Abstract
Background
The engagement of companion animal owners into the process of collecting epidemiological data can be facilitated through smartphone applications. In April 2018, the “tekenscanner“ (Dutch for tick scanner) app was launched with the aim of engaging pet owners and veterinarians to record ticks removed from their pets and submit these ticks for identification and pathogen testing. Tick-borne pathogens identified in ticks removed from dogs and cats during the first 6 months after the app was launched in the Netherlands are reported.
Methods
The tekenscanner app was used to record the geographical coordinates of ticks removed from dogs or cats onto a map of the Netherlands. A barcode was assigned to each tick for the easy tracking of each submission to our laboratory for taxonomic identification. Thereafter, DNA extracted from the ticks was PCR amplified, subjected to reverse line blot hybridization (RLB) and screened for a broad range of tick-borne pathogens. Results were added to the same app, usually within 2 weeks after the submission of each tick.
Results
The app was downloaded 5591 times and resulted in the collection of 1273 georeferenced and barcoded ticks, with a peak submission in May and June of 2018. There were 1005 ticks collected from 406 dogs and 268 ticks collected from 111 cats. Ixodes ricinus was the predominant species (90.0%), with all stages found on dogs as well as on cats. Ixodes hexagonus (7.3%) female and nymphal ticks were also identified on both hosts, whereas adults of Dermacentor reticulatus (2.4%) and Rhipicephalus sanguineus (0.2%) were exclusively found on dogs. Nearly 15% of the ticks recovered from dogs carried one or more pathogens, whereas 13.8% of the ticks removed from cats were infected. Ixodes ricinus collected from dogs contained Borrelia spp. (1.9%), Babesia spp. (0.7%), Anaplasma phagocytophilum (1.3%), “Candidatus Neoehrlichia mikurensis” (2.9%) and Rickettsia helvetica (7.3%). Ixodes ricinus recovered from cats were infected with Borrelia spp. (1.9%), Babesia spp. (0.4%), A. phagocytophilum (1.9%), “Ca. Neoehrlichia mikurensis” (2.6%) and R. helvetica (6.7%). Ixodes hexagonus ticks (n = 93) were not infected. Dermacentor reticulatus ticks, found only in autumn, were infected with Rickettsia raoultii (16 %) and A. phagocytophilum. Three R. sanguineus, on dogs from France and the USA imported into the Netherlands, were all negative.
Conclusions
The tekenscanner app is a versatile tool to use for submission of ticks and facilitated the fast feedback of test results. Community engagement through the app is suitable for identifying hotspots for ticks and tick-borne pathogens and provided an early warning system for exotic ticks invading the Netherlands.
Similar content being viewed by others
Background
Ticks (Acari: Ixodidae) are important vectors of a broad range of pathogens affecting both human and animal health worldwide [1]. For companion animals, the importance of ticks as vectors of Babesia, Borrelia, Anaplasma and Ehrlichia species has been well documented [2]. Moreover, changes in the epidemiology due to climate and tick habitat modifications as well as increasing host availability and movement of people with their companion animals, increases the importance of tick surveillance [3]. This has resulted in studies targeting ticks on companion animals in many areas throughout western Europe. For instance, surveillance of ticks from domestic dogs and/or cats has been conducted in nation-wide studies in the UK [4, 5], Belgium [6], Germany [7], Poland [8], as well as in a multinational European study including Hungary, France and Italy, Germany and Belgium [9].
In the Netherlands, monitoring of tick-borne diseases in dogs started in 2004 when outbreaks of autochthonous Babesia canis infections affected 23 dogs, including four fatal cases [10]. The prediction that the introduction of Dermacentor reticulatus ticks into the Netherlands may result in the establishment of permanent resident populations has become a reality. This was recently confirmed with the identification of novel foci of D. reticulatus in the Netherlands as well as in Belgium [11]. The outbreak of babesiosis in dogs was a starting point for a national campaign promoted by the Royal Netherlands Society of Veterinary Medicine (KNMvD). Over 200 veterinary clinics throughout the Netherlands were requested to submit ticks collected from their companion animal patients to our research centre. Information packages including brochures and collection tubes were widely distributed to facilitate the submission of ticks. From the results obtained with the first 4298 ticks collected in 2005 and 2006, it was concluded that a much broader spectrum of ticks and tick-borne pathogens, including several zoonotic pathogens, was present in the Netherlands than previously thought [12]. Over the past 10 years, veterinarians have continued submitting ticks removed from their companion animal patients and all of these ticks were taxonomically identified as a free service, but they were only tested by PCR upon request.
It is interesting to compare our approach with similar initiatives conducted in other parts of Europe. For instance, in the UK it was realized that systematic surveillances of tick-borne diseases in companion animals are not routinely undertaken [3]. As a result, a large-scale surveillance programme was initiated whereby more than 1000 veterinary practices were recruited through a media campaign, resulting in 6555 tick samples from infested dogs over a period of 16 weeks in 2015 [4]. As part of the same campaign, 278 veterinary practices submitted ticks removed from cats, which eventually resulted in the identification of a range of Babesia and Borrelia burgdorferi (sensu lato) species [13]. Another pet-owner-based survey was conducted in Switzerland, where dog owners in a rural town were sent postal requests to send ticks from their dogs and cats over 2 consecutive years. In total, 3003 ticks were received for identification which had been removed by the owners from 249 dogs and 117 cats [14].
Both examples are in line with our experiences, which indicate that active involvement of the companion animal owner and/or veterinary community is essential to conduct surveys on ticks and tick-borne diseases on companion animals. Here, we take it one step further by creating a much closer link between the citizen science community and testing laboratories. To facilitate this, we introduced a novel smartphone app to engage companion animal owners as well as veterinarians into active surveillance activities targeting ticks and tick-borne diseases in the Netherlands. This app, named “Tekenscanner” (Dutch for “Tick scanner”) was launched in April 2018 and the results of the first 6 months are presented here.
Methods
Study design
After downloading the Tekenscanner app, users were asked to create an account and enter the age, sex and breed of their pet into their account. The geographical coordinates of the location where the tick was removed from the dog or cat was recorded and plotted onto a map of the Netherlands. For the next step, each participant received a sample submission set containing a tick tube and a barcoded letter with instructions how to send the sample to our laboratory (UCTD). After arrival, the barcode was scanned and used to track each submission through eLabjournal (Groningen, The Netherlands), an electronic laboratory notebook wherein all test procedures and results were recorded for each tick. Prior to DNA extraction, each tick was identified using a binocular microscope with 80× magnification while consulting a recent taxonomic reference book wherein all European ticks have been described in detail [15]. Through the tekenscanner app, pet owners could submit ticks and receive feedback about tick identification and infection status within a very short time interval (usually less than 2 weeks).
DNA extraction
Ixodes ricinus ticks of the same stage (larvae/nymphs) and either males or females from the same host were pooled, with an average number of 4.5 ticks per pooled sample. All other ticks, such as I. hexagonus, D. reticulatus and R. sanguineus were tested individually. For DNA extraction, ticks were placed in sterile 2 ml microcentrifuge tubes containing 180 μl of lysis buffer and frozen at -20 °C. Thereafter, metal beads (5 mm in diameter) were added to the frozen samples, which were subsequently disrupted in a TissueLyser (Qiagen Benelux BV, Venlo, the Netherlands) at 50 Hz for 3 min. DNA was extracted from the triturated ticks using a GeneJet genomic DNA purification kit (Thermo Fisher Scientific, Landsmeer, the Netherlands) according to the manufacturer’s instructions. Extracted DNA was eluted in 150 μl of elution buffer, and either used directly or stored at -20 °C. After DNA extraction, DNA was PCR amplified and tested by reverse line blot hybridisation (RLB).
PCR
For Babesia/Theileria species PCR, the primer pair RLB-F2 (5′-GAC ACA GGG AGG TAG TGA CAA G-3′) and RLB-R2 (5′-biotin-CTA AGA ATT TCA CCT CTG ACA GT-3′) was used to amplify the V4 variable region of the 18S rRNA gene [16, 17]. The length of the PCR amplicon was 460 bp. For Anaplasma/Ehrlichia and Rickettsia PCR, the primer pair Ehr-F2 (5′-AGA GTT TGA TCC TGG CTC AG-3′) and Ehr-R2 (5′-biotin-GAG TTT GCC GGG ACT TYT TCT-3′) was used to amplify the V1 variable region of the 16S rRNA gene [18]. The length of the PCR amplicon was 460–500 bp. For Borrelia PCR, the primer pair Bor-F (5′-ACC ATA GAC TCT TAT TAC TTT GAC CA-3′) and Bor-R (5′-biotin-GAG AGT AGG TTA TTG GCC AGG G-3′) was used to amplify the 5S-23S rDNA spacer region gene [19]. The length of the PCR amplicon was 180–230 bp. Each PCR was performed in a total volume of 20 μl, containing 10 μl of 2× Phusion Hot Start High Fidelity Master Mix (Thermo Fisher Scientific), 0.5 μM of each primer, 2 μl of extracted genomic DNA and the remaining volume was double-distilled water. PCR primers were purchased from Life Technologies Europe BV, Bleiswijk, the Netherlands.
As positive controls, genomic DNA from B. canis, Babesia gibsoni, Ehrlichia canis, A. phagocytophilum and B. burgdorferi was used. Distilled water was used as negative control.
Reverse line blot (RLB) hybridization
Reverse Line Blot (RLB) hybridization assay has the advantage of being able to analyse multiple samples against multiple probes simultaneously, and it was first applied to differentiate tick-borne Borrelia species [19]. All probes used to differentiate Babesia, Theileria [20], Anaplasma and Ehrlichia [21] are listed in Table 1. Moreover, probes for the differentiation of Rickettsia species were also added to the membrane (Table 1) [22].
Oligonucleotide probes containing an N-terminal N-(trifluoracetamidohexyl-cyanoethyl,N,N-diisopropyl phosphoramidite [TFA])-C6 amino linker were synthesized by Thermo Fisher Scientific. Specific probes targeted 10 Babesia species. Furthermore, two catch-all Theileria/Babesia probes were included to capture possible unknown species or variants of species. In addition to one catch-all probe for Ehrlichia/Anaplasma, specific probes for E. canis, Ehrlichia ewingii, A. phagocytophilum, Anaplasma platys, and “Candidatus Neoehrlichia mikurensis” were also included.
For Borrelia species detection, B. burgdorferi (s.l.) was included as a catch-all probe together with specific probes for differentiating eight Borrelia species. Finally, Rickettsia conorii, R. helvetica, R. massiliae, R. raoultii, a catch-all probe for Rickettsia detection [22] plus a specific probe for “Candidatus Midichloria mitochondria” detection completed the membrane.
RLB hybridization was conducted as described previously [20]. In brief, a Biodyne C membrane was activated using 16% (wt/wv) 1-ethyl-3-(3-dimethyl-amino-propyl) carbodiimide (EDAC) (Carl Roth GmbH, Karlsruhe, Germany) for 10 min, after which the oligonucleotide probes were covalently linked to the membrane in 0.5 M NaHCO3 in a mini-blotter. Thereafter, the membrane was inactivated in 100 mM NaOH after washing in 2× SSPE/0.1% SDS at 60 °C and then stored in 20 mM EDTA, pH 8.0. For RBL assays, 10 µl of PCR product was added to 150 µl of 2× SSPE/0.1% SDS after denaturing at 100 °C for 10 min, followed by immediate cooling on ice. Denatured PCR products were subsequently hybridized to a Biodyne C membrane at 42 °C for 60 min. Thereafter, each membrane was washed twice in 2× SSPE/0.5% SDS at 50 °C for 10 min, incubated for 30 min at 42 °C in 2× SSPE/0.5% SDS with 5 µl of streptavidin-POD conjugate (Roche Diagnostic, Germany), again washed twice in 2× SSPE/0.5% SDS at 42 °C for 10 min, and finally washed twice in 2× SSPE for 5 min at room temperature. Hybridization detection was carried out by using chemiluminescence using Amersham ECL detection reagents [16].
Results
Tick collections
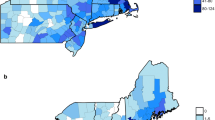
The app was downloaded 5591 times and resulted in the collection of 1273 georeferenced and barcoded ticks, with a peak submission in May and June 2018. A screenshot of the app is provided as an illustration in Fig. 1. There were 1004 ticks removed from 406 dogs and 268 ticks removed from 111 cats. Ixodes ricinus was the predominant species (90%), with all stages found on dogs as well as on cats. Ixodes hexagonus (7.3%) female and nymphal ticks were also identified on both hosts, whereas adults of D. reticulatus (2.4%) and R. sanguineus (0.2%) were exclusively found on dogs. Rhipicephalus sanguineus ticks were removed from dogs that had travelled in France and the USA. A distribution map of tick species recorded on dogs and cats based on postal codes of the Netherlands is presented in Fig. 2.
Pathogen detection
Almost 15% of the ticks recovered from dogs carried one or more pathogens, whereas 13.8% of the ticks removed from cats were infected. Ixodes ricinus collected from dogs contained Borrelia spp. (1.9%), Babesia spp. (0.7%), A. phagocytophilum (1.3%), “Ca. Neoehrlichia mikurensis” (2.9%) and R. helvetica (7.3%) (Table 2). Ixodes ricinus recovered from cats were infected with Borrelia spp. (1.9%), Babesia spp. (0.4%), A. phagocytophilum (1.9%), “Ca. Neoehrlichia mikurensis” (2.6%) and R. helvetica (6.7%). Ixodes hexagonus ticks (n = 93) collected primarily from cats, but also from dogs, all tested negative. Dermacentor reticulatus ticks were infected with R. raoultii (16%) and with A. phagocytophilum and were detected only in autumn (Table 3). Rhipicephalus sanguineus ticks were not infected (n = 4). Rickettsia helvetica was found in I. ricinus females together with B. venatorum, “Ca. Neoehrlichia mikurensis” or Borrelia species. Finally, seven I. ricinus ticks were recorded through the app by owners while travelling outside the Netherlands, e.g. in Belgium (4 ticks), Germany (1 tick), Italy (1 tick) and the Ukraine (1 tick). One tick from Italy was infected with “Ca. Neoehrlichia mikurensis”.
Discussion
Since the launch of the Tekenscanner app in April 2018, there were over 5000 downloads, which resulted in the collection of 1273 ticks. There were 1004 ticks removed from 406 dogs and 268 ticks from 111 cats. The lower number of ticks from cats versus dogs probably reflects differences in their behaviour and biology; however, there were no significant differences between the mean number of ticks collected from dogs versus cats (2.48 vs 2.41; P < 0.05).
Mapping predominant I. ricinus (90%) ticks together with I. hexagonus (7.3%) and D. reticulatus (2.4%) on a chart of the Netherlands divided into postal codes revealed specific clustering for D. reticulatus, whereas Ixodes ticks were much more widely distributed. However, this map is preliminary and requires improvement from more ticks collected during the forthcoming tick seasons (Fig. 2).
Importantly, D. reticulatus continues to broaden its distribution with novel locations since those already reported in 2015 [11] and 2016 [23]. New locations where dogs encountered these ticks remain to be surveyed to confirm the presence of significant populations of resident ticks in the vegetation. Although all D. reticulatus (n = 31) collected from dogs submitted thus far were negative for B. canis, the infection may be present in field ticks. Previously, ticks collected from novel foci were indeed found infected with B. canis, whereas all ticks removed from dogs visiting those foci tested negative [11].
It was found that nearly 15% of the ticks from dogs carried one or more pathogens, whereas 13.8% of the ticks from cats were infected. Almost 2% of I. ricinus collected from dogs (Table 2) and from cats (Table 3) contained six different Borrelia species. A similar diversity of Borrelia species was found in a previous study conducted in the Netherlands more than a decade ago, although the percentage of ticks (7.2%) harbouring spirochetes belonging to the B. burgdorferi (s.l.) group was higher [12]. Furthermore, B. microti and B. venatorum, two parasites with possible zoonotic implications, were detected in ticks derived from dogs and cats at a similar frequency as reported previously [12]. Finally, between 1–2% of ticks carried A. phagocytophilum in both studies (Tables 2, 3) [12].
In this study, I. ricinus (n = 1145) were tested in a pooled sample structure containing an average of 4.5 ticks, whereas in other studies ticks (n = 251) were previously tested individually [12]. Although the methods used in both studies differ, the results are quite similar. However, it is possible that pooling of ticks has masked additional infections that would have been detected if the ticks were tested individually. Since there is no standardized procedure, both approaches are justified, but direct comparison is limited. There are many other approaches used in the literature. For instance, Claerebout et al. [6] selected one tick (nymphs or adult) for DNA analysis, but when different tick species were present on the same host, one tick of each tick species was randomly selected for analysis. Another approach was followed by Geurden et al. [9] who pooled all ticks between one and 10 ticks of the same species.
It is interesting to note that despite the continuous challenge of companion animals by infected ticks, clinical cases of borreliosis, anaplasmosis and babesiosis are relatively rare. A thorough discussion of Lyme borreliosis in dogs and cats is beyond the scope of this paper. However, it is worth mentioning that there is much to gain by applying available serological and molecular tests combined with clinical observations and known infectious tick challenges as conducted in the UK [13] and elsewhere in Europe [24].
As far as canine anaplasmosis in the Netherlands is concerned, a recent study clearly demonstrated subclinical and clinical A. phagocytophilum infections in a pack of resident Rhodesian ridgeback dogs [25]. At least one additional clinical case with typical cytoplasmic inclusion bodies in circulating neutrophils was confirmed in a dog diagnosed in a veterinary clinic in The Hague in the Netherlands (F. Jongejan, unpublished data, 2015).
As far as Spotted Fever Group rickettsiae are concerned, R. helvetica was co-infecting I. ricinus female ticks together with B. venatorum, “Ca. Neoehrlichia mikurensis” or Borrelia species. Sixteen percent of D. reticulatus ticks collected from dogs in this study were infected with R. raoultii (Table 3), which is similar to 14% of those ticks reported positive in 2007 [12]. Likewise, “Ca. Neoehrlichia mikurensis” [26] has been identified in approximately 2–3% of all I. ricinus ticks in this study, confirming a similar percentage documented a decade ago [12].
Our current range of probes do encompass all Anaplasma, Ehrlichia and Borrelia species as well as all Babesia and Theileria species, and if DNA is amplified which does not hybridize with one of the species-specific probes, sequencing of the catch-all signal will determine whether there is a variant of an existing species or even a new species involved. This is key to RLB, which has resulted in the discovery of Babesia bicornis and Theileria bicornis [16]. Interestingly, clinical cases of Cytauxzoon have recently been reported in cats in several western European countries [27]. Moreover, Hepatozoon canis associated with the ingestion of ticks by dogs has very recently been reported from the UK [28]. New probes designed to facilitate parasite detection using RLB (Table 1) are currently expanded to include probes for the detection and differentiation of Cytauxzoon and Hepatozoon species. Screening of extracted DNA from ticks targeting those additional species is ongoing.
The role of companion animals in the dissemination of ticks and consequently possible tick-borne pathogens needs to be taken into further consideration. Seven I. ricinus ticks were recorded through the app by owners while travelling outside the Netherlands. This highlights the international travel of tick species with their hosts within Europe. Moreover, one of the R. sanguineus ticks that was reported through the app had entered the Netherlands on a dog from Texas, USA. In a comprehensive review, Fooks & Johnson [29] discussed the zoonotic risks of the international travel of pets and correctly mentions both R. sanguineus as well as D. reticulatus ticks which could possibly accompany these jet-set pets [29]. However, the possibility that the Asian longhorned tick, Haemaphysalis longicornis, could also have travelled on dogs from Asia and then been introduced into the USA was never contemplated. Now, this tick has already invaded nine different states in the USA [30, 31].
It is relevant to discuss here the possible scenarios with respect to the outbreaks of canine babesiosis, caused by B. canis, in southern England [32]. The probability that an asymptomatic dog entering into the UK, which subsequently infects a local population of D. reticulatus ticks is lower than that of a Babesia canis-infected Dermacentor reticulatus female tick being introduced by a dog. If the infected engorged tick drops into fertile soil, adults of the subsequent generation will readily transmit the potentially fatal infection to passing dogs. This is what most likely also happened in the outbreak of babesiosis in the Netherlands.
In any case, identification of ticks on companion animals is of prime importance. If this is done through the companion animal owner app, a link between a positive (introduced) tick and a potential patient can be established quickly. On the other hand, in most traditional surveys, this link is completely lost since usually ticks are tested years after they are collected.
Another example of the use of a smartphone app was recently evaluated for the prevention of tick bites in the Netherlands [33] and subsequently further analysed [34, 35]. It was concluded that this app facilitated an increase in public awareness, although the actual ticks were not identified and a link between people bitten by ticks and laboratories testing them was not established.
Importantly, in studies wherein tick surveillance depends on community engagement, there is a bias towards individuals who decide to participate versus those that discard the tick in disgust. Further public awareness on the usefulness of the approach through social media and rapid feedback of results are factors expected to increase the number of ticks reported in the upcoming tick seasons. Finally, the positive experience with the Tekenscanner app in the Netherlands has created opportunities to continue and launch the app as part of a coordinated European tick and tick-borne pathogen surveillance programme. This will include an early warning system for exotic ticks with the ultimate aim to improve control of ticks and associated diseases in companion animals.
Conclusions
The launch of the tekenscanner app stimulated companion animal owners to operate our tick and tick-borne pathogen surveillance programme. Feedback of the results into the app was formatted as a map of ticks in the Netherlands. The Dutch tick fauna is dominated by I. ricinus, which is prevalent throughout the country, whereas I. hexagonus is more restricted. Dermacentor reticulatus is continuing its spread into novel areas, which justifies year-round tick control measures, in particular, because adult D. reticulatus are active outside the regular tick season dominated by I. ricinus ticks. Our preliminary findings concur with those published a decade ago and confirm that a broad spectrum of tick-borne pathogens is established in the Netherlands, including several zoonotic pathogens.
Abbreviations
- RLB:
-
reverse line blot
- SDS:
-
sodiumdodecylsulfate
- PCR:
-
polymerase chain reaction
- Streptavidine-POD:
-
streptavidine-peroxidase
- SSPE:
-
sodium chloride-sodium phosphate-EDTA
References
Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl. 1):S3–14.
Beugnet F, Marié J-L. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305.
Wall R. Ectoparasites: future challenges in a changing world. Vet Parasitol. 2007;148:62–74.
Abdullah S, Helps C, Tasker S, Newbury H, Wall R. Ticks infesting domestic dogs in the UK: a large-scale surveillance programme. Parasit Vectors. 2016;9:391.
Duplan F, Davies S, Filler S, Abdullah S, Keyte S, Newbury H, et al. Anaplasma phagocytophilum, Bartonella spp., haemoplasma species and Hepatozoon spp. in ticks infesting cats: a large-scale survey. Parasit Vectors. 2018;11:201.
Claerebout E, Losson B, Cochez C, Casaert S, Dalemans A-C, De Cat A, et al. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit Vectors. 2013;6:183.
Schreiber C, Krücken J, Beck S, Maaz D, Pachnicke S, Krieger K, et al. Pathogens in ticks collected from dogs in Berlin Brandenburg, Germany. Parasit Vectors. 2014;7:535.
Król N, Obiegala A, Pfeffer M, Lonc E, Kiewra D. Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration, South-West Poland. Parasit Vectors. 2016;9:351.
Geurden T, Becskei C, Six RH, Maeder S, Latrofa MS, Otranto D, Farkas R. Detection of tick-borne pathogens in ticks from dogs and cats in different European countries. Ticks Tick Borne Dis. 2018;9:1431–6.
Matjila TP, Nijhof AM, Taoufik A, Houwers D, Teske E, Penzhorn BL, et al. Autochthonous canine babesiosis in the Netherlands. Vet Parasitol. 2005;131:239.
Jongejan F, Ringenier M, Putting M, Berger L, Burgers S, Kortekaas R, et al. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasit Vectors. 2015;8:232.
Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, et al. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007;7:585–95.
Davies S, Abdullah S, Helps C, Tasker S, Newbury H, Wall R. Prevalence of ticks and tick-borne pathogens: Babesia and Borrelia species in ticks infesting cats of Great Britain. Vet Parasitol. 2017;244:129–35.
Eichenberger RM, Deplazes P, Mathis A. Ticks on dogs and cats: a pet owner-based survey in a rural town in northeastern Switzerland. Ticks Tick Borne Dis. 2015;6:267–71.
Estrada-Peña A, Mihalca AD, Petney TN, editors. Ticks of Europe and North Africa. Cham: Springer International Publishing; 2017.
Nijhof AM, Penzhorn BL, Lynen G, Mollel JO, Morkel P, Bekker CPJ, Jongejan F. Babesia bicornis sp. nov. and Theileria bicornis sp. nov.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). J Clin Microbiol. 2003;41:2249–54.
Nijhof AM, Pillay V, Steyl J, Prozesky L, Stoltsz WH, Lawrence JA, et al. Molecular characterization of Theileria species associated with mortality in four species of African antelopes. J Clin Microbiol. 2005;43:5907–11.
Hailemariam Z, Rgen Krücken J, Baumann M, Ahmed JS, Clausen P-H, Nijhof AM, et al. Molecular detection of tick-borne pathogens in cattle from southwestern Ethiopia. PLoS One. 2017;12:e0188248.
Rijpkema SGT, Molkenboer MJC, Schouls LM, Jongejan F, Schellekens JF. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–5.
Gubbels JM, de Vos AP, Van Der Weide M, Viseras J, Schouls LM, de Vries E, Jongejan F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J Clin Microbiol. 1999;37:1782–9.
Bekker CP, de Vos S, Taoufik A, Sparagano OA, Jongejan F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet Microbiol. 2002;89:223–38.
Olivieri E, Wijnveld M, Bonga M, Berger L, Manfredi MT, Veronesi F, Jongejan F. Transmission of Rickettsia raoultii and Rickettsia massiliae DNA by Dermacentor reticulatus and Rhipicephalus sanguineus (s.l.) ticks during artificial feeding. Parasit Vectors. 2018;11:494.
Hofmeester TR, van der Lei P-B, van Leeuwen AD, Sprong H, van Wieren SE. New foci of Haemaphysalis punctata and Dermacentor reticulatus in the Netherlands. Ticks Tick Borne Dis. 2016;7:367–70.
Pantchev N, Pluta S, Huisinga E, Nather S, Scheufelen M, Vrhovec MG, et al. Tick-borne diseases (borreliosis, anaplasmosis, babesiosis) in German and Austrian dogs: status quo and review of distribution, transmission, clinical findings, diagnostics and prophylaxis. Parasitol Res. 2015;114:19–54.
Hovius E, de Bruin A, Schouls L, Hovius J, Dekker N, Sprong H. A lifelong study of a pack Rhodesian ridgeback dogs reveals subclinical and clinical tick-borne Anaplasma phagocytophilum infections with possible reinfection or persistence. Parasit Vectors. 2018;11:238.
Wennerås C. Infections with the tick-borne bacterium “Candidatus Neoehrlichia mikurensis”. Clin Microbiol Infect. 2015;21:621–30.
Nentwig A, Meli ML, Schrack J, Reichler IM, Riond B, Gloor C, et al. First report of Cytauxzoon sp. infection in domestic cats in Switzerland: natural and transfusion-transmitted infections. Parasit Vectors. 2018;11:292.
Attipa C, Maguire D, Solano-Gallego L, Szladovits B, Barker EN, Farr A, et al. Hepatozoon canis in three imported dogs: a new tickborne disease reaching the United Kingdom. Vet Rec. 2018;183:716.
Fooks AR, Johnson N. Jet set pets: examining the zoonosis risk in animal import and travel across the European Union. Vet Med (Auckland, NZ). 2015;6:17–25.
Rainey T, Occi JL, Robbins RG, Egizi A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J Med Entomol. 2018;55:757–9.
Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, et al. Multistate infestation with the exotic disease-vector tick Haemaphysalis longicornis - United States, August 2017 - September 2018. MMWR Morb Mortal Wkly Rep. 2018;67:1310–3.
de Marco MDMF, Hernández-Triana LM, Phipps LP, Hansford K, Mitchell ES, Cull B, et al. Emergence of Babesia canis in southern England. Parasit Vectors. 2017;10:241.
Antonise-Kamp L, Beaujean DJMA, Crutzen R, van Steenbergen JE, Ruwaard D. Prevention of tick bites: an evaluation of a smartphone app. BMC Infect Dis. 2017;17:744.
Garcia-Martí I, Zurita-Milla R, van Vliet AJH, Takken W. Modelling and mapping tick dynamics using volunteered observations. Int J Health Geogr. 2017;16:41.
Garcia-Marti I, Zurita-Milla R, Harms MG, Swart A. Using volunteered observations to map human exposure to ticks. Sci Rep. 2018;8:15435.
Yisaschar-Mekuzas Y, Jaffe CL, Pastor J, Cardoso L, Baneth G. Identification of Babesia species infecting dogs using reverse line blot hybridization for six canine piroplasms, and evaluation of co-infection by other vector-borne pathogens. Vet Parasitol. 2013;191:367–73.
Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.
Matjila PT, Leisewitz ALL, Oosthuizen MC, Jongejan F, Penzhorn BL. Detection of a Theileria species in dogs in South Africa. Vet Parasitol. 2008;157:34–40.
Georges K, Loria GR, Riili S, Greco A, Caracappa S, Jongejan F, Sparagano O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet Parasitol. 2001;99:273–86.
Moustafa MAM, Taylor K, Nakao R, Shimozuru M, Sashika M, Rosà R, et al. Dynamics, co-infections and characteristics of zoonotic tick-borne pathogens in Hokkaido small mammals, Japan. Ticks Tick Borne Dis. 2016;7:922–8.
Matjila PT, Penzhorn BL, Bekker CPJ, Nijhof AM, Jongejan F. Confirmation of occurrence of Babesia canis vogeli in domestic dogs in South Africa. Vet Parasitol. 2004;122:119–25.
Schötta A-M, Wijnveld M, Stockinger H, Stanek G. Approaches for reverse line blot-based detection of microbial pathogens in Ixodes ricinus ticks collected in Austria and impact of the chosen method. Appl Environ Microbiol. 2017;83:174–8.
Gern L, Douet V, López Z, Rais O, Cadenas FM. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick Borne Dis. 2010;1:23–9.
Hovius JWR, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658.
Christova I, van de Pol J, Yazar S, Velo E, Schouls L. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and Spotted Fever Group rickettsiae in ticks from southeastern Europe. Eur J Clin Microbiol Infect Dis. 2003;22:535–42.
Hornok S, Mulvihill M, Szőke K, Gönczi E, Sulyok KM, Gyuranecz M, Hofmann-Lehmann R. Impact of a freeway on the dispersal of ticks and Ixodes ricinus-borne pathogens: forested resting areas may become Lyme disease hotspots. Acta Vet Hung. 2017;65:242–52.
Acknowledgements
Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th Companion Animal Vector-borne Diseases (CVBD) World Forum Symposium.
Funding
The “Tekenscanner” is an initiative funded by Bayer Animal Health (Benelux) and executed by the Utrecht Centre for Tick-borne Diseases (UCTD).
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The datasets used in this study, as well as the extracted DNA from the collected ticks, are available upon request.
Authors’ contributions
FJ and HH conceptualized the study. CIJ developed the “tekenscanner” app. LB identified the ticks and performed PCR analysis, whereas SJ, TV, LH and RB carried out the DNA extractions from the ticks and conducted the reserve line blot hybridization experiments. RB performed the molecular phylogenetic analysis of selected catchall samples. FJ wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Removal of ticks was done according to approved World Organization for Animal Health (OIE) international standards and was carried out with the consent of the dog owners.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jongejan, F., de Jong, S., Voskuilen, T. et al. “Tekenscanner”: a novel smartphone application for companion animal owners and veterinarians to engage in tick and tick-borne pathogen surveillance in the Netherlands. Parasites Vectors 12, 116 (2019). https://doi.org/10.1186/s13071-019-3373-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3373-3