Abstract
Background
There is a paucity of data about the susceptibility status of malaria vectors to Public Health insecticides along the Thailand-Myanmar border. This lack of data is a limitation to guide malaria vector-control in this region. The aim of this study was to assess the susceptibility status of malaria vectors to deltamethrin, permethrin and DDT and to validate a simple molecular assay for the detection of knock-down resistance (kdr) mutations in the study area.
Methods
Anopheles mosquitoes were collected in four sentinel villages during August and November 2014 and July 2015 using human landing catch and cow bait collection methods. WHO susceptibility tests were carried out to measure the mortality and knock-down rates of female mosquitoes to deltamethrin (0.05%), permethrin (0.75%) and DDT (4%). DNA sequencing of a fragment of the voltage-gated sodium channel gene was carried out to identify knock-down resistance (kdr) mutations at position 1014 in mosquitoes surviving exposure to insecticides.
Results
A total of 6295 Anopheles belonging to ten different species were bioassayed. Resistance or suspected resistance to pyrethroids was detected in An. barbirostris (s.l.) (72 and 84% mortality to deltamethrin (n = 504) and permethrin (n = 493) respectively), An. hyrcanus (s.l.) (33 and 48% mortality to deltamethrin (n = 172) and permethrin (n = 154), respectively), An. jamesii (87% mortality to deltamethrin, n = 111), An. maculatus (s.l.) (85 and 97% mortality to deltamethrin (n = 280) and permethrin (n = 264), respectively), An. minimus (s.l.) (92% mortality, n = 370) and An. vagus (75 and 95% mortality to deltamethrin (n =148) and permethrin (n = 178), respectively). Resistance or suspected resistance to DDT was detected in An. barbirostris (s.l.) (74% mortality, n = 435), An. hyrcanus (s.l.) (57% mortality, n = 91) and An. vagus (97% mortality, n = 133). The L1014S kdr mutation at both heterozygous and homozygous state was detected only in An. peditaeniatus (Hyrcanus Group).
Conclusion
Resistance to pyrethroids is present along the Thailand-Myanmar border, and it represents a threat for malaria vector control. Further investigations are needed to better understand the molecular basis of insecticide resistance in malaria vectors in this area.
Similar content being viewed by others
Background
Vector-borne diseases account for 17% of all infectious diseases, causing more than 1 million deaths annually [1]. Malaria causes more than 600,000 deaths every year, mostly in children under 5 years [2]. Vector-control is an essential component of malaria control, and it relies mainly on long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [3]. In low transmission settings, especially where residual transmission (i.e. transmission that is not prevented by LLINs and IRS) is prominent, LLINs and IRS must be supplemented by other methods to target early feeding, exophagic and zoophagic malaria vectors [4].
Pyrethroids are widely used for vector control because of their strong insecticidal effect and their low toxicity to mammals [5]. They induce a rapid “knock-down” (KD), and they have irritant and excito-repellent properties against susceptible mosquitoes [6]. Sadly, pyrethroid resistance in malaria vectors has been identified in at least 64 countries [5] and is spreading worldwide [7] because of the intense selection pressure caused by agricultural practices [8] and the large-scale implementation of malaria vector control interventions [7]. Resistance has been implicated in the reduced efficacy of vector control interventions such as IRS and LLIN [9, 10] and malaria resurgence [11–17]. Routine monitoring of resistance and detection of temporal changes in both prevalence and intensity of resistance are needed to guide malaria vector interventions and resistance management plan [18].
Compared to Africa, there is a notable lack of data on insecticide resistance in Southeast Asian malaria vectors. Previous report of resistance in malaria vectors from the GMS are summarised in Table 1. Resistance to several pyrethroids and decreased susceptibility to DDT were described for An. minimus (s.s.) in Vietnam, Thailand and Cambodia [19, 20]. Resistance to organophosphate was reported in An. maculatus and An. sawadwongporni in northern Thailand [21]. Suspected resistance to pyrethroids (Vietnam) and DDT (Cambodia) was described in An. dirus (s.s.) [19] and suspected resistance to DDT and fenitrothion were reported in laboratory reared An. cracens from Thailand and Malaysia [22]. High levels of resistance to DDT and permethrin were reported in An. vagus in the Greater Mekong Subregion (GMS) [19, 23].
The mechanisms of insecticide resistance in mosquitoes are multiple and include behavioural and physiological changes leading to insecticide avoidance, reduced penetration, sequestration, target site modification (knock-down resistance or kdr mutation for pyrethroids and DDT) and increased biodegradation [13, 24, 25]. With the exception of An. sinensis [26–30], resistance mechanisms remain largely unknown in Southeast Asian malaria vectors. In this region, metabolic resistance is thought to play a major role compared to target site mutations [27, 31]. Only a few studies have been conducted to understand the pathways involved in metabolic resistance, and they were limited to a laboratory adapted deltamethrin-resistant strain of An. minimus (s.s.) from Thailand [32–34]. Kdr mutations were reported in several anopheline species [23, 35] but were not detected in the major malaria vectors in the GMS [31].
The aim of the present study is to investigate the susceptibility status of malaria vectors to Public Health insecticides (deltamethrin, permethrin and DDT) along the Thailand-Myanmar border (TMB), and to assess the presence of kdr mutations [35]. Resistance data are deemed important to design more effective vector control interventions in the area.
Methods
Study sites and mosquito collection
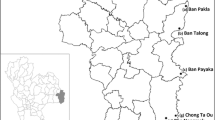
Mosquito collections were carried out in four Karen villages located on the Myanmar side of the TMB, namely Htoo Pyin Nyar (TPN, 17°14'N, 98°29'E), Tar Au Ta (TOT, 16°36'N, 98°57'E), Ka Nu Hta (KNH, 17°18'N, 98°24'E) and Htee Kaw Taw (HKT, 16°85'N, 98°47'E) (Fig. 1). Three entomological surveys were conducted in August and November 2014 and July 2015 using human landing catch (HLC) and cow-bait collection (CBC) methods. Mosquitoes were collected individually using 5 ml plastic tubes and shipped daily to the Shoklo Malaria Research Unit (Mae Sot, Thailand). Anopheles were individually transferred to a clean and transparent plastic tube while still alive and identified by morphology [36]. The mosquitoes belonging to different species were separated into different cups and reared for 1–4 days until enough specimens were collected to run the bioassay. Mosquitoes collected by HLC and CBC were pooled together to increase the sample size.
Map of the study sites. Three entomological surveys were conducted in four villages situated on the Myanmar side of the Thai-Myanmar border (HKT, Htee Kaw Taw; KNH, Ka Nu Hta; TPN, Htoo Pyin Nyar; and TOT, Tar Au Ta). Mosquitoes were collected using indoor and outdoor Human Landing Catch (HLC) in five sites and using Cow Bait Collection (CBC) in one site. Mosquitoes were shipped daily at the Shoklo Malaria Research Unit (SMRU, Mae Sot, Thailand) for identification, rearing and bioassays
Susceptibility tests
Susceptibility tests were performed following the WHO guidelines for insecticide resistance monitoring in malaria vectors [37]. All materials used in this study were provided by the Vector Control Research Unit (VCRU), Universiti Sains Malaysia. Wild caught female Anopheles were exposed for 60 min to papers impregnated with deltamethrin (0.05%), permethrin (0.75%) or DDT (4%). The susceptibility status of two Anopheles laboratory strains [An. minmus (s.s.) from Kasetsart University, Bangkok, Thailand and An. scanloni, Shoklo Malaria Research Unit, Mae Sot, Thailand] were also determined (50 mosquitoes for each insecticide and the control). The number of knocked-down (KD) mosquitoes was recorded every 5 min for 60 min. The females were then transferred into holding tubes, provided with a sugar solution (10%), and kept at 26 °C with a relative humidity of 80%. Mortality was recorded after a 24 h observation period. Mosquitoes exposed for 1 h to paper impregnated with the carrier (silicone oil mixed with acetone) were used as controls. Tests were replicated when a sufficient number of specimens were collected. Results were interpreted as per WHO guidelines: confirmed resistance (mortality below 90%), suspected resistance (mortality between 90 and 98%) and susceptible (mortality over 98%) [37].
kdr mutation detection
DNA was extracted from a leg of each specimen as previously described [38]. Amplification of a 300 bp segment of the voltage-gated sodium channel (VGSC) gene flanking the 1014 position was performed using the primer pair Ag-F kdr (5'-GAC CAT GAT CTG CCA AGA TGG AAT-3') and An-kdr-R2 (5'-GAG GAT GAA CCG AAA TTG GAC-3') described by Syaffrudin et al. [35]. The PCR mix was composed of 1 unit of Tfi DNA polymerase (Invitrogen™, Carlsbad, United States), 200 μM of dNTP mix (Invitrogen) which corresponded to 200 μM of each dNTP, 1.5 mM of MgCl2 (Invitrogen), and 400 μM of each primer. The PCR was conducted in a total reaction volume of 50 μl (3 μl of DNA template and 47 μl of PCR mix). The thermocycling protocol consisted in a first cycle of 5 min at 94 °C then 30 s at 45 °C, followed by 29 cycles of 30 s at 94 °C, 30 s at 50 °C and 1 min at 72 °C. The PCR product was sequenced by Macrogen™ (Seoul, South-Korea) using both primers. Each sequence was checked and cleaned manually using the Bioedit software version 7.1.9 (http://www.mbio.ncsu.edu//BioEdit/bioedit.html). A consensus sequence was generated for each specimen using the CAPS3 sequence assembly program [39] and then aligned using the Clustal Omega multiple sequence alignment program [40–42] (GenBank accession numbers KY677707–KY677716).
Molecular identification of Anopheles by ITS2 sequencing
Amplification of the ITS2 was performed using the primer pair ITS2A (5'-TGT GAA CTG CAG GAC ACA T-3') and ITS2B (5'-ATG CTT AAA TTY AGG GGG T-3') described by Beebe et al. [43]. The PCR mix was composed of 1 unit of Goldstar DNA polymerase (Eurogentec™, Seraing, Belgium) and 400 μM of each primer. The PCR was conducted in a total reaction volume of 25 μl (4 μl of DNA template and 21 μl of PCR mix). The thermocycling protocol consisted in an initial activation step of 1 min at 94 °C, followed by 40 amplifcation cycles of 20 s at 94 °C, 20 s at 51 °C and 30 s at 72 °C, and a final elongation step of 30 s at 72 °C. The PCR product was sequenced by Macrogen™ (Seoul, South-Korea) using the ITS2A primer. Anopheles species was determined using the blastn algorithm of the online BLAST™ software [44] (accession numbers KY677698–KY677706).
Data analysis
Adult mortality rate was corrected by the formula of Abbott [45] in the case of mortality > 5% in the control. Tests with > 20% mortality in the control were excluded from the analysis. Knock-down time 50 (KDT50) was determined by the log-probit method described by Finney [46] using R software [47]. The R code used to determine the knock-down time 50 (KDT50) and its confidence interval were adapted from Johnson et al. 2013 [48].
Results
Bioassays
In total, 5896 Anopheles (belonging to 9 groups of species) were collected in the villages and used to assess the insecticidal activity of deltamethrin 0.05% (n = 1,805), permethrin 0.75% (n = 1483) and DDT 4% (n = 1202) (Tables 2, 3 and 4). A total of 1406 specimens were used for the control (Tables 2, 3 and 4). Overall, the mean mortality in the control batch was 8.4% (119/1406), 3 of 46 tests were excluded from the analysis because the control mortality was > 20% (91/5896 specimens).
Mortality and knock-down (KD) rates are reported in Fig. 2 whereas the kinetic of KD (evolution of the KD rate over a 60 min observation period) is shown in Fig. 3 (data were analysed at the species complex or group when accurate identification at species level could not be done). Laboratory strain of An. minimus (s.s.) was susceptible to all insecticides whereas laboratory strain of An. scanloni was resistant to DDT (mortality of 84%, 42/50). The KDT50 of these strains were 10, 13 and 38 min for An. minimus (s.s.) and 20, 26 and 63 min for An. scanloni with deltamethrin, permethrin and DDT, respectively. Suspected resistance to deltamethrin was detected in wild caught An. minimus (s.l.) (92% mortality, 339/370). Resistance to deltamethrin and suspected resistance to permethrin were detected in An. maculatus (s.l.) (85 and 97% mortality, 239/280 and 257/264, respectively). Anopheles barbirostris (s.l.) and An. hyrcanus (s.l.) were resistant to all insecticides (mortality rates < 90%). Anopheles vagus was resistant to deltamethrin (75% mortality, 111/148) and suspected resistance to permethrin and DDT was also reported for this species (95 and 97% mortality, 169/178 and 123/133, respectively). Resistance to deltamethrin was detected in An. jamesii (s.l.) (87%, 97/111) whereas An. annularis (s.l.), An. tessellatus and An. kochi were susceptible to this pyrethroid insecticide. The susceptibility status of these latter species to permethrin and DDT could not be determined due to a very low sample size. Overall, the KDT50 varied from 10 to 131 min, from 13 to 116 min and 23 to 84 min for deltamethrin, permethrin and DDT, respectively (Tables 2, 3 and 4).
Mortality and knock-down (KD) rate determined following the WHO susceptibility test procedure for insecticide monitoring in malaria vectors. Alive female Anopheles were exposed during 1 h to insecticides (deltamethrin 0.05%, permethrin 0.75% and DDT 4%). KD rate was recorded at the end of the exposition period; the mortality rate was recorded after a 24 exposition period. Abbreviation: NA, not available
Kdr detection
A PCR assay for kdr detection developed by Syaffrudin et al. [35] was first validated on ten species complexes collected along the TMB using two specimens per species (Fig. 4). The presence of kdr mutations was assessed in specimens surviving exposure to insecticide (post bioassays) to increase the chance to detect SNPs. The presence of kdr mutation was not investigated in An. annularis (s.l.), An. kochi and An. tessellatus considering the high mortality rates observed in bioassays (Table 5). The sequence alignment of the PCR product is presented in Fig. 4a. The non-synonymous L1014S mutation was found in An. hyrcanus (s.l.) (Fig. 4a, b) at both heterozygous (19%, 6/31) or homozygous state (10%, 3/31) (Table 5). No kdr mutations were detected in other anopheline species (Table 5).
Molecular detection of the 1014 knock-down resistance (kdr) mutation on the voltage-gated sodium channel (VGSG) gene. a DNA sequence alignment of the fragment of VGSC gene encompassing nucleotides corresponding to the codon 1014 in various Anopheles species collected on the TMB. Bold character indicates the coding part of the DNA sequence (exon 20); codon 1014 is figured in red. b Consensus amino-acid sequence of the exon 20 determined for each anopheline taxa. The non-synonymous TCG polymorphism detected in An. hyrcanus (s.l.) is responsible for the L1014S kdr mutation; both heterozygous and homozygous mutations were detected. Other polymorphisms are either synonymous or located on non-coding part of the DNA sequence (GenBank Accession numbers KY677707–KY677716)
Discussion
In this study, we assessed the susceptibility status of several anopheline taxa to three public health insecticides (deltamethrin, permethrin and DDT) along the TMB. To our knowledge, this is the first study reporting the susceptibility status of Anopheles mosquitoes to pesticides used for malaria control in this area. These data are important in the context of malaria elimination to select the most appropriate insecticides for vector control.
Bioassays were performed following the WHO guidelines for insecticide resistance monitoring in malaria vectors [37]. However, the WHO recommends the use of unfed 2–5 days old female Anopheles to assess the susceptibility of wild malaria vectors populations to insecticides. This implies to collect a sufficient number of larvae, which was not possible in the present study considering the difficulty of accessing to the collection sites. Since only adults were collected to run the bioassays, the mortality might be underestimated [49, 50]. Another limitation of the study was the identification of malaria vectors at the species level. Indeed most of Anopheles collected in the villages belong to complexes of sibling species i.e. groups of several species that are not distinguishable using conventional morphology criterions [51]. Previous data in the same study villages showed that An. minimus (s.s.) represents > 99% of the Minimus complex whereas An. maculatus (s.s.) and An. sawadwongporni were predominant within Maculatus group (unpublished data). Moreover, it was not always possible to reach the sample size recommended by the WHO for each test (80 mosquitoes for each insecticide and at least 20 mosquitoes for the control). Thus the interpretation of the results must take into account the sample size and the corresponding error bars.
In this study, suspected resistance to deltamethrin was detected in An. minimus (s.s.). Resistance to DDT was previously reported in Cambodia, Vietnam and Thailand [19, 20, 52] whereas resistance to pyrethroids (permethrin, alpha-cypermethrin and lambda-cyhalothrin) was only detected in Vietnam [19]. Few data have been collected so far on the susceptibility of An. maculatus (s.s.) and An. sawadwongporni to insecticides. In Thailand, resistance to DDT was documented by the Offices of Vector-Borne Diseases Control [52]. Overgaard et al. [21] later reported resistance of both sibling species to methyl-parathion in the Northern part of the country. No resistance to organophosphates, pyrethroids and DDT was detected however in neighbouring Malaysia [53, 54].
Anopheles barbirostris (s.l.) (confirmed secondary vector) and An. hyrcanus (s.l.) (suspected vector) were resistant to all insecticides. Pyrethroid resistance in An. barbirostris has only been described before in studies conducted in Indonesia [35, 55] and Sri Lanka [56]. Confirmed resistance to deltamethrin and suspected resistance to permethrin and DDT in An. vagus (suspected vector) are in agreement with a previous report showing a high level of resistance within the Hyrcanus group and in An. vagus [19, 23]. Unfortunately, it was not possible to generate data on the susceptibility of primary vectors belonging to the Dirus complex because of the low number of specimens collected.
Overall resistance to pyrethroids was reported in six out of the ten species complexes tested along TMB which suggests a strong selection pressure in the studied area. In northern Thailand, Overgaard et al. [21] previously demonstrated that resistance is likely to arise from the intense use of pesticides for agriculture (especially for organophosphates compounds, a class of insecticides that has never been used for vector control purposes). This must be taken into account by policy makers as additional use of insecticide (especially pyrethroids) for vector-control may lead to a rapid selection of a resistant phenotype as observed previously in Africa [8, 57]. Further efforts should be made to document the susceptibility status of malaria vectors to other classes of insecticides (such as carbamates, organophosphates and insect growth regulators) that could be used as an alternative to pyrethroids in the frame of resistance management strategies [58–60].
In this study, we briefly investigated the molecular mechanisms involved in pyrethroid and DDT resistance by using a PCR assay adapted from Syafruddin et al. [35]. The kdr mutation is known to induce a cross-resistance to both DDT and pyrethroids, and to be associated with an increase in the KDT50 values [6, 27]. Therefore we suspected this mechanism to be involved in the resistance of An. hyrcanus (s.l.) to the insecticides tested. We reported the occurrence of the L1014S kdr mutation in An. hyrcanus (s.l.) at a low frequency in the specimens surviving insecticide exposure. Specimens carrying a kdr mutation were further identified as An. peditaeniatus using molecular methods (data not shown) (Accession numbers: KY677698–KY677706). This finding confirms the previous report of the L1014S mutation in An. peditaeniatus populations from Southern Vietnam and Cambodia [23]. However, the occurrence of kdr mutations in other positions cannot be ruled out. For example, another kdr mutation (N1575Y) occurring within the domain VIII of the VGSG gene was found in An. gambiae from West Africa occurring in a V1014F haplotypic background [61]. Several authors have stressed previously that the absence of kdr mutations in most of the Anopheles species tested suggests that metabolic resistance is probably the main route of insecticide resistance in malaria vectors in Southeast Asia (i.e. An. minimus (s.l.), An. maculatus (s.l.) and An. dirus (s.l.)) [27, 31]. The metabolic basis of insecticide resistance in malaria vectors in the GMS remains largely unknown. The only metabolic mechanism described so far is the over-expression of two P450 isoforms (CYP6P7 and CYP6AA3) suspected to metabolise several pyrethroids [62] in a laboratory colony of deltamethrin-resistant An. minimus (s.s.) from Thailand [34, 63]. Further efforts should be made to decipher the molecular basis of insecticide resistance in Southeast Asian malaria vectors.
Conclusion
Pyrethroid resistance seems to be widespread in Anopheles populations from the Thailand-Myanmar border. Documenting the susceptibility to other classes of insecticides is important in the framework of malaria control and elimination. Molecular basis of the resistance remains largely unknown in most of the Southeast Asian malaria vectors. Additional efforts should be made to identify molecular markers allowing the routine monitoring of insecticide resistance in this area.
Abbreviations
- DDT:
-
Dichlorodiphenyltrichloroethane
- DNA:
-
Deoxyribonucleic acid
- GMS:
-
Greater Mekong Subregion
- IRS:
-
Indoor residual spraying
- KD:
-
Knock-down
- kdr mutation:
-
Knock-down resistance mutation
- KDT50:
-
Knock-down time 50
- LLINs:
-
Long-lasting insecticide-treated nets
- TMB:
-
Thailand-Myanmar border
- VCRU:
-
Vector Control Research Unit
- VGSC:
-
Voltage-gated sodium channel
- WHO:
-
World Health Organisation
References
Prüss-Üstün A, Corvalán CF, World Health Organization. Preventing disease through healthy environments: towards an estimate of the environmental burden of disease. Geneva: World Health Organization; 2006.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11.
Killeen GF. Characterising, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330.
WHO. Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization; 2012.
Hougard JM, Duchon S, Darriet F, Zaim M, Rogier C, Guillet P. Comparative performances, under laboratory conditions, of seven pyrethroid insecticides used for impregnation of mosquito nets. Bull World Health Organ. 2003;81(5):324–33.
Corbel V, N’Guessan R. Distribution, mechanisms, impact and management of insecticide resistance in malaria vectors: a pragmatic review. In: Manguin S, editor. Anopheles mosquitoes - New insights into malaria vectors. Rijeka: InTech; 2013.
Diabate A, Baldet T, Chandre F, Akoobeto M, Guiguemde TR, Darriet F, et al. The role of the agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67(6):617–22.
N’Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13(2):199–206.
Asidi A, N’Guessan R, Akogbeto M, Curtis C, Rowland M. Loss of household protection from use of insecticide-treated nets against pyrethroid-resistant mosquitoes, benin. Emerg Infect Dis. 2012;18(7):1101–6.
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122.
Harrus S, Baneth G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int J Parasitol. 2005;35(11–12):1309–18.
Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–59.
McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 2014;90(4):597–604.
Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. Plos Med. 2014;11(3):e1001619.
Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11(12):925–32.
Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5. doi:10.7554/eLife.16090.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32(3):187–96.
Van Bortel W, Trung HD, Thuan le K, Sochantha T, Socheat D, Sumrandee C, et al. The insecticide resistance status of malaria vectors in the Mekong region. Malar J. 2008;7:102.
Somboon P, Prapanthadara LA, Suwonkerd W. Insecticide susceptibility tests of Anopheles minimus s.l., Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus in northern Thailand. Southeast Asian J Trop Med Public Health. 2003;34(1):87–93.
Overgaard HJ, Sandve SR, Suwonkerd W. Evidence of anopheline mosquito resistance to agrochemicals in northern Thailand. Southeast Asian J Trop Med Public Health. 2005;36 Suppl 4:152–7.
Hii JL. Insecticide susceptibility studies of three cryptic species of the Anopheles balabacensis complex. Southeast Asian J Trop Med Public Health. 1984;15(1):104–11.
Verhaeghen K, Van Bortel W, Trung HD, Sochantha T, Keokenchanh K, Coosemans M. Knockdown resistance in Anopheles vagus, An. sinensis, An. paraliae and An. peditaeniatus populations of the Mekong region. Parasit Vectors. 2010;3(1):59.
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91.
David JP, Ismail HM, Chandor-Proust A, Paine MJ. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos Trans R Soc Lond B Biol Sci. 2013;368(1612):20120429.
Zhu G, Zhong D, Cao J, Zhou H, Li J, Liu Y, et al. Transcriptome profiling of pyrethroid resistant and susceptible mosquitoes in the malaria vector, Anopheles sinensis. BMC Genomics. 2014;15:448.
Chang X, Zhong D, Fang Q, Hartsel J, Zhou G, Shi L, et al. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China. Plos Negl Trop Dis. 2014;8(5):e2889.
Chang X, Zhong D, Lo E, Fang Q, Bonizzoni M, Wang X, et al. Landscape genetic structure and evolutionary genetics of insecticide resistance gene mutations in Anopheles sinensis. Parasit Vectors. 2016;9:228.
Feng X, Yang C, Yang Y, Li J, Lin K, Li M, et al. Distribution and frequency of G119S mutation in ace-1 gene within Anopheles sinensis populations from Guangxi, China. Malar J. 2015;14:470.
Zhou D, Liu X, Sun Y, Ma L, Shen B, Zhu C. Genomic analysis of detoxification supergene families in the mosquito Anopheles sinensis. Plos One. 2015;10(11):e0143387.
Verhaeghen K, Van Bortel W, Trung HD, Sochantha T, Coosemans M. Absence of knockdown resistance suggests metabolic resistance in the main malaria vectors of the Mekong region. Malar J. 2009;8:84.
Chareonviriyaphap T, Rongnoparut P, Chantarumporn P, Bangs MJ. Biochemical detection of pyrethroid resistance mechanisms in Anopheles minimus in Thailand. J Vector Ecol. 2003;28(1):108–16.
Pemo D, Komalamisra N, Sungvornyothin S, Attrapadung S. Efficacy of three insecticides against Anopheles dirus and Anopheles minimus, the major malaria vectors, in Kanchanaburi Province, Thailand. Southeast Asian J Trop Med Public Health. 2012;43(6):1339–45.
Rodpradit P, Boonsuepsakul S, Chareonviriyaphap T, Bangs MJ, Rongnoparut P. Cytochrome P450 genes: molecular cloning and overexpression in a pyrethroid-resistant strain of Anopheles minimus mosquito. J Am Mosq Control Assoc. 2005;21(1):71–9.
Syafruddin D, Hidayati AP, Asih PB, Hawley WA, Sukowati S, Lobo NF. Detection of 1014 F kdr mutation in four major anopheline malaria vectors in Indonesia. Malar J. 2010;9:315.
Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37 Suppl 2:1–128.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2nd ed. Geneva: WHO; 2016.
Chaumeau V, Andolina C, Fustec B, Tuikue Ndam N, Brengues C, Herder S, et al. Comparison of the performances of five primer sets for the detection and quantification of Plasmodium in anopheline vectors by Real-Time PCR. Plos One. 2016;11(7):e0159160.
PRABI-Doua: CAP3 Sequence assembly program. http://doua.prabi.fr/software/cap3 Accessed 27 Feb 2017.
Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38(Web Server issue):W695–9.
Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, Mcwilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8.
McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41(Web Server issue):W597–600.
Beebe NW, Saul A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction—restriction fragment length polymorphism analysis. Am J Trop Med Hyg. 1995;53(5):478–81.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10.
Abbott WS. A method of computing the effectiveness of an insecticide. 1925. J Am Mosq Control Assoc. 1987;3(2):302–3.
Finney D. Probit analysis. 3rd edition edn. New York: Cambridge University Press; 1971.
R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016.
Johnson RM, Dahlgren L, Siegfried BD, Ellis MD. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). Plos One. 2013;8(1):e54092.
Lines JD, Nassor NS. DDT resistance in Anopheles gambiae declines with mosquito age. Med Vet Entomol. 1991;5(3):261–5.
Hodjati MH, Curtis CF. Effects of permethrin at different temperatures on pyrethroid-resistant and susceptible strains of Anopheles. Med Vet Entomol. 1999;13(4):415–22.
Tainchum K, Kongmee M, Manguin S, Bangs MJ, Chareonviriyaphap T. Anopheles species diversity and distribution of the malaria vectors of Thailand. Trends Parasitol. 2015;31(3):109–19.
Chareonviriyahpap T, Aum-Aung B, Ratanatham S. Current insecticide resistance patterns in mosquito vectors in Thailand. Southeast Asian J Trop Med Public Health. 1999;30(1):184–94.
Ho LY, Zairi J. Species composition and pyrethroid susceptibility status of Anopheles mosquitoes from two different locations in Malaysia. Trop Biomed. 2013;30(1):125–30.
Rohani A, Aziz I, Zurainee MN, Rohana SH, Zamree I, Lee HL. Current insecticide susceptibility status of Malaysian Anopheles maculatus Theobald to malathion, permethrin, DDT and deltamethrin. Trop Biomed. 2014;31(1):159–65.
Asih PB, Syahrani L, Rozi IE, Pratama NR, Marantina SS, Arsyad DS, et al. Existence of the rdl mutant alleles among the Anopheles malaria vector in Indonesia. Malar J. 2012;11:57.
Perera MD, Hemingway J, Karunaratne SP. Multiple insecticide resistance mechanisms involving metabolic changes and insensitive target sites selected in anopheline vectors of malaria in Sri Lanka. Malar J. 2008;7:168.
Yadouleton A, Martin T, Padonou G, Chandre F, Asidi A, Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroid resistance in Anopheles gambiae population in northern Benin. Parasit Vectors. 2011;4:60.
Curtis CF, Miller JE, Hodjati MH, Kolaczinski JH, Kasumba I. Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors? Philos Trans R Soc Lond B Biol Sci. 1998;353(1376):1769–75.
Djènontin A, Chabi J, Baldet T, Irish S, Pennetier C, Hougard JM, et al. Managing insecticide resistance in malaria vectors by combining carbamate-treated plastic wall sheeting and pyrethroid-treated bed nets. Malar J. 2009;8:233.
Pennetier C, Corbel V, Hougard JM. Combination of a non-pyrethroid insecticide and a repellent: a new approach for controlling knockdown-resistant mosquitoes. Am J Trop Med Hyg. 2005;72(6):739–44.
Jones CM, Liyanapathirana M, Agossa FR, Weetman D, Ranson H, Donnelly MJ, et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci USA. 2012;109(17):6614–9.
Duangkaew P, Kaewpa D, Rongnoparut P. Protective efficacy of Anopheles minimus CYP6P7 and CYP6AA3 against cytotoxicity of pyrethroid insecticides in Spodoptera frugiperda (Sf9) insect cells. Trop Biomed. 2011;28(2):293–301.
Rongnoparut P, Boonsuepsakul S, Chareonviriyaphap T, Thanomsing N. Cloning of cytochrome P450, CYP6P5, and CYP6AA2 from Anopheles minimus resistant to deltamethrin. J Vector Ecol. 2003;28(2):150–8.
Acknowledgements
We acknowledge the UMR-224 (MIVEGEC) of the Institut de Recherche pour le Développement (IRD), the Shoklo Malaria Research Unit (SMRU) and the Centre Hospitalier Régional Universitaire (CHRU) de Montpellier for providing human resources to the project. We also thank the Thailand International Development Cooperation Agency (TICA) from the Ministry of Foreign Affairs (MoFA) through the STOPVEC programme for their technical and administrative assistance. This work was partially supported by the Centre for Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand.
Funding
This work was supported by The Global Fund Thailand (THA-M-DDC) through the MAEL research programme. This work was partially supported by the Center for Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand. Victor Chaumeau received a PhD scholarship by the Centre Hospitalier Régional Universitaire de Montpellier (CHRU Montpellier). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article. The raw data used and analysed during the current study are available from the corresponding author on reasonable request. Sequences are submitted to the GenBank database under accession numbers KY677698–KY677706.
Authors’ contributions
Conceived and designed the experiments: VIC, VC, FN and TC. Performed the bioassays: JZ and VIC. Performed mosquito identification: JZ, PK and VIC. Performed molecular detection of kdr mutation: VIC. Analysed the data: VIC and VC. Contributed reagents/materials/analysis tools: DC and CA. Wrote the paper: VIC and VC. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The protocols for mosquito collection were approved by the Ethics Review Committee for Research Involving Human Research Subjects, Health Science Group, Chulalongkorn University (No 096.1/56) and Oxford Tropical Research Ethic Committee (1015-13, dated 29 April 2013).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chaumeau, V., Cerqueira, D., Zadrozny, J. et al. Insecticide resistance in malaria vectors along the Thailand-Myanmar border. Parasites Vectors 10, 165 (2017). https://doi.org/10.1186/s13071-017-2102-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2102-z