Abstract
Background
Species of Canidae in Russia can be infested with up to 24 different tick species; however, the frequency of different tick species infesting domestic dogs across Russia is not known. In addition, tick-borne disease risks for domestic dogs in Russia are not well quantified. The goal of this study was to conduct a nationwide survey of ticks collected from infested dogs admitted to veterinary clinics in Russian cities and to identify pathogens found in these ticks.
Methods
Ticks feeding on dogs admitted to 32 veterinary clinics in 27 major cities across Russia were preserved in ethanol and submitted to a central facility for examination. After identification, each tick was evaluated for infection with known tick-borne pathogens using PCR.
Results
There were 990 individual ticks collected from 636 dogs. All collected ticks belonged to the Ixodidae (hard ticks) and represented 11 species of four genera, Dermacentor, Ixodes, Rhipicephalus and Haemaphysalis. Four most common tick species were D. reticulatus, followed by I. persulcatus, I. ricinus and R. sanguineus. Ixodes persulcatus ticks were found to be infected with 10 different pathogens, and ticks of this species were more frequently infected than either D. reticulatus or I. ricinus. Ixodes persulcatus females were also more frequently co-infected with two or more pathogens than any other tick. Pathogenic species of five genera were detected in ticks: Anaplasma centrale, A. phagocytophilum and A. marginale; Babesia canis, B. microti, B. venatorum, B. divergens, B. crassa and B. vogeli; Borrelia miyamotoi, B. afzelii and B. garinii; Ehrlichia muris, E. canis and E. ruminantum; and Theileria cervi. Anaplasma marginale, E. canis, B. crassa, B. vogeli and T. cervi were detected in I. persulcatus, and Babesia canis in D. marginatum, for the first time in Russia.
Conclusions
Multiple ticks from four genera and 11 species of the family Ixodidae were collected from domestic dogs across Russia. These ticks commonly carry pathogens and act as disease vectors. Ixodes persulcatus ticks present the greatest risk for transmission of multiple arthropod-borne pathogens.
Similar content being viewed by others
Introduction
Hard ticks are dangerous ectoparasites of domestic dogs because of their blood-feeding, toxicoses (including paralysis), irritation, allergy, and potential pathogen transmission. Many ticks are found in Russia, and over a hundred different tick species of the genera Haemaphysalis Koch, Dermacentor Koch, Rhipicephalus Koch and Ixodes Latreille are reported from moderate and subtropical climate zones; of these, 24 species are known to infest Canidae [1, 2]. However, there are no data regarding the species composition of ticks infesting urban dogs in Russia and additional information on tick attachment risks for dogs in Russia is important because of the potential for tick-borne disease spread [3]. In addition, tick populations are growing and their geographical range is changing in association with climate change, leading to tick infestation of naïve populations [4,5,6,7,8]. Tick populations can spread quickly over large distances, facilitated by the mobility of people and their companion animals [9, 10]. Dog owners also face tick-borne disease risks while out walking with their animals. There were 6439 cases of human tick-borne Lyme disease reported in Russia in 2014, or 4.41 cases per 100,000 people [11].
Many organisations in multiple Russian cities need to be coordinated to manage a programme that collects and identifies ticks, and then completes the detection of pathogen presence. However, it is important to record the current geographical distribution of tick- and tick-borne disease risks for dogs and also gain insight into regional zoonotic infection risks. Therefore, the goal of this study was to conduct a nationwide survey of ticks infesting dogs admitted to veterinary clinics in multiple Russian cities. Collected ticks were identified to species and then evaluated for vector-borne canine, and potentially zoonotic, pathogens.
Methods
Sample collection and parasitological analysis
Veterinary practices in Russian cities were invited to register for the study through voluntary completion of an on-line questionnaire at a custom web site, now closed. The questionnaire collected data on: location (city); clinic name; sample ID; tick removal date; dog breed; age; clinical signs; and diagnosis. All registered practices were provided with tubes containing 70% ethanol for tick collection and submission.
Veterinarians in participating practices collected attached ticks from dogs presented voluntarily to the clinic by their owners. The veterinarian completed a thorough external physical exam to remove ticks attached to the dog and then selected two ticks for preservation and submission. Each saved tick was placed in a separate tube with 70% ethanol and assigned a unique identification number.
Questionnaire responses were entered into a spreadsheet (Excel, Microsoft, Redmond, WA, USA) published online and the initial 1000 submitted ticks were submitted for pathogen analysis in addition to tick identification. The ticks were identified to the species level using morphological keys and the life-cycle stage and sex were determined [1, 2, 12]. Where tick identification based on morphology was uncertain, sequencing was used; if the species could still not be reliably identified, the tick was excluded from the study. All ticks were then processed for pathogen identification using qPCR.
DNA extraction, amplification and sequencing
Frozen ticks were homogenized (MagNALyser Instrument, MagNaLyserGreenBeads, Roche Diagnostics, Switzerland) and total DNA was extracted (RealBest Extraction 100, Vector-Best, Novosibirsk, Russia) according to the manufacturer’s protocol.
Oligonucleotide sequences were designed (PrimerQuest online software, Integrated DNA Technologies, USA) and produced (Vector-Best, Novosibirsk, Russia). Real-time PCR was performed (CFX96 thermal cycler, Bio-Rad Laboratories, USA) and tests were manually set-up. Each reaction required 50 μl total volume and included 1× PCR buffer (Vector-Best, Novosibirsk, Russia), 0.4 mM of dNTP (Biosan, Russia), 1% BSA and 1U Taq polymerase (Vector-Best, Novosibirsk, Russia) pre-mixed with active center-specific monoclonal antibody (Takara Bio, Mountain View, CA, USA), 0.5 units of uracil-DNA glycosylase (Vector-Best, Novosibirsk, Russia), 0.5 μM of each primer and 0.25 μM of dual-labeled probe. PCR cycling conditions were: 2 min incubation at 50 °C; 2 min pre-denaturation step at 94 °C; 50 denaturation cycles (94 °C for 10 s) alternated with annealing and elongation (60 °С for 20 s).
For screening analysis, B. miyamotoi and B. burgdorfgeri (s.l.) DNA was detected by TaqMan real-time PCR (RealBest DNA Borrelia miyamotoi and RealBest DNA Borrelia burgdorfgeri (s.l.), Vector-Best, Novosibirsk, Russia) according to the manufacturer’s protocol. Babesia spp and Anaplasma spp./Ehrlichia spp. were detected by the real-time PCR method, using oligonucleotides for the detection of Babesia and Anaplasma/Ehrlichia. Borrelia spp. were then determined by species-specific qPCR and Ehrlichia spp., Anaplasma spp., and Babesia spp. were identified using sequencing. Hepatozoon spp. were not screened for in this study.
PCR products were purified (GFX Columns, Amersham Biosciences, USA) and sequenced (ABI 3500 Genetic Analyzer, Applied Biosystems, USA), aligned (BioEdit v7.2.5, Ibis Biosciences, USA) and analyzed (BLASTN software; http://www.ncbi.nlm.nih.gov/BLAST).
Nucleotide sequences of pathogens detected in Russia for the first time were deposited in the GenBank database under the accession numbers MG028598 (A. marginale), MG066535 (E. canis), MG062780 (B. microti), MG062781 (B. venatorum), MG062782 (B. crassa), MG041384 (B. vogeli) and MG041373 (Theileria cervi).
Statistics
Pathogen prevalence differences in ticks between sites and among pathogens within tick species were computed by the Pearson’s Chi-square goodness-of-fit test, when conditions were met, or otherwise by Fisher’s exact test (IBM SPSS Statistics, version 22; Statistica software, version 12). P < 0.05 was considered significant; 95% confidence intervals for tick pathogen prevalence were computed using a bootstrap technique.
Results
Tick distribution
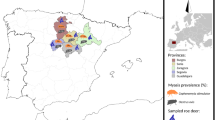
The study was completed in 2016, with participation of 32 veterinary clinics from 27 cities located across Russia (Fig. 1). There were 1010 ticks removed and submitted from 636 dogs. Of these, 990 ticks were identified to species including 11 species of the Ixodidae (Table 2), although 20 submitted ticks could not be analysed because of damage during collection or transport. Submitted ticks were semi-engorged or fully-engorged and most were adults, with 64.9% adult females and 28.7% adult males. Juvenile ticks were 3.9% nymphs and 2.4% larvae.
The most common tick identified on dogs was Dermacentor reticulatus with a prevalence of 40.7% (411/990; 95% CI: 37–43%) (Table 1). This tick species was identified in 17 cities, with the westernmost location in Smolensk (54°46'58"N, 32°2'42"E) and the easternmost in Krasnoyarsk (56°0'38"N, 92°51'9"E). Ixodes persulcatus ticks were less commonly identified with a prevalence of 23.8% (218/990; 95% CI: 20–30%) (χ2 = 64.442, df = 1, P < 0.001). This species was identified in 12 widely separated cities with the westernmost location at Vologda (59°13'14"N, 39°53'30"E). Ixodes ricinus ticks (prevalence 13.1%; 130/990; 95% CI: 10–20%) were submitted from 8 cities (Fig. 1) and significantly less often (χ2 = 37.661, df = 1, P < 0.01) than I. persulcatus. The easternmost locations for I. ricinus were Moscow (55°45'21"N, 37°37'3"E) and Voronezh (51°39'42"N, 39°12'1"E). The prevalence of Rhipicephalus sanguineus was slightly lower than that of I. ricinus (11.3%; 112/990) (Fig. 1, Table 2) with an easternmost location of Blagoveshchensk (50°17'26"N, 127°31'38"E). Three species of Haemaphysalis were identified with a prevalence of 2.5–2.7%. Haemaphysalis concinna (27 ticks) was collected in Blagoveshchensk, Khabarovsk (48°28'49"N, 135°4'19"E), and Sochi (43°35'8"N, 39°43'23"E). Haemaphysalis parva (25 ticks) was collected in Simferopol (44°56'54"N, 34°6'1"E); and H. japonica (27 ticks) in Artem (44°56'54"N, 34°6'1"E). Ticks recorded with lower prevalences included R. turanicus (2.1%; 29 ticks) from Grozniy (43°19'4"N, 45°41'42"E); Dermacentor silvarum (1 male and 3 female ticks) from Blagoveshchensk and Irkutsk (52°17'11"N, 104°16'50"E); and, D. marginatus (3 female ticks) from Moscow. One I. pavlovskyi female was submitted from Novosibirsk (55°1'49"N, 82°55'14"E), and one from Vladivostok (43°6'55"N, 131°53'7"E).
Pathogen detection
Sixteen pathogens were detected: 3 Anaplasma species; 1 Ehrlichia species; Borrelia miyamotoi; 2 Borrelia burgdorferi (sensu lato) genospecies; 6 Babesia species; and 1 species of Theileria cervi, a cosmopolitan apicomplexan haemoparasite of domestic and wild ungulates (Fig. 2). Pathogen prevalences in all submitted ticks were: Anaplasma spp. 1.5% (15/990; 95% CI: 0.8–2.5%); Ehrlichia spp. 1.1% (11/990; 95% CI: 0.6–2.0); Borrelia spp. 7.3% (90/990; 95% CI: 5.7–9.0); and Babesia spp. 10.1% (100/990; 95% CI: 8.3–12.1%). There were 199 ticks (prevalence 20.1%; 95% CI: 17.6–22.7%) from 6 different species in which a single pathogen species was identified (Table 2).
Anaplasma spp. prevalence in tick species varied between 0.1–3.1%: A. centrale was detected in H. parva (1/25) from Simferopol; A. phagocytophilum was detected in 3.0% (7/238) of I. persulcatus and 3.1% (4/130) of I. ricinus, a non-significant difference (Fisher’s exact test: P > 0.05); A. phagocytophilum was identified in 1 D. reticulatus (1/404) from Moscow and A. marginale was detected in 2 I. ricinus from Moscow.
Ehrlichia spp. were detected in 11 ticks with a prevalence between 0.77–3.8% in different tick species. Ehrlichia muris was detected in I. persulcatus only, collected from dogs from widely separated cities including: 3 in Irkutsk; 2 each in Kirov and Vologda; and 1 each in Khabarovsk and Perm. Ehrlichia canis and E. ruminantum were detected in 1 R. sanguineus from Sevastopol and in 1 I. ricinus from Stavropol. Borrelia miyamotoi prevalence was 0.5–3.1% in tick species submitted. Infected ticks included: 3 I. ricinus from Moscow and 1 from St Petersburg; 2 I. persulcatus from Vladivostok; 1 D. reticulatus from Novosibirsk; and 1 from Simferopol. Borrelia afzelii and B. garinii were only detected in I. ricinus and I. persulcatus, with a prevalence between 4.6–14.2%. The overall prevalence of B. burgdorferi (s.l.) in I. persulcatus (20.8%; 49/238; 95% CI: 15.8–26.5%) was significantly greater (χ2 = 4.943, df = 1, P < 0.005) than in I. ricinus (11.5 %; 15/130; 95% CI: 6.6–18.3%). Borrelia garinii (prevalence 11.2%; 41/368; 95% CI: 8.2–14.9%) was detected in Ixodes spp. significantly more frequently (χ2 = 4.658, df = 1, P < 0.005) than B. afzelii (prevalence 6.3%; 23/368; 95% CI: 4.0–9.3%). Borrelia burgdorferi (s.l.) infected ticks were widespread and identified in multiple cities. Cities with few submitted I. ricinus and I. persulcatus included: Blagoveshchensk, Bryansk, Rostov-on-Don, Sevastopol, Simferopol and Yaroslavl. There were 6 tick species infected with Babesia spp. and prevalence varied from 0.4 to 20.3%. The highest B. canis prevalence was among D. reticulatus (Table 3), which were collected from 10 cities. The highest prevalences were in Ekaterinburg (35.8%; 19/53; 95% CI: 23.1–50.2%) and Tyumen (30.8%; 20/65; 95% CI: 20.0–43.4%). Three D. reticulatus from Krasnodar tested positive for B. canis and 1 D. reticulatus from Ekaterinburg was positive for B. venatorum. Babesia microti was detected in 1 I. persulcatus from Kirov and 1 from Vologda while B. divergens was detected in I. persulcatus from Irkutsk. Babesia crassa and B. vogeli were detected in ticks from Simferopol and Grozniy, respectively. Theileria cervi was detected in 1 I. persulcatus tick from Artem.
Ixodes persulcatus was the tick species that was most commonly pathogen infected with an infection prevalence significantly greater than D. reticulatus (χ2 = 9.282, df = 1, P < 0.01) although not significantly greater than I. ricinus (χ2 = 2.830, df = 1, P > 0.05). Ixodes persulcatus also carried the greatest diversity of detected arthropod-borne pathogens, with 10 different species identified (Table 2).
Pathogen co-infection in ticks
The prevalence of co-infection in ticks (2.7%; 19/316; 95% CI: 1.8–3.9%), was significantly lower (χ2 = 167.012, df = 1, P < 0.01) than the prevalence of a single pathogen infection and only 2 species had co-infections (Table 3). Ixodes persulcatus adult females were the most commonly co-infected ticks (Fisher’s exact test: P < 0.05) and 21 (prevalence 8.9%; 95% CI: 5.6–13.3%) were infected with more than one pathogen including A. phagocytophilum, E. muris, B. miyamotoi, 2 genospecies of B. burgdorferi (s.l.), B. microti and B. divergens (Table 3). Three female ticks had a triple infection involving A. phagocytophilum, B. burgdorferi (s.l.), B. microti, and B.venatorum (prevalence 1.3%; 95% CI: 0.3–3.7%). Co-infections with B. burgdorferi (s.l.) and another pathogen were common among I. persulcatus ticks, while double infections with E. muris and B. divergens were observed only once. Ixodes ricinus females co-infected with 2 or more tick-borne pathogens were collected from 2 cities (Table 3) and pathogens involved were A. marginale, B. miyamotoi, B. afzelii, B. garinii or B. canis in various combinations.
Discussion
This is the first comprehensive study of ticks found attached to domestic dogs and their associated tick-borne pathogens conducted in multiple Russian cities. The results show that there are a wide variety of infesting ixodid ticks and that there are multiple potential pathogens that can be transmitted through a tick bite, with a frequent risk of more than one pathogen from each tick. This result is generally consistent with observations on ticks and pathogens from dogs in other countries, although there are some unique results in this study.
The most common tick species from domestic dogs in Russian cities were D. reticulatus, I. persulcatus and I. ricinus. In this study, D. reticulatus-infested dogs were found in the widest geographical range, with reports from Smolensk to Krasnoyarsk. Previously, the distribution of D. reticulatus was linked to the southern taiga in the eastern part of the geographical range and the eastern limit of distribution was previously reported as the upper reaches of the River Yenisei [2]. Ixodes persulcatus had the second greatest prevalence in urban dogs in this study although ticks were not found on dogs outside the tick’s previously described geographical range. However, there have been reports of I. persulcatus to the south and east of the main geographical range [13, 14]. Ixodes ricinus ticks were submitted from dogs in 8 cities. This tick is known to be widely distributed in Russia; it inhabits European deciduous and mixed forests and can be found in the subregions of southern and sometimes northern taiga [1]. Additional tick genera collected from dogs in this study included species of Haemaphysalis, Dermacentor and Rhipicephalus. Rhipicephalus spp. ticks were submitted in a similar proportion to I. ricinus; however, unlike the more widely distributed I. ricinus, Rhipicephalus spp. were primarily found on dogs from cities in humid and arid subtropical regions, although R. sanguineus was recovered from the northeastern city of Blagoveshchensk (Fig. 1). Rhipicephalus sanguineus is a globally widespread tick that preferentially infests dog hosts and is also called the “kennel” or “brown dog” tick [15]. Rhipicephalus sanguineus in southern Italy has induced tick paralysis outbreaks in dogs [16]. Rhipicephalus turanicus was submitted from Grozniy in this study, and although this tick was previously reported in Russia and the former USSR, available information is fragmentary and collected in the mid-20th century [2]. This tick is morphologically similar to R. sanguineus [17] but has very different behavioural, ecological and vector characteristics [18]. The present study contributes new information about the risks of H. concinna and H. japonica infestation of dogs in eastern Russia. Haemaphysalis concinna is found in the moderate climate zone of Eurasia and is common in China and Mongolia [19, 20]. Haemaphysalis japonica infests dogs in Japan, Mongolia and China [21,22,23] and H. parva is also known as a parasite of dogs [24]; however, this is the first known report of H. parva on dogs in Russia. The previous limited experience with these two tick species on dogs in Russia could reflect a bias toward examination of domestic dogs in urban biotopes, where there is a lower risk of encountering these species of ticks. However, growth of cities, increased tourist movement, and a greater opportunity for owners to walk dogs in rural areas, may lead to more frequent encounters with these tick species. Haemaphysalis parva feeds on a wide range of large mammals and it is likely that this tick was not reported previously on dogs in Russia because there are few previous investigations on the spectrum of ticks parasitizing Russian dogs. The possibility that this tick plays a significant role in vector borne disease transmission to dogs where it is endemic requires further investigation. Three female D. marginatus were collected from dogs in Moscow, and this thermophilic species is reported to be widespread in the Mediterranean region. In Russia, D. marginatus is widely distributed in the south, while the geographical range in the east reaches the southern forest steppes of Western Siberia. Dermacentor silvarum, similar to D. marginatus, prefers forest steppes and is common in eastern Russia, Mongolia and China [23,24,25]. These tick species are uncommon in ecosystems transformed by human activity, which likely explains their low prevalence in this study of dogs from urban areas.
Ticks in this study were feeding on dogs at the time of collection and were therefore potentially transmitting any carried pathogen to the dogs while feeding. Detected pathogens are also an indication of the tick-transmitted pathogens circulating in the local community. Specific tick species represent a greater risk for some pathogens, for example, pathogens were detected in D. reticulatus at variable prevalences: B. canis (20.3%); B. miyamotoi (0.5%); A. phagocytophilum (0.25%); and B. venatorum (0.25%). Previous studies show that the most significant and widespread pathogen transmitted by D. reticulatus ticks is B. canis which is found from western Europe to Siberia [26, 27] and the present study confirms that these ticks and pathogens are widespread across Russia. Veterinarians practising in Khabarovsk in eastern Russia (Fig. 1) have diagnosed canine babesiosis although no ticks from this city were B. canis-positive indicating that the vector and specific pathogen in this region are not yet identified. Field-collected D. reticulatus ticks from prior years and from other areas of Europe have had B. canis infection prevalence between 0.7–15.0% [28]. In contrast, little is known about the significance of D. reticulatus-transmitted B. venatorum in Russia. Ixodes persulcatus and I. ricinus are believed to be the main vectors for the transmission of B. venatorum [29]. Babesia gibsoni DNA was not detected in any ticks in this study; however, this pathogen has been reported in dogs from Moscow, the Lipetsk Oblast, Ufa, St Petersburg and Oryol [30].
Ixodes persulcatus ticks carried the widest array of tick-borne pathogens, including A. phagocytophilum and E. muris detected in ticks submitted from seven cities. The zoonotic cycles of these pathogens are established in these locations and these Anaplasmataceae pathogens were previously detected using molecular methods in unfed I. persulcatus in several regions in Russia [31]. The prevalence of I. persulcatus with Borrelia spp. DNA in this study was 21.6%, a value similar to a previous report of 14.9% prevalence of Borrelia spp. in I. persulcatus [32]. Ixodes persulcatus are known to be primary carriers and reservoirs for B. burgdorferi (s.l.) and B. miyamotoi in Russia [33]. Where it is present, I. persulcatus is known as the main source for these pathogens for both humans and companion animals. Babesia canis DNA was detected in one I. persulcatus from Ekaterinburg and this adult female may have ingested the pathogen while feeding on the dog, which was reported to be showing clinical signs of babesiosis with a positive blood smear for B. canis. Babesia microti, B. venatorum and B. divergens DNA were detected in I. persulcatus collected from dogs in Irkutsk and Kirovin this study, while B. microti and B. venatorum DNA were reported in a previous study in I. persulcatus ticks collected from vegetation in the Russian Far East [31]. Ixodes persulcatus was proposed as the main vector for babesiosis in China [34]. Theileria cervi DNA was detected in one I. persulcatus in the eastern city of Artem and T. cervi was previously reported from white-tailed deer blood and I. scapularis in North America [35].
Ixodes ricinus were second to I. persulcatus in the prevalence and diversity of pathogens carried, with A. phagocytophilum and A. marginale DNA detected. Ixodes ricinus is known as the major vector for Anaplasma spp. in Europe [36]. Ehrlichia ruminantium, the pathogen that causes “heart water” disease in cattle, sheep and goats [37], was detected in one I. ricinus from Stavropol. The mean prevalence of I. ricinus infected with one Borrelia pathogen, including B. miyamotoi or two Borrelia genospecies was 11.5%, and this tick is reported to be the main vector of B. miyamotoi, B. afzelii and B. garinii in western Russia [38]. The prevalence in the present study is similar to the 13.9% mean prevalence of B. burgdorferi (s.l.) reported in female I. ricinus in Europe [39]. Five I. ricinus ticks in this study were positive for pathogens that cause canine babesiosis, although these ticks are not considered to be a vector for canine babesiosis [40]. It is possible that these ticks ingested Babesia infected canine peripheral blood cells from infected dogs leading to subsequent pathogen DNA detection. Rhipicephalus sanguineus were positive for E. muris DNA (Sevastopol) or B. vogeli (Grozniy). Babesia vogeli DNA was previously reported in R. sanguineus in the Rostov district [41] and experiments have shown that R. sanguineus transmits Babesia spp. (e.g. B. vogeli) to dogs. Confirmation of B. vogeli in this study convincingly demonstrates the presence of this pathogen in Russia although its distribution in Russia is not well known. Babesia vogeli and E. muris are pathogens that could pose a serious danger to animals and their detection in ticks attached to dogs is also an indicator of a possible infection risk to the dog owner.
This study found previously unreported tick infections in Russia: B. crassa was detected in H. parva in Russia for the first time, while previously, B. crassa DNA was only reported in H. parva attached to humans in Ankara, Turkey [42]; and B. canis DNA was also detected for the first time in two D. marginatus from Moscow.
The true prevalence of co-infections with human pathogens among hard ticks in most regions of Russia is unknown [43, 44]. In this study, the risk of tick co-infection with more than one pathogen was found to be greatest in regions where I. persulcatus is the predominant tick. This tick was submitted from veterinary practices in nine cities and I. persulcatus ticks with more than one pathogen were primarily submitted from known borreliosis-endemic areas. Ixodes persulcatus co-infected with B. burgdorferi (s.l.) and two species of the Anaplasmataceae were submitted from six cities across Russia. However, results of multiple previous studies indicate that in most regions a minority of Ixodes ticks are co-infected [45]. The present study found B. venatorum and B. divergens (0.42%) infection hotspots in Irkutsk; however, these pathogens were only detected in co-infection with zoonotic bacteria. Two ticks co-infected with B. microti (0.85 %) and B. garinii or B. afzelii were reported, one each in Kirov and Vologda. Babesia microti co-infections with Borrelia spp. may increase the risk of babesiosis in Lyme disease endemic areas [46]. Ticks with three co-infecting pathogens were submitted from Irkutsk and Vologda, where Babesia spp. DNA was detected in combination with B. burgdorferi (s.l.) and two Anaplasmataceae spp. Overall, these results reflect the complicated tick-borne pathogen epizootology reported throughout the wide geographical range of I. persulcatus [22, 47, 48]. These ticks therefore represent a pathogen transmission risk for both dogs and humans [49] and results of this study confirm the risk of tick-borne disease transmission from I. persulcatus in Russia.
Single I. ricinus co-infected with pathogens were detected in two cities, Moscow and St Petersburg, and these co-infected ticks always had Borrelia spp. pathogens. Previous studies in Russia reported I. ricinus with B. burgdorferi (s.l.) genospecies, A. phagocytophylum, Rickettsia monacensis and R. helvetica [50]. The present study indicates the potential presence of a wider selection of pathogens, including a tick found to have three different pathogen infections therefore underlining the risk for transmission of multiple pathogens to canine hosts with every feeding tick.
In general, owners of dogs need to be made aware of the risks of tick infestation that they face during walks. Pathogens carried by ticks can infect both dogs and people and monitoring of ticks and the pathogens they carry provides insight into the occurrence and spread of zoonotic diseases. Veterinarians in all areas of Russia should keep these risks in mind and educate owners regarding the risks as well as developing optimal approaches for tick protection protocols that maximize owner compliance.
Conclusions
Multiple ticks from four genera and 11 species of the family Ixodidae were collected from domestic dogs across Russia. These ticks commonly carry pathogens and act as disease vectors. Ixodes persulcatus ticks present the greatest risk for transmission of multiple arthropod-borne pathogens. New tick pathogen transmission risks identified in Russia are B. crassa infecting H. parva and B. canis in D. marginatus.
References
Filippova NA. Fauna of Russia and Neigbouring Countries. Arachnoidea. Ixodid ticks of subfamily Ixodinae. Leningrad: Nauka; 1977.
Filippova NA. Fauna of Russia and Neigbouring Countries. Arachnoidea. Ixodid ticks of subfamily Amblyomminae. Leningrad: Nauka; 1997.
Daniel M, Kříž B, Danielová V, Beneš Č. Sudden increase in tick-borne encephalitis cases in the Czech Republic, 2006. I J Med Microbiol. 2008;298:81–7.
Lindgren E, Tälleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Env Health Persp. 2000;108:119–23.
Zygner W, Górski P, Wedrychowicz H. New localities of Dermacentor reticulatus tick (vector of Babesia canis canis) in central and eastern Poland. Pol J Vet Sci. 2009;12:549–55.
Beugnet F, Marié JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305.
Livanova NN, Tikunov AY, Kurilshikov AM, Livanov SG, Fomenko NV, Taranenko DE, et al. Genetic diversity of Ixodes pavlovskyi and I. persulcatus (Acari: Ixodidae) from the sympatric zone in the south of Western Siberia and Kazakhstan. Exp Appl Acarol. 2015;67:441–56.
Korotkov Y, Kozlova T, Kozlovskaya L. Observations on changes in abundance of questing Ixodes ricinus, castor bean tick, over a 35-year period in the eastern part of its range (Russia, Tula region). Med Vet Entomol. 2015;29:129–36.
Otranto D, Wall R. New strategies for the control of arthropod vectors of disease in dogs and cats. Med Vet Entomol. 2008;22:219–302.
Khasnatinov NA, Liapunov AV, Manzarova EL, Kulakova NV, Petrova IV, Danchinova GA. The diversity and prevalence of hard ticks attacking human hosts in eastern Siberia (Russian Federation) with first description of invasion of non-endemic tick species. Parasitol Res. 2015;115:501–10.
State Report “On the sanitary-epidemiological welfare of the population of the Russian Federation in 2014”. 2014. http://rospotrebnadzor.ru/upload/iblock/22c/gd_2014_seb_dlya-sayta.pdf Accessed 28 Nov 2017.
Walker A, Bouattour A, Camicas J-L, Estrada-Peña A, Horak IG, Latif AA, et al. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Edinburgh, UK: Bioscience Reports; 2014.
Tokarevich NK, Tronin AA, Blinova OV, Buzinov RV, Boltenkov VP, Yurasova ED, et al. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Global Health Act. 2011;4:8448.
Jaenson TG, Värv K, Fröjdman I, Jääskeläinen A, Rundgren K, Versteirt V, et al. First evidence of established populations of the taiga tick Ixodes persulcatus (Acari: Ixodidae) in Sweden. Parasit Vectors. 2016;9:377.
Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26.
Otranto D, Dantas-Torres F, Tarallo VD, Ramos RA, Stanneck D, Baneth G, et al. Apparent tick paralysis by Rhipicephalus sanguineus (Acari: Ixodidae) in dogs. Vet Parasitol. 2012;188:325–9.
Estrada-Peña A, Bouattour A, Camicas J-L, Walker AR. Ticks of Domestic Animals in the Mediterranean Region. In: Guide to Identification of Species. Zaragoza, Spain: University of Zaragoza; 2004.
Walker JB, Keirans JE, Horak IG. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World. Cambridge: Cambridge University Press; 2000.
Meng H, Xu S, Yu Z, Li N, Wang R, Gao X, et al. Abundance and seasonal activity of Haemaphysalis concinna (Acari: Ixodidae) at the border between China and Russia in Northern Inner Mongolia, China. Parasit Vectors. 2016;9:1.
Fuehrer HP, Biro N, Harl J, Worliczek HL, Beiglböck C, Farkas R, et al. Molecular detection of Theileria sp. ZS TO4 in red deer (Cervus elaphus) and questing Haemaphysalis concinna ticks in eastern Austria. Vet Parasitol. 2013;197:653–7.
Iwakami S, Ichikawa Y, Inokuma H. A nationwide survey of ixodid tick species recovered from domestic dogs and cats in Japan in 2011. Ticks Tick Borne Dis. 2014;5:771–9.
Kim BJ, Kim H, Won S, Kim HC, Chong ST, Klein TA, et al. Ticks collected from wild and domestic animals and natural habitats in the Republic of Korea. Korean J Parasitol. 2014;52:281–5.
Chen Z, Yang X, Bu F, Yang X, Yang X, Liu J. Ticks (Acari: Ixodoidea: Argasidae, Ixodidae) of China. Exp Appl Acarol. 2010;51:393–404.
Papadopoulos B, Morel PC, Aeschlimann A. Ticks of domestic animals in the Macedonia region of Greece. Vet Parasitol. 1996;63:25–40.
Dash M, Biambaa B, Neronov VM. The ixodid tick fauna of the Mongolian People’s Republic. The species distribution. Med Parazitol (Mosk). 1988;3:37–42 (in Russian).
Rar VA, Fomenko NV, Dobrotvorsky AK, Livanova NN, Rudakova SA, Fedorov EG, et al. Tickborne pathogen detection, Western Siberia, Russia. Emerg Infect Dis. 2005;11:1708–15.
Matijatko V, Torti M, Schetters TP. Canine babesiosis in Europe: how many diseases? Trends Parasitol. 2012;28:99–105.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314.
Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15:196–203.
Konyayev SV, Akimov IA, Fomenko NV, Ivanov MK. Cases of Hepatozoon canis and Babesia gibsoni infection in Russia. Proceedings of 2th International Parasitology Symposium “Modern problems general and special parasitology”, St Petersburg, Russia, 6 –8 December 2017, vol. 2017. p. 136–8.
Rar VA, Epikhina TI, Yakimenko VV, Malkova MG, Tancev AK, Bondarenko EI, et al. Genetic variability of Anaplasma phagocytophilum in ticks and voles from Ixodes persulcatus/Ixodes trianguliceps sympatric areas from Western Siberia, Russia. Ticks Tick Borne Dis. 2014;5:854–63.
Fomenko NV, Viu B, Panov VV. Genetic features of Borrelia miyamotoi transmitted by Ixodes persulcatus. Mol Gen Mikrobiol Virusol. 2011;2:12–7 (In Russian).
Rar V, Livanova N, Tkachev S, Kaverina G, Tikunov A, Sabitova Y, et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit Vectors. 2017;10:258.
Wei FR, Lan QX, Zhu D, Ye JH, Liu Q, Zhang Y. Investigation on Babesia in ticks infested on police dogs in selected areas of China. Chinese J Parasitol Paras Dis. 2012;30:390–2 (In Chinese).
Fritzen C, Mosites E, Applegate RD, Telford SR, Huang J, Yabsley MJ, et al. Environmental investigation following the first human case of babesiosis in Tennessee. J Parasitol. 2014;100:106–9.
Atif FA. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol Res. 2015;114:3941–57.
Zhang J, Kelly P, Guo W, Xu C, Wei L, Jongejan F, et al. Development of a generic Ehrlichia FRET-Qpcr and investigation of ehrlichioses in domestic ruminants on five Caribbean islands. Parasit Vectors. 2015;8:506.
Masuzawa T, Kharitonenkov IG, Okamoto Y, Fukui T, Ohashi N. Prevalence of Anaplasma phagocytophilum and its co-infection with Borrelia afzelii in Ixodes ricinus and Ixodes persulcatus ticks inhabiting Tver Province (Russia) - a sympatric region for both tick species. J Med Microbiol. 2008;57:986–91.
Strnad M, Hönig V, Rüžek D, Grubhoffer L, Rego ROM. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol. 2017;83:e609–17.
Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336.
Kartashov SI, Ermakov, Klyuchnikov AG, Mironova AA, Yaroshenko NV, Boyko VP. Babesiosis of dogs: new ecological, molecular-genetic and clinical-laboratory aspects. Veterinary of Kuban. 2010;5:22–4.
Orkun Ö, Karaer Z, Çakmak A, Nalbantoğlu S. Identification of tick-borne pathogens in ticks feeding on humans in Turkey. PLOS Negl Trop Dis. 2014;8:e3067.
Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708–27.
Movila A, Dubinina HV, Sitnicova N, Bespyatova L, Uspenskaia I, Efremova G, et al. Comparison of tick-borne microorganism communities in Ixodes spp. of the Ixodes ricinus species complex at distinct geographical regions. Exp Appl Acarol. 2014;63:65–76.
Belongia EA. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002;2:265–73.
Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32:30–42.
Hiraoka H, Shimada Y, Sakata Y, Watanabe M, Itamoto K, Okuda M, et al. Detection of Borrelia garinii, Borrelia tanukii and Borrelia sp. closely related to Borrelia valaisiana in Ixodes ticks removed from dogs and cats in Japan. Vet Parasitol. 2007;144:188–92.
Wei F, Song M, Liu H, Wang B, Wang S, Wang Z, et al. Molecular detection and characterization of zoonotic and veterinary pathogens in ticks from northeastern China. Front Microbiol. 2016;29:1913.
Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, et al. Babesia divergens-like infection, Washington State. Emerg Infect Dis. 2004;10:622–9.
Movila A, Alekseev AN, Dubinina HV, Toderas I. Detection of tick-borne pathogens in ticks from migratory birds in the Baltic region of Russia. Med Vet Entomol. 2013;27:113–7.
Acknowledgements
The authors are deeply grateful to all of the study participants. These results were obtained thanks to a large network of people across Russia who supported this project. In particular, there were many volunteer veterinarians who collected and submitted ticks from their patients. Only with their help were we able to gain this additional information about the spread of pathogens and ticks in such a vast territory. We are also indebted to the work of Dr O. N. Filippova, who recently passed away, who provided immense support in determining the species of ticks and to Dr S. G. Medvedev for the opportunity to work with the parasitology laboratory and with the museum collection at the Zoological Institute of the Russian Academy of Science.
Funding
This study was funded by MSD Animal Health.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. The raw data will be shared with legitimate investigators providing an appropriate request documenting the study objective and investigator affiliations.
Author information
Authors and Affiliations
Contributions
NNL and SVK developed the study protocol. NVF, IAA and MJI developed and implemented the molecular identification methodology. SVK and NVT worked with participating veterinary hospitals on tick submission and identification. SVK and RA wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participating veterinary hospitals provided their consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
RA is employed by MSD Animal Health; NVF, IAA, and MJI are employees of AO Vector-Best. NNL, NVT and SVK declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Livanova, N.N., Fomenko, N.V., Akimov, I.A. et al. Dog survey in Russian veterinary hospitals: tick identification and molecular detection of tick-borne pathogens. Parasites Vectors 11, 591 (2018). https://doi.org/10.1186/s13071-018-3161-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3161-5