Abstract
Background
Phlebotomine sand flies (Diptera: Psychodidae) are insects of medical importance due to their involvement in the zoonotic transmission of Leishmania spp. to vertebrates. The aim of this work was to study the ecology of the sand fly fauna of two types of environments, a rural environment (the Transacreana Road) and an urban park (Horto Florestal Park), both located in the municipality of Rio Branco in the state of Acre, Brazil. Additionally, this study intended to investigate Leishmania infection and blood meal sources of these sand flies using molecular techniques.
Methods
The sand fly fauna was studied in different environments (i.e. forest and peridomestic environments in a rural area, and an urban forest) using Shannon traps and HP light traps to collect sand fly specimens over 13 consecutive months (December 2014 to January 2016). For investigating natural infection by Leishmania and the source of sand fly blood meals, DNA samples were extracted from female sand flies and subjected to polymerase chain reaction targeting ITS1 and cytb genes. DNA sequencing was subsequently used to identify species of Leishmania and the source of blood meals.
Results
A total of 2515 individual sand flies of 43 species were collected and identified, Trichophoromyia auraensis (839; 33.35%), Trichophoromyia spp. (537; 21.35%) and Evandromyia saulensis (187; 7.43%) were more abundant in the rural area (S = 41 species) than in the urban forest. No significant differences were found in species richness between forest and peridomestic environments in the rural area (H = 0.04; P > 0.05), but a larger number of species was found in the forest. Leishmania DNA was sequenced in 13 samples, confirming the presence of L. (V.) braziliensis in Th. auraensis (n = 1), Ev. saulensis (n = 2), Ev. walkeri (n = 1), Ps. llanosmartinsi (n = 1), Pi. nevesi (n = 2), Ps. davisi (n = 1), Ps. ayrozai (n = 1), Pa. aragaoi (n = 1), Ny. antunesi (n = 1) and Ev. infraspinosa (n = 1). Only Ps. ayrozai possessed a sequence similar to that of L. (V.) guyanensis (99%). Through microscopic analysis, five specimens of Ev. saulensis were found to possess flagellate forms in the hindgut, with an infection rate of 2.4%. Samples from 33 fed females were submitted to cytb gene amplification, for which sequencing determined that all were similar to the sequence deposited on GenBank for Gallus gallus (domestic chicken).
Conclusions
The high abundance of Trichophoromyia auraensis and Ev. saulensis, and the detection of L. (V.) braziliensis DNA, suggests that both species may be vectors of American tegumentary leishmaniasis. Psychodopygus ayrozai was found to be infected by L. (V) braziliesnsis and L. (V.) guyanensis, and although collected in low abundance, it may be a potential vector in the region. The sand fly fauna was found to be rich and diverse with predominance of the genus Psychodopygus. Identification of food sources of fed females showed that 100% amplified a gene region compatible with the domestic chicken, which although considered refractory in the disease transmission cycle, may have an influence on the population dynamics of sand flies.
Similar content being viewed by others
Background
Leishmaniasis is a complex of vector-transmitted zoonotic diseases, of great clinical importance and epidemiological diversity. It is caused by protozoan parasites of the genus Leishmania (order Kinetoplastida, family Trypanosomatidae) in vertebrate hosts including man. Transmission is performed by hematophagous insects of the subfamily Phlebotominae (family Psychodidae), known as sand flies, which have complex ecological behavior [1,2,3]. There are two main clinical types of leishmaniasis, which are caused by different protozoan species of the genus Leishmania: visceral leishmananiasis (VL) in which the etiological agent is Leishmania infantum and tegumentary leishmaniasis (TL) that affect the skin and mucous membranes [1, 4]. American tegumentary leishmaniasis (ATL) is a significant disease in Brazil due in part to the great abundance of sand fly vectors of the various species of Leishmania that occur in the country [3,4,5,6]. With 20,187 cases reported in 2015 [7], ATL has been reported in all states, suggesting adaptation of the parasites and their vectors to anthropic environments [8,9,10,11]. They have adapted to urban environments that are surrounded by to forest remnants, also because of the greater supply of food sources for the vectors in the form of domestic animals in peridomestic areas [11, 12]. In the North Region of Brazil, ATL is endemic with 45% (9278) of the confirmed cases for the country in 2015 [7]. This region is characterized by human occupation associated with agricultural activities, mineral extraction and intense deforestation. It is also a region possessing low human development indices, with the absence of basic services such as sanitation in most residences, which are considered potential sites for the occurrence of tropical diseases [12,13,14].
In the state of Acre, located southwest of the Amazon in Brazil, ATL is considered endemic with high rates of incidence and prevalence [11, 15, 16]. Of the few studies that have been undertaken in the region, there have been reports of Leishmania (Viannia) guyanensis, Leishmania (V.) braziliensis, Leishmania (V.) lainsoni and Leishmania (Leishmania) amazonensis [17,18,19]. Likewise, these studies have reported the sand fly species Bichromomyia flaviscutellata, Migonemyia migonei, Nyssomyia whitmani, Ny. antunesi, Ny. umbratilis, Psychodopygus ayrozai, Ps. davisi, Ps. paraensis and Trichophoromyia ubiquitalis as the main vectors of the etiological agents of leishmaniasis [11, 16, 20,21,22]. Rio Branco is a municipality in Acre with the greatest occurrence of the disease, with 500 ATL cases reported between 2013 and 2014 [7]. However, studies of leishmaniasis are still incipient, especially with regard to sand fly ecology and food sources, and the transmission cycle of Leishmania spp. In addition, little information has been acquired regarding natural infection of possible vectors by dissection and observation of trypanosomatids [23,24,25], or by molecular methods such as polymerase chain reaction (PCR), which are capable of identifying Leishmania DNA, such the studies carried out in Assis Brasil [22] and Rio Branco [26].
The objective of this study was to investigate the sand fly fauna of rural and urban environments, and determine their food source and natural infection by Leishmania in an endemic area of ATL in Rio Branco.
Methods
Study area
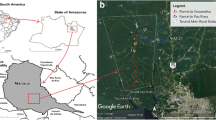
The municipality of Rio Branco (09°59′11"S, 67°49′52"W), capital of the state of Acre (AC), is located in the southwestern part of the Amazon Region of northern Brazil (Fig. 1a). It encompasses an area of 8835.52 km2 [27], and possesses an estimated population of 377,057 inhabitants [27]. The vegetation is composed of dense and open tropical forest with predominance of bamboo and palm trees. The climate is equatorial, with temperatures varying from 24 °C to 32 °C and annual rainfall of between 1877 and 1982 mm. The hottest period of the year corresponds to the months of July to August, marked by intense fires due to the conversion of forest to pastures [28].
Map of the study area with (a) location of the collection area in the municipality of Rio Branco, and (b) sand fly sampling areas in the urban park (1 and 2, Horto Florestal park) and rural (3 and 5, Dom Joaquim’s settlement, 5 and 6, Riozinho Branch), environments, municipality of Rio Branco Acre, Brazil (Map data ©2017 Google)
Sand fly sampling
Sand flies were collected in forest and peridomestic environments in a rural area, and in an urban forest, between December 2014 and January 2016. Sampling was carried out once a month using six HP light traps, set at 6:00 pm and retrieved at 6:00 am, distributed among the following sampling sites: Dom Joaquim Settlement Pole - Highway AC-90 (9°59′46.6"S, 67°58′51.0"W); Ramal do Riozinho - Highway AC-90 (10°05′00.1"S, 67°53′56.0"W); and Horto Florestal Urban Environmental Park (9°56′39.0"S, 67°49′43.6"W) (Fig. 1b).
To determine sand fly anthropophily, nocturnal sampling (06:00 pm to 08:00 pm) was performed by 2 persons using a Shannon trap in an area of peridomiciliar forest at Dom Joaquim Settlement during 13 nights. The females collected during this sampling were killed with ethyl acetate and dissected on slides with a drop of sterile saline solution to expose and examine the digestive tract and spermathecae for flagellate research and species identification, respectively. The specimens were then stored, in microtubes containing absolute ethanol, in pools of up to 10 females according to the month of collection and species, for subsequent molecular detection of Leishmania spp.
Fed females collected in HP light traps had only their genitalia exposed for species identification, and then were stored in 90% absolute ethanol for food source analysis.
All the remaining specimens collected in the Shannon trap were clarified according to Forattini [29] and identified according to the taxonomic key proposed by Galati [30]. The abbreviation of species names followed Marcondes [31].
DNA extraction and PCR conditions
After being identified, female sand flies were subjected to DNA extraction using the Gentra Puregene® Cell and Tissue Extraction Kit (QIAGEN, Hilden, Germany), following the manufacturer’s protocol.
After DNA extraction, PCR was performed targeting the internal transcribed spacer 1 (ITS1) using the primers LITSR - Forward (5′-CTG GAT CAT TTT CCG ATG-3′) and L5.8S - Reverse (5′-TGA TAC CAC TTA TCG CAC TT-3′) [32], to detect natural infection by Leishmania spp. In non-engorged females, the region amplifies a 350 bp fragment by means of the following reaction: 1× buffer solution (200 mM Tris-HCl, pH 8.4; 500 mM KCl), 1.5 mM MgCl2, 0.2 mM dNTPs, 0.5 pmol of the LITSR primer, 0.5 pmol of the L5.8S primer, 1 U of Taq DNA Polymerase Platinum (Invitrogen, California, USA) of 5 μl template DNA in a total volume of 25 μl. Samples were amplified in an automatic thermocycler (MaxyGene Gradient - AXYGEN, Corning, USA) with 33 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 1 min and extension at 72 °C for 1 min. In negative control, pure water was used with DNA from male sand flies. The amplified product was analyzed on a 2% agarose gel stained with GelRed (Nucleic Acid Gel Stain - Biotium, Fremont, USA) and compared with amplified material from positive samples from reference strains of Leishmania braziliensis (MHOM/BR/75/M2903). Positive samples were cloned into competent Escherichia coli (DH5-α) cells using the CloneJet PCR cloning kit (Thermo Scientific, California, USA) following the manufacturer’s protocol. The colonies of transformed bacteria were selected, re-submitted to PCR of the ITS1 gene and sequenced.
Blood-feeding study and PCR conditions
DNA extracted from blood-fed female sand flies was submitted to PCR for amplification of the Cytochrome B gene, which amplifies a fragment of approximately 359 bp using the cytb primers (forward: 5′-CCA TCC AAC ATC TCA GCA TGA TGA AA-3′; and reverse: 5′-GCC CCT CAG AAT GAT ATT TGT CCT CA-3′) [33]. DNA extraction from the females followed the same protocol as described previously but in a laminar flow booth and adopting disinfection measures with care not to contaminate samples with human DNA. The PCR reaction was performed using 1× buffer solution (200 mM Tris-HCl, pH 8.4; 500 mM KCl), 1.5 mM MgCl2, 0.2 mM dNTPs, 0.5 pmol LITSR primer, 0.5 pmol L5.8S primer, 1 U of Taq DNA polymerase platinum (Invitrogen, California, USA) and 5 μl template DNA, in a total volume of 50 μl. Samples were amplified in an automatic thermocycler (MaxyGene Gradient - AXYGEN, Corning, USA) with 32 cycles of 95 °C for 20 s (denaturation), 53 °C for 30 s (annealing) and 72 °C for 1 min (extension). As negative control, pure autoclaved water and DNA of male and female sand flies without the presence of intestinal blood were used. Five μl of the amplified product was analyzed on a 2% agarose gel stained with 0.1 μl of GelRed (Biotium Acid Gel Stain - Biotium, Fremont, USA) and compared to the band obtained by positive control (DNA amplified from canine clot).
Sequencing
For both the study of natural infection and that of food source, amplified PCR products were sent to Macrogen (South Korea) for sequencing using a 3730XL Applied Biosystems. The sequences obtained were edited using the program Sequencher, compared by BLAST with sequences available in GenBank.
Data analysis
Kruskal-Wallis was used to test the hypothesis of equality among the ecological descriptors in the different ecotopes studied. The measures of diversity among the environments were obtained using the Shannon-Wiener’s (H′) diversity index, the Pielou’s equitability index (J’), Margalef’s richness index and the Berger-Parker’s dominance index [34], followed by comparisons and Spearman’s correlation tests between species and climatic variables (temperature, relative humidity and rainfall). The analyses were performed using the Past 2.17c program and IBM SPSS 20 software, with a 5% level of significance. The minimum natural infection rate was calculated considering the number of positive pools multiplied by 100 and divided by the number of insects tested [35].
Results
A total of 2515 sand flies were collected of 43 species belonging to 13 genera: Brumptomyia, Bichromomyia, Evandromyia, Lutzomyia, Micropygomia, Migonemyia, Nyssomyia, Pintomyia, Pressatia, Psathiromyia, Psychodopygus, Sciopemyia and Tricophoromyia (Table 1). The genus Psychodopygus was the most frequently collected (17.0% of the total), followed by Evandromyia (10.0%), Thricophoromyia (10.0%) and Brumptomyia (10.0%). Evandromia saulensis was the third most abundant species in the collections (7.4%), and occurred in all of the studied ecotopes, but predominantly in the rural area (Table 2).
Evandromia saulensis (n = 66) was the most frequently captured species in Shannon traps, indicating the anthropophilic behavior of this species, followed by Psychodopygus davisi (n = 48). A total of 2304 specimens of 41 species were collected at rural sites, with the most abundant being Th. auraensis, Trichophoromyia sp., Ev.saulensis, Pr. calcarata and Ps. carrerai carrerai. In the urban park, 213 specimens of 26 species were collected, with the most abundant being Th. auraensis, Trichophoromyia sp. and Ny. antunesi. Sand fly abundance was higher in the forest and peridomestic environments in the rural area than in the urban forest (H = 17.9, df = 42, P < 0.05) (Fig. 2). In the rural area, mean species richness was 5.16 (S = 41) and mean equitability (J’) 0.61, whereas in the urban park the mean richness was lower at 4.66 (S = 26), and equitability higher (J’ = 0.72), since it is an environment with a more uniform pattern of abundance. The diversity index was higher in the urban forest, but not significantly different from that of the rural area according to the Kruskal-Wallis test (H = 1, df = 1, P > 0.05) for two samples. Species dominance, according to the Berger-Parker’s index, averaged 0.33 and 0.30 for the rural area and urban forest, respectively. There was a significant correlation between the total number of sand flies collected and mean temperature on the days of collection (Spearman’s rho = 0.58, n = 13, P = 0.03) (Table 3); however, there was no significant correlation between the number of sand flies collected by HP light traps and relative humidity (Spearman’s rho = -0.66, n = 13, P = 0.831) or monthly rainfall (Spearman’s rho = -0.38, n = 13, P = 0.45) during the study period. Species richness for forest and peridomestic environments in the rural area did not differ significantly (H = 0.04, df = 1, P > 0.05), although there was a greater number of species in the forest. The greatest diversity (Shannon index, H′) of species was in the primary forest of the urban forest, with 17 species (H′ = 2.39), followed by rural peridomestic environments (H′ = 2.11) with 26 species, rural forest (H′ = 1.99) with 32 species and secondary forest in the urban forest (H′ = 1.85) with 20 species (Table 2).
Leishmania infection in sand flies
A total of 206 female sand flies were collected using Shannon traps in a forest environment and used to determine natural infection by microscopy and molecular analysis. Sand fly species were grouped into 39 pools and 43 individual samples. Of these, 17 pools (Th. auraensi, 3 pools; Ev. saulensis, 2; Ev. walkeri, 2; Bi. flaviscutellata, 1; Ps. llanosmartinsi, 1; Pi. nevesi, 3; Ps. davisi, 2; Ps. ayrozai, 2; Lu. sherlocki, 1) and 8 individual samples (Pi. nevesi, 1; Ny. whitmanni, 2; Pa. aragaoi, 1; Ev. saulensis, 1; Ny. antunesi, 1; Lu. sherlocki, 1; Ev. infraspionsa, 1) were amplified for the target ITS1 region (Fig. 3). The microscopic analysis revealed that five specimens of Ev. saulensis had flagellated forms in the hindgut, with an infection rate of 2.4%. Of the analyzed samples, 13 sequences showed similarity (99%) with L. (V.) braziliensis as follows: Th. auraensis (n = 1); Ev. saulensis (n = 2); Ev. walkeri (n = 1); Ps. llanosmartinsi (n = 1); Pi. nevesi (n = 2); Ps. davisi (n = 1); Ps. ayrozai (n = 1); Pa. aragaoi (n = 1); Ny. antunesi (n = 1); and Ev. infraspinosa (n = 1). Only a specimen of Ps. ayrozai exhibited similarity (94%) to the L. (V.) guyanensis sequence deposited in the GenBank database (MF802812–MF802824).
PCR-ITS1-2% agarose gel, colored with GelRed showing ITS1 PCR amplification product from DNA extracted from female sand flies collected in the municipality of Rio Branco, Acre, Brazil. Lanes 1–4, 6, 10, 11 and 14: collected female sand flies positive for natural infection with Leishmania sp.; Lanes C1- and C2-: negative controls (Milli-Q ultrapure water); Lane C+: positive control (Leishmania (Viannia) braziliensis DNA) (MHOM/BR/75/M2903)
Sand fly blood sources
A total of 33 fed females were submitted to amplification of the cytb gene, of which 25 were collected in rural areas (Trichophoromyia sp.: 10; Pressatia sp.: 2; Ev. saulensis: 7; Ev. walkeri: 1; Ny. antunesi: 1; Ps. davisi: 1; Ps. hirsutus: 1; Ps. carrerai: 1; and Pi. nevesi: 1) and eight collected in the urban park (Trichophoromyia sp.: 1; Ev. saulensis: 1; Ev. walkeri: 1; Ny. antunesi: 2; Ps. davisi: 1; Pi. serrana: 1; and Mig. migonei: 1 (Fig. 4). After sequencing, all samples were found to be similar to the sequence deposited in GenBank (MG027586–MG027618) for Gallus gallus (domestic chicken).
PCR-cytb- 2% agarose gel colored with GelRed showing cytb PCR amplification product from DNA extracted from female sand flies collected in the municipality of Rio Branco, Acre, Brazil. Lanes 1–16: female sand flies collected containing a blood meal; Lane C+: positive control (canine blood clot); Lane C-: negative control (Milli-Q ultrapure water)
Discussion
In the state of Acre, ATL and environmental impacts have historically been related to the processes of human occupation, which from the 1970’s experienced a gradual conversion from the extractive model of the region to agricultural activity [28]. These environmental changes, associated with the construction of rural settlements, urbanization and massive logging with a view to human occupation, played a major role in the dissemination of this disease in the region and the state. The impacts resulting from the dynamics of land use in the Amazon have had environmental consequences such as high rates of deforestation, loss of biodiversity, adaptation of vector species to peridomestic environments, potentially leading to extinction of rare species or increasing others.
The increase in ATL cases reported in the North Region of Brazil suggests an adaptation of sand flies to transmit ATL agents in the peridomestic environment [11], also as a consequence of the impact of anthropic activities on the environment, including animal farming near houses. This may have favored the adaptation of the vectors to human dwellings and modified the transmission cycle of ATL [36]. Studies of geographical distribution [16], ecological indices of the fauna and rates of natural infection rates are scarce in Brazil. In this study, a total of 2515 sand flies were captured and the most abundant species sampled were Th. auraensis, indistinguishable females of Trichophoromyia sp., Ev. saulensis and Pressatia calcarata, altogether representing 73.8% of the collections. Of these, L. (V.) braziliensis was detected in Th. auraensis and Ev. saulensis, corroborating other studies in the state that point to Th. auraensis as an abundant species in the sampled environments in Acre [20,21,22, 26] and its potential involvement in the transmission cycle of Leishmania spp. Recently, in the municipality of Assis Brasil, this species was found infected with L. (V.) braziliensis and L. (V.) guyanensis by PCR-RFLP [22], indicating its importance in maintaining the circulation of Leishmania spp., although its vectorial competence has not yet been proven [37]. In Madre de Dios, Peru, Th. auraensis has been found infected with L. (V.) lainsoni and L. (V.) braziliensis [38], reinforcing the importance of this species and its possible capacity as a vector in these regions of the Amazon Basin.
Among potential vectors of ATL agents to humans, Ev. saulensis has not been considered of medical importance. Although it has a large distribution from central Brazil to Central America, this species is not commonly collected in sand fly studies. However, this species has been found infected with L. (V.) braziliensis in Rio Branco [26], and in the present study was the third most frequent species in light-trap collections, the most abundant species in the collections using Shannon, being present in all environments sampled and with dominance in the forest environment, confirming its anthropophilic behavior. We also found flagellated forms in five specimens of Ev. saulensis, reinforcing its participation in the enzootic transmission cycle of ATL and the need for studies of the vectorial capacity and competence in transmitting Leishmania to humans. Furthermore, the potential participation of Ev. saulensis in the transmission of ATL agents, in addition to the vectors already incriminated needs to be investigated within the state of Acre. The genus Psychodopygus was the most dominant genus in the collections, with a total of seven species collected, of which Ps. carrerai carrerai and Ps. davisi were the most abundant in the study. Species of this genus are frequent in faunal studies of this region and have been found naturally infected in the Amazon Region [39,40,41,42]. In our studies, Psychodopygus davisi was found infected with L. (V.) braziliensis, which corroborate a previous study carried out in Assis Brasil where parasite of the L. (V.) braziliensis complex was found [22]. Reinforcing the importance of this species in the region is that it has been found naturally infected with Leishmania sp. in the state of Rondônia [43, 44], and also infected by L. (V.) braziliensis in the Serra dos Carajás, Pará and in the neighboring country of Peru [38, 42]. Psychodopygus davisi has been frequently found in peridomesitc environments, suggesting its adaptation to anthropic environments. Based on its anthropophilic behavior, density and occurrence of natural infection of Leishmania, this species is incriminated in the transmission cycle of ATL in the Amazon Region [37, 43,44,45].
The species Ny. whitmani, Ny. antunesi, Bi. flaviscutellata, Ps. hirsutus hirsutus, Ps. ayrozai and Migonemyia migonei were found in low abundance in the different environments studied, yet these are considered vectors of species of Leishmania in the region [20]. Psychodopygus ayrozai was found naturally infected with L. (V.) guyanensis, reinforcing its role in the etiological agent’s circulation of ATL in the state. In other studies, L. (V.) guyanensis was isolated from human cases and in sand flies already described in other epidemiological studies [18, 22]. Psychodopygus ayrozai is common in the North Region of Brazil [6], especially in peridomiciliar areas, indicative of its high degree of anthropophilia [14]. In the Amazon Region, species of Psychodopygus are associated with ATL transmission and can be important vectors in zoonotic and enzootic cycles [46]. Thricophoromyia ubiquitalis was described as a vector of L. (V.) lainsoni in the state of Pará [16, 46]. In a study carried out near the Solimões River (AM), Th. ubiquitalis was found to be the predominant species, accounting for 57.94% of flies sampled, and was also found naturally infected by L. (V.) lainsoni [47]. In our study, only one specimen of Th. ubiquitalis was captured in a Shannon trap. Faunal studies of sand flies in three municipalities of Acre identified 52 species, among them Th. auraensis, Ny. antunesi, Ny. whitmani, and Ps. davisi were responsible for 66.95% of the collected flies, with Ny. whitmani being the most abundant [20]. In the present study, these species represented 40.7% of the species collected, with the most frequent being Th. auraensis (33.3%). In 2008, Silva-Nunes et al. [16] investigating the epidemiology of ATL and surveying the phlebotomine fauna in order to identify possible vectors in the municipality of Acrelândia, collected 14 species, three of which are known vectors of the disease: Ny. antunesi (59.1%), in the peridomestic environment and forests edges; Ny. whitmani, more frequent in peridomestic environment (15%) and the only one within a house; and Th. ubiquitalis, also in peridomestic environment. In this study, Ny. whitmany and Ny. antunesi were more frequent in forest environments.
In a faunal survey of three areas of the city of Rio Branco during the course of a single year, Araújo-Pereira et al. [21] found high diversity of sand flies, with the most abundant being Th. auraensis and Ny. whitmani, representing 72% of the collected specimens. Some of the species collected by these authors were already known as vectors of the parasite that causes ATL (i.e. Ny. whitmani, Ny. antunesi and Bi. flaviscutellata). These data corroborate the high frequency of Th. auraensis (33.3%) and other species known to be potentially vectors of Leishmania parasites to humans.
In Assis Brasil, Teles et al. [22] evidenced a rich and diverse sand fly fauna of 67 species, of which Th. auraensis/ruifreitasi and Ps. davisi were dominant. These species were collected in domestic and peridomestic forest environments, demonstrating their potential as vectors of ATL agents in the region.
In the present study, species diversity was lower in the rural area than in the urban forest, with a lower index of equitability due to high species dominance. Thus, sand fly frequency seems to be related to levels of environmental degradation and food supply provided by animals in peridomestic environments, with changing the behavior of those populations compared with sand flies in unaltered environments [48]. Thus, an environmental park interconnected to an area of environmental protection, even with low frequency and lower richness, suffers less anthropic effects, and thus tends to maintain the balance on species distribution in the ecosystem. The ecological indices in the different studied ecotopes did not show any significant differences, having the greatest diversity for primary forest in the urban park. Silva et al. [48] suggested that areas with vegetation in different successional stages may influence the dominance and frequency of sand flies. Also, high species diversity is common in the Amazon Region, as observed by other authors [37, 43, 47].
There was an increase in the abundance of sand flies collected with light traps at the beginning of the rainy season, from December to April, with smaller quantities during the dry months with low humidity and the occurrences of Amazonian cool periods. The species frequency had a significant positive correlation with temperature variation (Spearman’s rho = 0.58, P = 0.030), indicating that with increasing temperature there is an increase for abundance of collected sand flies. Other studies have recognized a similar pattern of seasonal variation in sand flies such in Campo Grande, Mato Gross do Sul, with an increase in the abundance of captured specimens after the months of highest rainfall and humidity [49]. It should be noted that there are distinct seasonal patterns for sand flies in different regions of Brazil. In Rio Grande do Norte, a study found that sand flies were most abundant during the dry season, and declined in abundance in the rainy season when temperatures decreased and relative humidity was high [50].
All fed female sand flies submitted to analysis of blood sources exhibited an amplified gene region compatible with Gallus gallus (domestic chicken). Although chickens do not act as reservoirs of Leishmania spp., their presence may influence on the population dynamics of sand flies with increasing population density in the peridomestic environment, which could contribute to increase the risk of the transmission of Leishmania to humans [51]. The presence of animal shelters (e.g. stables and chicken pen) in the peridomestic environment represents a permanent food supply and provides shelter for sand flies, as observed in studies in the State of Maranhão, where sand fly sampling was unsuccessful in environments without the presence of domestic animals [52]. Again, the presence of large numbers of chickens in peridomestic environments can be highly attractive to sand flies and may contribute to the maintenance of their life cycle, and thus constitute an epidemiological problem by keeping the vector near the human environment [52, 53]. It is worth noting that the presence of infected sand flies and the presence of synanthropic reservoirs can increment the transmission cycle in the peridomestic environment of the present study. Thus, in this study, domestic chicken appears to be a common blood source for sand flies, probably because it does not offer resistance and are kept in large number in the study area [53].
Conclusions
The sand fly fauna found in the present study was composed of 43 species and included known vectors of ATL, such as Nyssomyia whitmani, Ny. antunesi, Bichromomyia flaviscutellata and Mg. migonei. The high frequency of Trichophoromyia auraensis and Evandromyia saulensis, and the detection of L. (V.) braziliensis DNA, and Ps. ayrozai with L. (V.) guyanensis DNA, indicate that these species could be putative vectors for ATL in this Amazonian region. Investigation of blood sources of sand flies revealed a preference among female sand flies collected in this area for domestic chicken, which may be participating in the population dynamics of these insects.
References
Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde.Guia de Vigilância em Saúde / Ministério da Saúde, Secretaria de Vigilância em Saúde. – Brasília: Ministério da Saúde; 2014. p. 812
Lessa MM, Lessa HA, Castro TWN, Oliveira A, Scherifer A, Machado P, et al. Leishmaniose mucosa: aspectos clínicos e epidemiológicos. Rev Bras Otorrinolaringol. 2007;73:843–7.
Gontijo B, Carvalho MDL. Leishmaniose tegumentar americana. Rev Soc Bras Med Trop. 2003;36(1):71–80.
Lainson R, Shaw JJ. New world Leishmaniasis. In: Cox FEG, Kreier JP, Wakelin D, editors. Topley & Wilson’s Microbiology and Microbial Infections. Wiley; 2005. p. 313–49.
Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, West Indies, central and South America. Mem Am Entomol Inst. 1994;54:1–881.
Brazil RP, Rodrigues AAF, Filho JDA. Sand fly vectors of Leishmania in the Americas - a mini review. Entomol Ornithol Herpetol. 2015;4:144.
Brasil. Ministério da Saúde. Sistema de Informação de Agravos de Notificação - SINAN. http://portalsinan.saude.gov.br/. Accessed 28 Nov 2016.
Patz JA, Graczyk TK, Geller N, Vittor YA. Effects of environmental changes on emerging parasitic disease. Int J Parasitol. 2000;30:1395–405.
Petney TN. Environmental, cultural and social changes and their influence on parasite infections. Int J Parasitol. 2001;31:919–32.
Molyneux DH. Control of human parasitic diseases: context and overview. Adv Parasitol. 2006;61:1–45.
Silva NS, Muniz VD. Epidemiologia da leishmaniose tegumentar americana no Estado do Acre, Amazônia brasileira. Cad de Saúde Pública. 2009;25:1325–36.
Ximenes MFFM, Silva VPM, Queiroz PVS, Rego MM, Cortez AM, Batista LMM, et al. Flebotomíneos (Diptera: Psychodidae) e leishmanioses no Rio Grande do Norte, Nordeste do Brasil – reflexos do ambiente antrópico. Neotrop Entomol. 2007;36:128–37.
Ambroise-Thomas P. Emerging parasite zoonoses: the role of host-parasite relationship. Int J Parasitol. 2000;30:1361–7.
Basano SA, Camargo LMA. American cutaneous leishmaniasis: history, epidemiology and prospects for control. Rev Bras Epidemio. 2004;7:328–37.
Silva NS, Viana AB, Cordeiro JA, Cavasini CE. Leishmaniose tegumentar americana no estado do Acre, Brasil. Rev Saúde Públ. 1999;33:554–9.
Silva-Nunes M, Cavasini CE, Silva NS, Galati EAB. Epidemiologia da leishmaniose tegumentar e descrição das populações de flebotomíneos no município de Acrelândia, Acre, Brasil. Rev Bras de Epidemiol. 2008;11(2):241–51.
Tojal AC, Romero GAS, Cupolillo EA. A diversidade das espécies causadoras de leishmaniose cutânea em Rio Branco - Acre. Rev Soc Bras Med Trop. 2003;36:49.
Tojal AC, Cupolillo E, Volpini AC, Almeida R, Romero GAS. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of acre, Brazil. Tropical Med Int Health. 2006;11:1388–98.
Oliveira JGS, Novais FO, Oliveira CI, Cruz-Júnior AC, Campos LF, Rocha AV, et al. Polymerase chain reacton (PCR) is highly sensitive for diagnosis of mucosal leishmaniasis. Acta Trop. 2005;94:55–9.
Azevedo ACR, Costa SM, Pinto MCG, Souza JL, Cruz HC, Vidal J, et al. Studies on the sandfly fauna (Diptera: Psychodidae: Phlebotominae) from transmission areas of American cutaneous leishmaniasis in state of Acre, Brazil. Mem Inst Oswaldo Cruz. 2008;103:760–7.
Araújo-Pereira T, Fuzari AA, Andrade-Filho JD, Pita-Pereira D, Britto C, Brazil RP. Sand fly fauna (Diptera: Psychodidade: Phlebotominae) in an area of leishmaniasis transmission in the municipality of Rio Branco, sate of Acre, Brazil. Parasit Vectors. 2014;7:360.
Teles CBG, Santos APA, Freitas RA, Oliveira AFJ, Ogawa GM, Rodrigues MS, et al. Phlebotomine sandfly (Diptera: Psychodidae) diversity and their Leishmania DNA in a hot spot of American cutaneous leishmaniasis human cases along the Brazilian border with Peru and Bolivia. Mem Inst Oswaldo Cruz. 2016;111:423–32.
Pessoa FAC, Medeiros JF, Barrett TV. Effects of timber harvest on phlebotomine sand flies (Diptera: Psychodidae) in a production forest: abundance of species on tree trunks and prevalence of trypanosomatids. Mem Inst Oswaldo Cruz. 2007;102:593–9.
Pinheiro FG, Luz SLB, Franco AMR. Infecção natural por tripanosomatídeos (Kinetoplastida: Trypanosomatidae) em Lutzomyia umbratilis (Diptera: Psychodidae) em áreas de leishmaniose tegumentar americana no Amazonas, Brasil. Acta Amaz. 2008;38:165–72.
Reis SR, Gomes LHM, Ferreira NM, Nery LR, Pinheiro FG, Figueira LP, et al. Ocorrência de flebotomíneos (Diptera: Psychodidae: Phlebotominae) no ambiente peridomiciliar em área de foco de transmissão de leishmaniose tegumentar no município de Manaus, Amazonas. Acta Amaz. 2013;43:121–3.
Araujo-Pereira T, Pita-Pereira D, Boité MC, Melo M, Costa-Rego TA, Fuzari AA, et al. First description of Laishmania (Viannia) infection in Evandromyia saulensis, Pressatia sp. and Trichophoromyia auraensis (Psychodidae: Phlebotominae) in a transmission area of cutaneous leishmaniases in Acre state, Amazon Basin, Brazil. Mem Inst Oswaldo Cruz. 2017;112:75–8.
IBGE. Instituto Nacional Brasileiro de Geografia e Estatística. Anuário Estatístico do Brasil. 2016. www.ibge.gov.br. Accessed 28 Nov 2016.
Acre. Programa Estadual de Zoneamento Ecológico Econômico Fase II: documento Síntese Rio Branco. Acre: SEMA; 2010.
Foranttini OP. Entomologia Médica IV. Psychodidae: Phlebotominae, Leishmaniose e Bartonelose. São Paulo: Edgar Blucher Ltda; 1983. p. 658.
Galati EAB. Morfologia e Taxonomia: Morfologia, terminologia de adultos e identificação dos táxons da América, Flebotomíneos do Brasil, Editora Fiocruz; 2003. p. 176.
Marcondes CB. A proposal of generic and subgeneric abbreviations of phlebotomines sandflies (Diptera: Psychodidae: Phlebotominae) of the world. Entomol News. 2007;118:351–6.
Schonian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, Jaffe CL. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349–58.
Steuber S, Abdel-Rady A, Clausen P-H. PCR-RFLP analysis: a promising technique for host species identification of blood meals from tsetse flies (Diptera: Glossinidae). Parasitol Res. 2005;97:247–54.
Magurran AE. Measuring biological diversity. Oxford: Blackwell Science; 2004.
Paiva BR, Secundino NFC, Pimenta PFP, Galati EAB, Andrade-Junior HF, Malafronte RDS. Standardization of conditions for PCR detection of Leishmania spp. DNA in sandflies (Diptera, Psychodidae). Cad Saude Pública. 2007;23:87–94.
Costa SM, Cechinel M, Banderia V, Zannuncio JC, Lainson R, Rangel EF. Lutzomyia (Nyssomyia) whitmani s.l. (Antunes & Coutinho, 1939) (Diptera: Psychodidae: Phlebotominae): geographical distribution and the epidemiology of American cutaneous leishmaniasis in Brazil mini-review. Mem Inst Oswaldo Cruz. 2007;102:149–53.
Ogawa GM, Pereira Júnior AM, Resadore F, Ferreira RGM, Medeiros JF, Camargo LMA. Sandfly fauna (Diptera: Psychodidae) from caves in the state of Rondônia, Brazil. Braz J Vet Parasitol. 2016;25(1):61–8.
Valdivia HO, De Los Santos MB, Fernandez R, Baldeviano GC, Zorrilla VO, Vera H, et al. Natural Leishmania infection of Lutzomyia (Trichophoromyia) auraensis in Madre de Dios, Peru, detected by a fluorescence resonance energy transfer-based real-time polymerase chain reaction. Am J Trop Med Hyg. 2012;87:511–7.
Silva DF, Freitas RA, Franco AM. Diversidade e abundância de flebotomíneos do gênero Lutzomyia (Diptera: Psychodidae) em áreas de mata do nordeste de Manacapuru, AM. Neotrop Entomol. 2007;36(1):138–44.
Neto GJL, Baima JM, Freitas RAD, Passos MAB. Fauna flebotomínica (Diptera: Psychodidae) em floresta preservada e alterada do Município de Caroebe, Estado de Roraima, Brasil. Rev Pan-Amaz Saude. 2012;3:41–6.
Neto GJL, Freitas RAD, Baima JM, Passos MAB. Fauna flebotomínica (Diptera: Psychodidae) da Serra do Tepequém, Município de Amajarí, Estado de Roraima, Brasil. Rev Pan-Amaz Saude. 2010;1:131–6.
Souza AAA, Silveira FT, Lainson R, Barata IDR, Silva MDGS, Lima JAN, et al. Fauna flebotomínica da Serra dos Carajás, Estado do Pará, Brasil, e sua possível implicação na transmissão da leishmaniose tegumentar americana. Rev Pan-Amaz Saude. 2010;1:45–51.
Gil LHS, Basano SA, Souza AA, Silva MGS, Barata I, Ishikawa EA, et al. Recent observations on the sand fly (Diptera: Psychodidae) fauna of the state of Rondônia, western Amazônia, Brazil: the importance of Psychdopygus davisi as a vector of zoonotic cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2003;98:751–5.
Grimaldi G, Momen H, Naiff RD, McMahon-Pratt D, Barrett TV. Characterization and classification of leishmanial parasites from humans, wild mammals, and sand flies in the Amazon region of Brazil. Am J Trop Med Hyg. 1991;44:645–61.
Alves VR, Freitas RA, Santos FL, Oliveira AFJ, Barrett TV, Shimabukuro PHF. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the state of Amazonas, Brazil. Rev Bras Entomol. 2012;56:220–7.
Silveira FT, Souza AAA, Lainson R, Shaw JJ, Braga RR, Ishikawa EA. Cutaneous leishmaniasis in the Amazon region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará state, Brazil. Mem Inst Oswaldo Cruz. 1991;186:127–30.
Pereira AM Jr, Teles CBG, dos Santos APA, Souza Rodrigues MD, Marialva EF, Pessoa FAC, et al. Ecological aspects and molecular detection of Leishmania DNA Ross (Kinetoplastida: Trypanosomatidae) in phlebotomine sandflies (Diptera: Psychodidae) in terra firme and várzea environments in the middle Solimões region, Amazonas state, Brazil. Parasit Vectors. 2015;8:180.
Silva AMD, Camargo NJD, Santos DRD, Massafera R, Ferreira AC, Postai C, et al. Diversidade, distribuição e abundância de flebotomíneos (Diptera: Psychodidae) no Paraná. Neotrop Entomol. 2008;37:209–25.
Oliveira AGD, Andrade Filho JD, Falcão AL, Brazil RP. Estudo de flebotomíneos (Diptera, Psychodidae, Phlebotominae) na zona urbana da cidade de Campo Grande, Mato Grosso do Sul, Brasil, 1999-2000. Cad Saúde Pública. 2003;19:933–44.
Pinheiro MPG, Silva JHT, Cavalcanti KB, de Azevedo PRM. Ecological interactions among phlebotomines (Diptera: Psychodidae) in an agroforestry environment of Northeast Brazil. J Vector Ecol. 2013;38(2):307–16.
Teodoro U, La Salvia FV, Lima EM, Spinosa RP, Barbosa OC, Ferreira MEMC, et al. Observações sobre o comportamento de flebotomíneos em ecótopos florestais e extraflorestais, em área endêmica de leishmaniose tegumentar americana, no norte do Estado do Paraná, sul do Brasil. Rev Saúde Pública. 1993;27:242–9.
Dias FDOP, Lorosa ES, Rebêlo JMM. Fonte alimentar sangüínea e a peridomiciliação de Lutzomyia longipalpis (Lutz & Neiva, 1912) (Psychodidae, Phlebotominae). Cad Saúde Pública. 2003;19:1373–80.
Brazil RP, De Almeida DC, Brazil BG, Mamede SM. Chicken house as a resting site of sandflies in Rio de Janeiro, Brazil. Parassitologia. 1991;33:113–7.
Acknowledgements
We thank the Municipal Government of Rio Branco, the Service of entomology and chemical Control of municipality, Janis Lunier de Souza and Acigelda da Silva Cardoso for their help and Arthur Alexandre de Souza de Vieira for support with field collections; to Cleilton Sampaio for making the map of the sampling sites; and to the Federal Institute of Acre (IFAC) and the Oswaldo Cruz Institute (IOC) through the Cooperation Instrument 004/2012 IFAC/IOC.
Funding
This study was supported by the Fundação de Amparo à Pesquisa do Estado do Acre (FAPAC) (grant agreement number PPSUS n°00011878); the Foundation for Scientific and Technological Development (FIOTEC), which provided the scholarship granted to Ávila MM; and Conselho Nacional de Pesquisas (CNPq).
Availability of data and materials
All data generated or analyzed during this study are included in the article and are available from the corresponding author upon reasonable request. Representative sequences are submitted in the GenBank database under accession numbers MF802812–MF802824 and MG027586–MG027618.
Author information
Authors and Affiliations
Contributions
MMA and RPB designed the study. AF, MMA, CFS and EABG performed the fieldwork. MMA, AFB, RPB and EABG prepared the materials and identified phlebotomine specimens. MMA, AFB and CFS performed the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
de Ávila, M.M., Brilhante, A.F., de Souza, C.F. et al. Ecology, feeding and natural infection by Leishmania spp. of phlebotomine sand flies in an area of high incidence of American tegumentary leishmaniasis in the municipality of Rio Branco, Acre, Brazil. Parasites Vectors 11, 64 (2018). https://doi.org/10.1186/s13071-018-2641-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-2641-y