method may affect the sensitivity of the parasite detection. Microfilariae in blood samples from the pet dogs were detected based on blood smear analysis, while in shelter dogs, Knott’s test was applied for parasite detection. Knott’s test is a more sensitive test because it concentrates the microfilaria, making it less likely to be missed during microscopic examination. Perhaps the most important factor is that shelter dogs are most often caught roaming free in streets and are in greater contact with vectors than dogs living in the human environment. Furthermore, shelter dogs do not usually receive prophylactic treatments and are therefore at a higher risk of infection. These findings suggest that shelter dogs may serve as major reservoirs for D. repens in urban areas in Lithuania.
Given that dogs are the most important source for human transmission of dirofilariosis [4], there should be stricter rules around the use of preventive measures and regulations for free-ranging dogs.
Human dirofilariosis has been diagnosed in five different areas in eastern, central, western and north-eastern Lithuania: Vilnius, Kaunas, Klaipėda, Ukmergė and Utena. Five human cases of autochthonous D. repens have been found in the central and eastern parts of the country (Kaunas, Vilnius) in areas where dirofilariosis has been diagnosed in dogs. A total of seven out of the nine people investigated had never travelled to endemic countries. These data show that human subcutaneous dirofilariosis is also endemic in Lithuania. Human Dirofilaria infections are sporadic in Lithuania and in neighbouring countries, where the first cases of dirofilariosis were noted at a similar time [61,62,63]. In the past few decades, the number of human infections in previously endemic countries has increased dramatically [9, 16, 50, 64]. The current epidemiological situation of dirofilariosis in Europe, including in the Baltic countries, suggests that previously non-endemic countries should expect an increase in human infections in future.
Both filarioid species (D. immitis and D. repens) require the same temperature and the same time interval for incubation in the same vector species under laboratory conditions [64, 65]. Recently reported autochthonous cases of heartworm infection in Central European countries [32, 44, 66, 67] and the documented imported case in the Baltic countries [68] demonstrate that veterinary practitioners should expect the expansion of life-threatening D. immitis in north-eastern Europe.
Arthropod-borne diseases are increasing in Lithuania [69, 70] and other European countries [71]. The samples examined in this study were also tested for the presence of tick-borne pathogens such as Anaplasma phagocytophilum and Babesia canis and possible co-infection with D. repens (data not shown). Out of 2180 blood samples from pet dogs, 3 (0.1%) were positive for A. phagocytophilum and 38 (1.7%) were positive for B. canis. Of 42 D. repens-positive samples, nine contained co-infection with B. canis and in one sample a triple-infection with D. repens, B. canis and A. phagocytophilum was detected. These findings suggest that co-infections with anaplasmosis and babesiosis in patients infected with Dirofilaria are suspected. Co-infected cases are complicated for practitioners, and may cause failures in diagnosis, treatment and prognosis [72,73,74].
More studies on Wolbachia have been carried out around the world on arthropods than on filarioids (family Onchocercidae) [17, 18, 75]. Based on the data thus far obtained, endosymbiontic Wolbachia has been detected in at least 28 filarioid species [17, 76]. To the authors’ knowledge, this is the first report on the detection of endosymbiontic Wolbachia bacteria in Lithuanian dogs infected with D. repens. Wolbachia DNA was detected in the blood of more than 80.0% of all the dogs infected with Dirofilaria. According to previous reports, Wolbachia endosymbionts are detected in 30.6–52.6% of dogs infected with D. repens or D. immitis in Europe [24, 77]. Meanwhile in the Asian part of Turkey, Wolbachia has been detected in just 6.2% of the tested dogs infected with D. immitis [78]. Phylogenetic analyses indicate that Wolbachia detected in D. repens and D. immitis represents one group [15, 73, 74, 79]. These studies will drive further investigations to improve understanding of the importance of symbiosis between Wolbachia and Dirofilaria. Wolbachia could therefore be used as a target of antibiotic therapy [16, 25, 79].
Conclusions
To the authors’ knowledge, this is the first report of autochthonous D. repens infection in dogs and humans in Lithuania. The present data extend the knowledge about the distribution of D. repens in shelter dogs and dogs living in the human environment and demonstrated that D. repens is the main etiological agent of dirofilariosis in Lithuania. The DNA of the filarioid endosymbiotic bacterium Wolbachia was detected in the vast majority of dogs infected with D. repens.
Abbreviations
- EDTA:
-
ethylenediamine tetraacetic acid
- ITS:
-
internal transcribed spacer
- PCR:
-
polymerase chain reaction
- 16S rDNA:
-
16S ribosomal RNA gene
- cox1:
-
cytochrome c oxidase subunit 1
References
Pakalniškis S, Bernotienė R, Lutovinovas E, Petrašiūnas A, Podėnas S, Rimšaitė J, et al. Checklist of Lithuanian Diptera. In: New and Rare for Lithuania Insect Species, vol. 18. Vilnius: Lithuanian Entomological Society; 2006.
Cancrini G, Magi M, Gabrielli S, Arispici M, Tolari F, Dell’Omodarme M, et al. Natural vectors of dirofilariasis in rural and urban areas of the Tuscan Region, central Italy. J Med Entomol. 2006;43:574–9.
Cancrini G, Pietrobelli M, Frangipane di Regalbono AF, Tampieri MP, della Torre A. Development of Dirofilaria and Setaria nematodes in Aedes albopictus. Parassitologia. 1995;37:141–5.
McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L. Heartworm disease in animals and humans. Adv Parasitol. 2008;66:193–285.
Mañas S, Ferrer D, Castellà J, López-Martín JM. Cardiopulmonary helminth parasites of red foxes (Vulpes vulpes) in Catalonia, northeastern Spain. Vet J. 2005;169:118–20.
Segovia JM, Torres J, Miquel J, Lianeza L, Feliu C. Helminths in the wolf, Canis lupus, from north-western Spain. J Helminthol. 2001;75:183–92.
Gortazar C, Castillo JA, Lucientes J, Blanco JC, Arriolabengoa A, Calvete C. Factors affecting Dirofilaria immitis prevalence in red foxes in northeastern Spain. J Wildl Dis. 1994;30:545–7.
Tarello W. Cutaneous lesions in dogs with Dirofilaria (Nochtiella) repens infestation and concurrent tick-borne transmitted diseases. Vet Dermatol. 2002;13:267–74.
Genchi C, Kramer LH, Rivasi F. Dirofilarial infections in Europe. Vector Borne Zoonotic Dis. 2011;11:1307–17.
Pampiglione S, Rivasi F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: an update of world literature from 1995 to 2000. Parassitologia. 2000;42:231–54.
Genchi C, Rinaldi L, Mortarino M, Genchi M, Cringoli G. Climate and Dirofilaria infection in Europe. Vet Parasitol. 2009;163:286–92.
Damle AS, Iravane Bajaj JA, Khaparkhuntikar MN, Maher GT, Patil RV. Microfilaria in human subcutaneous dirofilariasis: a case report. J Clin Diagn Res. 2014;8:113–4.
Petrocheilou V, Theodorakis M, Williams J, Prifti H, Georgilis K, Apostolopoulou I, et al. Microfilaremia from a Dirofilaria-like parasite in Greece. Case report. APMIS. 1998;106:315–8.
Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103.
Bandi C, Anderson TJC, Genchi C, Blaxter ML. Phylogeny of Wolbachia in filarial nematodes. Proc Biol Sci. 1998;265:2407–13.
Simón F, Siles-Lucas M, Morchón R, González-Miguel J, Mellado I, Carretón E, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev. 2012;25:507–44.
Lustigman S, Melnikow E, Anand SB, Contreras A, Nandi V, Liu J, et al. Potential involvement of Brugia malayi cysteine proteases in the maintenance of the endosymbiotic relationship with Wolbachia. Int J Parasitol Drugs Drug Resist. 2014;4:267–77.
Kozek WJ. What is new in the Wolbachia/Dirofilaria interaction? Vet Parasitol. 2005;133:127–32.
Jankauskaitė A, Pockevičius A, Petkevičius S. Roundworms in dog subcutaneous tissue in Lithuania - the first case. Vet Info. 2011;79:30–1 (In Lithuanian).
Rosenblatt JE. Laboratory diagnosis of infections due to blood and tissue parasites. Clin Infect Dis. 2009;49:1103–8.
Newton W, Wright WH. The occurrence of a dog filariid other than Dirofilaria immitis in the United States. J Parasitol. 1956;42:246–58.
Manfredi MT, Di Cerbo A, Genchi M. Biology of filarial worms parasitizing dogs and cats. In: Genchi C, Rinaldi L, Cringoli G, editors. Mappe parassitologiche: Dirofilaria immitis and D. repens in dog and cat and human infections. Naples: Rolando Editore; 2007. p. 39–45.
Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. 2006;135:303–14.
Landum M, Ferreira CC, Calado M, Alho AM, Maurício IL, Meireles JS, et al. Detection of Wolbachia in Dirofilaria infected dogs in Portugal. Vet Parasitol. 2014;204:407–10.
Foster JM, Kumar S, Ford L, Johnston KL, Ben R, Graeff-Teixeira C, Taylor MJ. Absence of Wolbachia endobacteria in the non-filariid nematodes Angiostrongylus cantonensis and A. costaricensis. Parasit Vectors. 2008;1:31.
Otranto D, Dantas-Torres F, Brianti E, Traversa D, Petrić D, Genchi C, Capelli G. Vector-borne helminths of dogs and humans in Europe. Parasit Vectors. 2013;6:16.
Tasić A, Rossi L, Tasić S, Miladinović-Tasić N, Ilić T, Dimitrijević S. Survey of canine dirofilariasis in Vojvodina, Serbia. Parasitol Res. 2008;103:1297–302.
Tasić A, Tasić-Otašević S, Gabrielli S, Miladinović-Tasić N, Ignjatović A, Đorđević J, et al. Canine Dirofilaria infections in two uninvestigated areas of Serbia: epidemiological and genetic aspects. Vector Borne Zoonotic Dis. 2012;12:1031–5.
Kosić LS, Simin S, Lalošević V, Lalošević D, Kuruca L, Nikolić S, et al. Updating the prevalence of canine dirofilariosis in pet dogs in Novi Sad, Vojvodina, Serbia. Contemp Agric/Savrem Poljopr. 2014;63:487–93.
Kosić LS, Lalošević V, Lalošević D, Simin S, Vasić I, Kuruca L. Prevalence of dirolirariosis in pet dogs in Novi Sad. Contemp Agric/Savrem Poljopr. 2012;61:247–54.
Demiaszkiewicz AW, Polańczyk G, Osińska B, Pyziel AM, Kuligowska I, Lachowicz J, et al. The prevalence and distribution of Dirofilaria repens in dogs in the Mazovian Province of central-eastern Poland. Ann Agric Environ Med. 2014;21:701–4.
Świątalska A, Demiaszkiewicz A. First autochthonous case of Dirofilaria immitis invasion in dog in Poland. Žycie Weterynaryjne. 2012;87:685–6 (In Polish).
Osińska B, Demiaszkiewicz AW, Pyziel AM, Kuligowska I, Lachowicz J, Dolka I. Prevalence of Dirofilaria repens in dogs in central-eastern Poland and histopathological changes caused by this infection. Bull Vet Inst Pulawy. 2014;58:35–9.
Demiaszkiewicz AW, Polańczyk G, Pyziel AM, Kuligowska I, Lachowicz J. The first foci of dirofilariosis of dogs evoked by Dirofilaria repens Railliet et Henry, 1911 in central Poland. Wiadomości Parazytol. 2009;55:367–70 (In Polish).
Miterpáková M, Antolová D, Hurníková Z, Dubinský P. Dirofilariosis in Slovakia - a new endemic area in central Europe. Helminthologia. 2008;45:20–3.
Iglódyová A, Miterpáková M, Hurníková Z, Antolová D, Dubinský P, Letková V. Canine dirofilariosis under specific environmental conditions of the Eastern Slovak Lowland. Ann Agric Environ Med. 2012;19:57–60.
Miterpáková M, Antolová D, Hurníková Z, Dubinský P, Pavlaĉka A, Németh J. Dirofilaria infections in working dogs in Slovakia. J Helminthol. 2010;84:173–6.
Ionică AM, Matei IA, Mircean V, Dumitrache MO, D’Amico G, Győrke A, et al. Current surveys on the prevalence and distribution of Dirofilaria spp. and Acanthocheilonema reconditum infections in dogs in Romania. Parasitol Res. 2015;114:975–82.
Ciocan R, Darăbuș G, Jascó O, Fok É. Detection of Dirofilaria spp. in dogs by PCR. Bull USAMV. 2010;67:40–4.
Ciocan R, Mederle N, Jacsó O, Tánczos B, Fok É. Autochthonous cases of Dirofilaria in dogs from Timiș County (western part) Romania. Glob J Med Res. 2013;13:29–34.
Ilie MS, Imre K, Hotea I, Darabuş G. Survey of canine dirofilariosis from south-western Romania - preliminary results. In: Grandi G, Kramer L, Genchi C, editors. Third European Dirofilaria Days, Parma, Italy, 2012. p 68.
Veksins A, Kruklite A, Keidane D, Houtana IM. Dirofilaria repens infection among dogs in Latvian animal shelters during 2013. In: Proceedings of Conference Research and Practice in Veterinary Medicine, 27–28 November; 2014. p. 118.
Fok E, Farkas R, Kiss G, Majoros G, Jacsó O, Gyurkovszky M. Preliminary results of an epidemiological survey on dirofilariosis of dogs and cats in Hungary. In: Genchi C, Rinaldi L, Cringoli G, editors. Mappe parassitologiche 8 - Dirofilaria immitis and D. repens in dog and cat and human infections. Naples: Rolando Editore; 2007. p. 195–6.
Svobodová Z, Svobodová V, Genchi C, Forejtek P. The first report of authochthonous dirofilariosis in dogs in the Czech Republic. Helminthologia. 2006;43:242–5.
Pantchev N, Norden N, Lorentzen L, Rossi M, Rossi U, Brand B, et al. Current surveys on the prevalence and distribution of Dirofilaria spp. in dogs in Germany. Parasitol Res. 2009;105(Suppl. 1):63–74.
Overgaauw P, van Dijk E. Autochthonous case of Dirofilaria repens in a dog in the Netherlands. Vet Rec. 2009;164:158.
Fuehrer HP, Auer H, Leschnik M, Silbermayr K, Duscher G, Joachim A. Dirofilaria in humans, dogs, and vectors in Austria (1978–2014) - from imported pathogens to the endemicity of Dirofilaria repens. PLoS Negl Trop Dis. 2016;10:e0004547.
Silbermayr K, Eigner B, Joachim A, Duscher GG, Seidel B, Allerberger F, et al. Autochthonous Dirofilaria repens in Austria. Parasit Vectors. 2014;7:226.
Șuleșco T, Volkova T, Yashkova S, Tomazatos A, von Thien H, Lühken R, et al. Detection of Dirofilaria repens and Dirofilaria immitis DNA in mosquitoes from Belarus. Parasitol Res. 2016;115:3535–41.
Sałamatin RV, Pavlikovska TM, Sagach OS, Nikolayenko SM, Kornyushin VV, Kharchenko VO, et al. Human dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: epidemiological report of 1465 cases. Acta Parasitol. 2013;58:592–8.
Jokelainen P, Mõtsküla PF, Heikkinen P, Ülevaino E, Oksanen A, Lassen B. Dirofilaria repens microfilaremia in three dogs in Estonia. Vector Borne Zoonotic Dis. 2016;16:136–8.
Pietikäinen R, Nordling S, Jokiranta S, Saari S, Heikkinen P, Gardiner C, et al. Dirofilaria repens transmission in southeastern Finland. Parasit Vectors. 2017;10:561.
Genchi C, Mortarino M, Rinaldi L, Cringoli G, Traldi G, Genchi M. Changing climate and changing vector-borne disease distribution: the example of Dirofilaria in Europe. Vet Parasitol. 2011;176:295–9.
Venco L. Heartworm (Dirofilaria immitis) disease in dogs. In: Genchi C, Rinaldi L, Cringoli G, editors. Mappe Parassitologiche 8 - Dirofilaria immitis and D. repens in dog and cat and human infections. Naples: Rolando Editore; 2007. p. 117–25.
Bernotienė R, Lučiūnaitė V. Mosquito (Diptera: Culicidae) species new for Lithuanian fauna. In: New and Rare for Lithuania Insect Species, vol. 23. Vilnius: Lithuanian Entomological Society; 2011.
Bocková E, Rudolf I, Kočišová A, Betášová L, Venclíková K, Mendel J, et al. Dirofilaria repens microfilariae in Aedes vexans mosquitoes in Slovakia. Parasitol Res. 2013;112:3465–70.
Zittra C, Kocziha Z, Pinnyei S, Harl J, Kieser K, Laciny A, et al. Screening blood-fed mosquitoes for the diagnosis of filarioid helminths and avian malaria. Parasit Vectors. 2015;8:16.
Rudolf I, Šebesta O, Mendel J, Betášová L, Bocková E, Jedličková P, et al. Zoonotic Dirofilaria repens (Nematoda: Filarioidea) in Aedes vexans mosquitoes, Czech Republic. Parasitol Res. 2014;113:4663–7.
Wróblewska P, Wilczyńska M, Zaleśny G. The evaluation of dirofilariosis among shelter dogs in Kraków. Ann Parasitol. 2016;62:140.
Giangaspero A, Marangi M, Latrofa MS, Martinelli D, Traversa D, Otranto D, et al. Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol Res. 2013;112:1357–61.
Cielecka D, Zarnowska-Prymek H, Masny A, Salamatin R, Wesołowska M, Gołab E. Human dirofilariosis in Poland: the first cases of autochthonous infections with Dirofilaria repens. Ann Agric Environ Med. 2012;19:445–50.
Zarnowska-Prymek H, Cielecka D, Salamatin R. Dirofilariasis-Dirofilaria repens-first time described in Polish patients. Przegl Epidemiol. 2008;62:547–51 (In Polish).
Melbarde-Gorkusa I, Abolins A, Strumfa I, Martinsons A, Gardovskis J. Human dirofilariasis in Latvia - the first case in surgical practice. Acta Chir Latv. 2011;11:172–4.
Genchi C, Kramer L. Subcutaneous dirofilariosis (Dirofilaria repens): an infection spreading throughout the old world. Parasit Vectors. 2017;10(Suppl. 2):517.
Webber WA, Hawking F. Experimental maintenance of Dirofilaria repens and D. immitis in dogs. Exp Parasitol. 1955;4:143–64.
Jacsó O, Mándoki M, Majoros G, Pétsch M, Mortarino M, Genchi C, et al. First autochthonous Dirofilaria immitis (Leidy, 1856) infection in a dog in Hungary. Helminthologia. 2009;46:159–61.
Svobodova V, Svobodova Z, Beladicova V, Valentova D. First cases of canine dirofilariosis in Slovakia: a case report. Vet Med (Praha). 2005;11:510–2.
Sabūnas V, Radzijevskaja J, Sakalauskas P, Paulauskas A. First report of heartworm (Dirofilaria immitis) infection in an imported dog in Lithuania. Helminthologia. 2018;1:1. https://doi.org/10.2478/helm-2018-0036.
Radzijevskaja J, Paulauskas A, Rosef O. Prevalence of Anaplasma phagocytophilum and Babesia divergens in Ixodes ricinus ticks from Lithuania and Norway. Int J Med Microbiol. 2008;298:218–21.
Paulauskas A, Radzijevskaja J, Karvelienø B, Grigonis A, Aleksandravičienø A, Zamokas G, et al. Detection and molecular characterization of canine babesiosis causative agent Babesia canis in the naturally infected dog in Lithuania. Vet Parasitol. 2014;205:702–6.
Beugnet F, Marié JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305.
Tuttle AD, Birkenheuer AJ, Juopperi T, Levy MG, Breitschwerdt EB. Concurrent bartonellosis and babesiosis in a dog with persistent thrombocytopenia. J Am Vet Med Assoc. 2003;223:1306–10.
Víchová B, Miterpáková M, Iglódyová A. Molecular detection of co-infections with Anaplasma phagocytophilum and/or Babesia canis canis in Dirofilaria-positive dogs from Slovakia. Vet Parasitol. 2014;203:167–72.
De Tommasi AS, Otranto D, Dantas-Torres F, Capelli G, Breitschwerdt EB, De Caprariis D. Are vector-borne pathogen co-infections complicating the clinical presentation in dogs? Parasit Vectors. 2013;6:97.
Lefoulon E, Bain O, Makepeace BL, d’Haese C, Uni S, Martin C, et al. Breakdown of coevolution between symbiotic bacteria Wolbachia and their filarial hosts. PeerJ. 2016;4:e1840.
Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol. 2005;60:245–84.
Tabar MD, Altet L, Martínez V, Roura X. Wolbachia, filariae and Leishmania coinfection in dogs from a Mediterranean area. J Small Anim Pract. 2013;54:174–8.
Simsek S, Ciftci AT. Serological and molecular detection of Dirofilaria species in stray dogs and investigation of Wolbachia DNA by PCR in Turkey. J Arthropod Borne Dis. 2016;10:445–53.
Bouchery T, Lefoulon E, Karadjian G, Nieguitsila A, Martin C. The symbiotic role of Wolbachia in onchocercidae and its impact on filariasis. Clin Microbiol Infect. 2013;19:131–40.
Acknowledgements
The authors are grateful to the veterinary and auxiliary staff at the “Lesė” and “Penkta koja” dog shelters and at the “Dr Leono Kriaučeliūno” and “Siaurio snauceris” (Kaunas) veterinary clinics for their collaboration.
Funding
This work was partly supported by the project “Promotion of Student Scientific Activities” (VP1-3.1-ŠMM-01-V-02-003) of the Research Council of Lithuania.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request. Representative sequences were submitted to the GenBank database under the accession numbers MH469230, MH469227-MH469229, MK050782 and MK050783.
Authors’ contributions
AP, JR and SP conceived and designed the research project. VS, PS and BK participated in the fieldwork and conducted the clinical examination and sample collection. VS, PS, JR and IL carried out the morphological identification of adult worms, blood smears and PCR reactions. JŽ was responsible for collecting data about human cases and helminth identification. VS, PS, JR, SP and AP wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by Research Council of Lithuania. All clinical procedures with the animals followed in this study were in accordance with Lithuanian (Republic of Lithuania Law on Welfare and Protection of Animals No. XI-2271) and European legislation for the protection of animals and met the International Guiding Principles for Biomedical Research Involving Animals by the Council for International Organizations of Medical Sciences. Human data were collected from the National Public Health Surveillance Laboratory (NVSPL).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sabūnas, V., Radzijevskaja, J., Sakalauskas, P. et al. Dirofilaria repens in dogs and humans in Lithuania. Parasites Vectors 12, 177 (2019). https://doi.org/10.1186/s13071-019-3406-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3406-y