Abstract
Background
Rickettsia species belonging to the spotted fever group (SFG) cause infections in humans, domestic animals and wildlife. At least ten SFG Rickettsia species are known to occur in China. However, the distribution of rickettsiae in ticks and fleas in the border region of northwestern China have not been systematically studied to date.
Results
A total of 982 ticks (Rhipicephalus turanicus, Dermacentor marginatus, D. nuttalli and Haemaphysalis punctata) and 5052 fleas (18 flea species from 14 species of wild mammals) were collected in ten and five counties, respectively, of Xinjiang Uygur Autonomous Region (northwestern China). Tick and flea species were identified according to morphological and molecular characteristics. Seven sets of primers for amplifying the 17-kDa antigen gene (17-kDa), citrate synthase gene (gltA), 16S rRNA gene (rrs), outer membrane protein A and B genes (ompA, ompB), surface cell antigen 1 gene (sca1) and PS120-protein encoding gene (gene D) were used to identify the species of rickettsiae. Nine Rickettsia species have been detected, seven of them in ticks: R. aeschlimannii, R. conorii, R. raoultii, Rickettsia sibirica, R. slovaca, R. massiliae and “Candidatus R. barbariae”. In addition, R. bellii and two genotypes of a rickettsia endosymbiont (phylogenetically in an ancestral position to R. bellii) have been detected from flea pools.
Conclusions
This study provides molecular evidence for the occurrence of several SFG rickettsiae in Rhipicephalus turanicus, Dermacentor nuttalli and D. marginatus. Furthermore, R. bellii and two ancestral rickettsia endosymbionts are present in fleas infesting wild rodents in the border regions of northwestern China. These data extend our knowledge on the diversity of rickettsiae in Central Asia.
Similar content being viewed by others
Background
Rickettsiae are obligate intracellular Gram-negative bacteria causing infection in humans, domestic animals and wildlife [1, 2]. Their vectors are typically ticks, fleas or mites, but rickettsiae were also shown to be present in several other arthropod groups [3, 4]. There is a great variety of clinical presentations of rickettsioses, and some pathogenic species, which cause debilitating diseases, are listed as bioterrorism agents [5]. Members of the genus Rickettsia are divided into four clades: spotted fever group (SFG); typhus group (TG); ancestral group (AG); and transitional group (TRG) [6]. To date, ten valid Rickettsia species have been detected in China [7,8,9,10]. In previous studies, seven species of rickettsiae (including R. aeschlimannii, R. conorii, R. raoultii, Rickettsia sibirica, R. slovaca, R. massiliae and “Candidatus R. barbariae”) were shown to be present in ticks or fleas in Xinjiang Uygur Autonomous Region (XUAR) [7, 8].
There is a great diversity of tick and flea species in XUAR, owing to the variability of geographical landscape and the availability of multiple vertebrate host species for these parasites [11, 12]. Therefore, the aim of this study was to systematically analyze the occurrence of Rickettsia species in ticks and fleas in the border region of XUAR.
Methods
Study area and sample collection
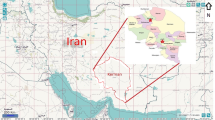
XUAR, located in northwestern China, covers 1.66 million square kilometers, and is bordered by eight countries (in the northeast Mongolia, northward the Russian Federation, in the northwest and west Kazakhstan, Kyrgyzstan, Tajikistan, and in the southwest Afghanistan, Pakistan and India) [13]. During the period between 2014 and 2016, late April to mid-May (coinciding with the peak activities of adult ticks), 982 adult ticks (675 males and 307 females) were collected from ten counties including Jimunai, Emin, Wenquan, Qapqal, Qinghe, Habahe, Wushi, Artux, Wuqia and Yecheng, while 5052 fleas were collected from Alataw, Burqin, Huocheng, Wenquan and Qapqal counties in the border area of XUAR (Fig. 1). The first six counties of tick sampling and the first three counties of flea sampling are located in the northern region, whereas the remaining counties are located in the southern part of XUAR. Jimunai, Emin, Wenquan, Qapqal, Artux and Huocheng counties are adjacent to Kazakhstan. Artux, Wushi and Wuqia counties are adjacent to Kyrgyzstan, Qinghe and Yecheng counties are neighboring on Mongolia and Pakistan, respectively, and Habahe county shares a border with Russia and Kazakhstan.
Ticks were collected from cattle and sheep after examining the entire body of each animal (including predilection sites such as ears, neck, armpits, thorax, abdomen, femurs, perianal region, etc.) [11, 14]. For flea sampling rodents were captured with Sherman traps (H.B. Sherman Traps, Tallahassee, Florida, USA), which were placed at the entrances of occupied burrows [15]. Each survey site included 150 traps that were checked twice a day. Each trap was removed before nightfall and replaced on the survey site the following day. The abundance and species of fleas were determined in each captured rodent. The animal fur was combed thoroughly until no additional fleas were recovered. After this, each rodent was released [16].
Morphological and molecular identification of ticks
All ticks were identified morphologically according to previous reports [17, 18]. To confirm tick species, 80 specimens (5–10 for each tick species representing every sampling county), were used for molecular taxonomic analysis. The genomic DNA was extracted from each tick individually, using the TIANamp Genomic DNA Kit (TIANGEN, Beijing, China). All 80 tick DNA extracts analyzed based on partial mitochondrial [12S rRNA, 16S rRNA and cytochrome c oxidase subunit 1 (cox1)] gene sequences [19, 20].
Morphological and molecular identification of fleas
Fleas were identified morphologically using a compound microscope and observing key structures [21, 22]. Depending on the flea species, host and sampling site, every 1–15 fleas were pooled together for DNA extraction by using Isolate II Genomic DNA Kit (BioLine, Sydney, Australia) as previously described [23]. The DNA extracts of 70 flea pools were included in multi-locus sequence analysis using four genes, i.e. the 18S ribosomal DNA (18S rDNA), 28S ribosomal DNA (28S rDNA), cox2 and elongation 1-alpha (EF-1a) as described previously [24].
Detection of rickettsial agents and sequence analysis
Seven sets of primers for amplifying the 17-kDa antigen gene (17-kDa), citrate synthase gene (gltA), 16S rRNA gene (rrs), outer membrane protein A and B genes (ompA, ompB), surface cell antigen 1 gene (sca1) and PS120-protein encoding gene (gene D) were used to identify the species of rickettsia [25,26,27]. An additional genetic marker 17-kDa2 was used to confirm the presence of the rickettsiae in fleas [28]. Sequence-confirmed rickettsia DNA amplified in our laboratory and double distilled water (Dongsheng, Guangzhou, China) were used as positive and negative controls, respectively. PCR products were purified using the TIANgel Midi Purification Kit (TIANGEN, Beijing, China) and cloned into the pGEM-T Easy vector and subjected to sequencing. A phylogenetic tree was constructed using the maximum-likelihood (ML) method with MEGA 6 software [29].
All sequences obtained in this study were compared with GenBank data using the nucleotide BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/). Representative sequences including 22 from ticks, 78 from fleas, 84 from rickettsiae in ticks and 12 from rickettsiae in fleas have been deposited in the GenBank database (accession numbers shown in Additional file 1: Table S1, sections A, B, C, D, respectively).
Results
A total of 982 ticks, belonging to three genera and four species (401 Rhipicephalus turanicus, 180 Dermacentor marginatus, 319 D. nuttalli and 82 Haemaphysalis punctata) were collected from ten counties of XUAR. Rhipicephalus turanicus (40.84%) was the most frequently collected species, followed by D. nuttalli (32.48%), D. marginatus (18.33%) and H. punctata (8.35%). Seven Rickettsia species including R. aeschlimannii, R. conorii, R. raoultii, Rickettsia sibirica, R. slovaca, R. massiliae and “Candidatus R. barbariae” were detected (Table 1, Fig. 2) in the ticks from cattle or sheep. “Candidatus R. barbariae” was mainly detected in the southern region, whereas R. raoultii was a dominant rickettsial agent in the northern region of XUAR.
Phylogenic analysis of Rickettsia species within ticks and fleas collected from border regions of northwestern China. The tree was constructed with the maximum likelihood (ML; bootstrap replicates: 1000) based on concatenated sequence data for gltA-rrs-17KDa-sca1-ompA-ompB-geneD genes with MEGA6.0. Sequences of the Rickettsia species from ticks and fleas obtained in this study are indicated by triangles and circles, respectively
In addition, a total of 5052 fleas, belonging to 6 families, 15 genera and 18 species were collected from 14 mammalian species, including Rhombomys opimus, Meriones meridianus, Meriones libycus, Meriones tamariscinus, Marmota baibacina, Vormela peregusna, etc. (Table 2). Among them, Xenopsylla gerbilli minax (52.40%) was the dominant species, followed by Paradoxopsyllus repandus (9.70%), Citellophilus tesquorum dzetysuensis (8.31%), Oropsylla silantiewi (7.52%) and Nosopsyllus laeviceps laeviceps (6.00%). Rickettsia bellii and two genotypes of a rickettsia endosymbiont (phylogenetically in an ancestral position to R. bellii) were molecularly detected in five flea species (Echidnophaga oschanin, Nosopsyllus laeviceps laeviceps, Paradoxopsyllus repandus, Rhadinopsylla cedestis and Xenopsylla gerbilli minax) of Alataw county. The BLAST analysis of rickettsial agents are shown in Additional file 2: Table S2.
Discussion
In this study, nine Rickettsia species have been molecularly detected in 982 ticks and 5052 fleas collected in 13 border counties of XUAR, neighboring Kyrgyzstan, Mongolia, Pakistan, Russia and Kazakhstan. Considering ticks, R. massiliae, R. aeschlimannii and “Candidatus R. barbariae” have been detected in Rh. turanicus, while R. raoultii, R. slovaca and R. sibirica were found in D. marginatus and D. nuttalli. Based on these findings, several SFG Rickettsia species occur in highly abundant tick species in the border regions of XUAR. In particular, Rh. turanicus, D. marginatus and D. nuttalli might play key roles in the propagation of rickettsiae across country borders in the region. In addition, the present data revealed significant differences in the spectrum and prevalence of rickettsiae between the north and south XUAR, most likely as a consequence of variations in the abundance of corresponding vectors and reservoirs.
Rickettsia bellii was previously detected in members of the genera Dermacentor and Amblyomma, in which it also undergoes transovarial transmission [30]. This Rickettsia species can be cultured in mammalian cells and may cause disease in mammals [30]. Here, R. bellii is reported for the first time in China. Detections of R. bellii in Xenopsylla gerbilli minax, Echidnophaga oschanin and Paradoxopsyllus repandus fleas are also novel findings. More interestingly, two genotypes of a rickettsia endosymbiont (ancestral to R. bellii) have been identified for the first time in Rhadinopsylla cedestis and Nosopsyllus laeviceps laeviceps fleas. These results indicate that some flea species infesting wild rodents in the border regions might carry different, probably ancient Rickettsia species or genotypes. Therefore, these data extend our knowledge on the geographical distribution and reservoir spectrum of AG rickettsiae.
XUAR has a great variety of landscape and habitats, maintaining a broad range of mammalian and avian species, which could serve as hosts of diverse tick and flea species. In this study, seven SFG and two AG Rickettsia species have been detected in border regions of northwest China. Surveillances of rickettsial agents in border regions of Central Asia are particularly useful and informative, because data from such monitoring studies are relevant to several countries, which are involved in international trade of livestock and livestock products, but may also be affected (in the context of rickettsioses) by movements of wildlife and migratory birds [11, 31].
Conclusions
In this study, nine Rickettsia species were molecularly detected in 982 ticks and 5052 fleas in the border regions of XUAR, northwestern China. The data indicate the occurrence of several SFG rickettsiae in Rh. turanicus, D. nuttalli and D. marginatus collected from ruminants, as well as of AG rickettsiae in fleas infesting wild rodents. These data extend our knowledge on the diversity of and potential vector/reservoir range of rickettsiae in central Asia.
Abbreviations
- 17-kDa :
-
17-kDa antigen
- cox1:
-
cytochrome c oxidase subunit 1
- gene D:
-
PS120-proteinencoding gene
- gltA:
-
citrate synthase
- ompA:
-
outer membrane proteins A
- ompB:
-
outer membrane proteins B
- rrs :
-
16S rRNA gene
- sca1:
-
cell surface antigen 1
- SFG:
-
spotted fever group
- XUAR:
-
Xinjiang Uygur Autonomous Region
References
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702.
Maina AN, Jiang J, Omulo SA, Cutler SJ, Ade F, Ogola E, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: implications for human health. Vector Borne Zoonotic Dis. 2014;14:693–702.
Oberoi A, Singh N. Rickettsiae infections-classification. Jk Sci J Med Edu Res. 2010;12:57.
Azad AF, Webb L, Carl M, Dasch GA. Detection of rickettsiae in arthropod vectors by DNA amplification using the polymerase chain reaction. Ann N Y Acad Sci. 1990;590:557–63.
Azad AF, Radulovic S. Pathogenic rickettsiae as bioterrorism agents. Ann N Y Acad Sci. 2003;990:734–8.
Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, et al. Plasmids and rickettsial evolution: insight from Rickettsia felis. PLoS One. 2013;2:e266.
Guo LP, Jiang SH, Liu D, Wang SW, Chen CF, Wang YZ. Emerging spotted fever group rickettsiae in ticks, northwestern China. Ticks Tick Borne Dis. 2016;7:1146–50.
Wei QQ, Guo LP, Wang AD, Mu LM, Zhang K, Chen CF, et al. The first detection of Rickettsia aeschlimannii and Rickettsia massiliae in Rhipicephalus turanicus ticks, in northwest China. Parasit Vectors. 2015;8:631.
Jia N, Zheng YC, Ma L, Huo QB, Ni XB, Jiang BG, et al. Human infections with Rickettsia raoultii, China. Emerg Infect Dis. 2014;20:866–8.
Li W, Liu L, Jiang X, Guo X, Garnier M, Raoult D, et al. Molecular identification of spotted fever group rickettsiae in ticks collected in central China. Clin Microbiol Infect. 2009;15(Suppl. 2):279–80.
Wang YZ, Mu LM, Zhang K, Yang MH, Zhang L, Du JY, et al. A broad-range survey of ticks from livestock in northern Xinjiang: changes in tick distribution and the isolation of Borrelia burgdorferi sensu stricto. Parasit Vectors. 2015;8:449.
Ye RY, Zhang JT, Yu X, Chen XR. Helminthes parasitized in fleas of Xinjiang, China. Acta Parasitol Med Entomol Sin. 1994;1:57–60.
Song R, Wang Q, Guo F, Liu X, Song S, Chen C, et al. Detection of Babesia spp., Theileria spp. and Anaplasma ovis in border regions, northwestern China. Transbound Emerg Dis. 2018. https://doi.org/10.1111/tbed.12894.
Zhang GL, Zheng Z, Sun X, Liu XM, Liu R, Li HL, et al. A survey of tick species and its distribution with the landscape structure in Xinjiang. Chin J Vector Biol Control. 2016;27:432–5.
Torres-Perez F, Wilson L, Collinge SK, Harmon H, Ray C, Medina RA, et al. Sin Nombre virus infection in field workers, Colorado, USA. Emerg Infect Dis. 2010;16:308–10.
Krasnov BR, Shenbrot GI, Medvedev SG, Vatschenok VS, Khokhlova IS. Host-habitat relations as an important determinant of spatial distribution of flea assemblages (Siphonaptera) on rodents in the Negev Desert. Parasitology. 1997;114:159–73.
Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the new and old worlds. Parasit Vectors. 2013;6:213.
Estrada-Peña A, Bouattour A, J-L C, Walker AR. Ticks of domestic animals in the Mediterranean region: a guide to identification of species. Zaragoza: University of Zaragoza; 2004.
Chen Z, Li Y, Ren Q, Luo J, Liu Z, Zhou X, et al. Dermacentor everestianus Hirst, 1926 (Acari: Ixodidae): phylogenetic status inferred from molecular characteristics. Parasitol Res. 2014;113:3773–9.
Szabó MP, Mangold AJ, João CF, Bechara GH, Guglielmone AA. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus, tick group (Acari: Ixodidae) in South America. Vet Parasitol. 2005;130:131–40.
Hopkins GHE, Eothschild M. An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History) with keys and short descriptions for the identification of families, genera, species and subspecies. Vol. I. Tungidae and Pulidae. London: Trustees of the British Museum; 1953.
Dunnet GM, Nardon D. A monograph of Australian fleas (Siphonaptera). Aust J Zool Suppl. 1974;22:1–273.
Lawrence AL, Brown GK, Peters B, Spielman DS, Morin-Adeline V, Šlapeta J. High phylogenetic diversity of the cat flea (Ctenocephalides felis) at two mitochondrial DNA markers. Med Vet Entomol. 2014;28:330–6.
Zhao SS, Li HY, Yin XP, Liu ZQ, Chen CF, Wang YZ. First detection of Candidatus Rickettsia barbariae in the flea Vermipsylla alakurt from north-western China. Parasit Vectors. 2016;9:325.
Anstead CA, Chilton NB. Detection of a novel Rickettsia, (Alphaproteobacteria: Rickettsiales) in rotund ticks (Ixodes kingi) from Saskatchewan, Canada. Ticks Tick Borne Dis. 2013;4:202–6.
Anstead CA, Chilton NB. A novel Rickettsia species detected in vole ticks (Ixodes angustus) from western Canada. Appl Environ Microbiol. 2013;79:7583–9.
Sekeyova Z, Roux V, Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D’, which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol. 2001;51:1353–60.
McIntosh D, Bezerra RA, Luz HR, Faccini JL, Gaiotto FA, Giné GA, et al. Detection of Rickettsia bellii and Rickettsia amblyommii in Amblyomma longirostre (Acari: Ixodidae) from Bahia state, northeast Brazil. Braz J Microbiol. 2015;46:879–83.
Tamura K, Stecher G, Peterson D, Fillpski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
Ogata H, La Scola B, Audic S, Renesto P, Blanc G, Robert C, et al. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet. 2006;2:e76.
Guo LP, Mu LM, Xu J, Jiang SH, Wang AD, Chen CF, et al. Rickettsia raoultii in Haemaphysalis erinacei from marbled polecats, China-Kazakhstan border. Parasit Vectors. 2015;8:461.
Acknowledgements
The authors thank the contributions by the staff at the School of Medicine and School of Animal Science and Technology, Shihezi University.
Funding
This research was supported in part by the National Key Research & Development Programme of China (2018ZX10101002-002-007, 2017YFD0500304 and 2017ZX10304402-002-005) and National Natural Science Foundation of China (81560338).
Availability of data and materials
The sequences obtained and analyzed during the present study are deposited in the GenBank database under the accession numbers KT878385-KT878387, KU364354-KU364359, KU364361-KU364367, KU364369-KU364371, KU364375, KU601752, KU757301-KU757306, KX254161, KX254163-KX254164, KX457946-KX457954, KX668365-KX668384, KX822749-KX822752, KX822754-KX822757, KX999715-KX999719, KY593298, KY593300-KY593301, KY593303, KY593306-KY593309, KY593311-KY593312, KY593314, KY593316-KY593317, KY593319, KY610534-KY610535, KY610537, KY610542-KY610545, MF000668, MF000670-MF000671, MF000678-MF000681, MF002497-MF002508, MF002515-MF002516, MF002518-MF002558, MF002560-MF002565, MF002567-MF002568, MF002570-MF002576, MF002578, MF002580-MF002583, MF002585-MF002591, MF045759-MF045765, MF045767, MF136071-MF136074. All other relevant data are included in the article and its additional files.
Author information
Authors and Affiliations
Contributions
SS, CC, MY and YW conceived and designed the study, and critically revised the manuscript. SZ, BW, BM and KR performed the experiments, analyzed the data. SS and SH contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Shihezi University (Approval No. AECSU2014-03).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. GenBank accession numbers of representative nucleotide sequences, including 22 from ticks, 78 from fleas, 84 from rickettsiae in ticks and 12 from rickettsiae in fleas, are shown in A, B, C and D, respectively. (DOCX 45 kb)
Additional file 2:
Table S2. Rickettsia endosymbiont and Rickettsia bellii detected in this study. (DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Song, S., Chen, C., Yang, M. et al. Diversity of Rickettsia species in border regions of northwestern China. Parasites Vectors 11, 634 (2018). https://doi.org/10.1186/s13071-018-3233-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3233-6