Abstract
Background
Previous surveys in dogs from Korea indicated that dogs are exposed to a variety of vector- borne pathogens, but perception for a nation-wide canine vector-borne disease (CVBD) occurrence has been missing. We report here results of both serological and molecular prevalence studies for major CVBDs of dogs from all over the South Korean Peninsula except for Jeju Island.
Results
Serological survey of 532 outdoor dogs revealed the highest prevalence for Dirofilaria immitis (25.2%), followed by Anaplasma phagocytophilum (15.6%), Ehrlichia canis (4.7%) whereas Borrelia burgdorferi showed the lowest prevalence (1.1%). The number of serologically positive dogs for any of the four pathogens was 216 (40.6%). Concurrent real-time PCR assay of 440 dogs in the study indicated that DNA of “Candidatus M. haematoparvum”, Mycoplasma haemocanis, Babesia gibsoni, A. phagocytophilum, and Hepatozoon canis was identified in 190 (43.2%), 168 (38.2%), 23 (5.2%), 10 (2.3%) and 1 (0.2%) dogs, respectively. DNA of Bartonella spp., Ehrlichia spp., Leishmania spp., Rickettsia spp. and Neorickettsia risticii was not identified. Analysis of questionnaires collected from owners of 440 dogs showed that the number of dogs with heartworm preventive medication was 348 (79.1%) among which dogs still positive to D. immitis infection were 60 (17.2%), probably due to the mean months of heartworm preventive medication being only 6.5. The high prevalence rates of both “Ca. M. haematoparvum” and Mycoplasma haemocanis in dogs from Korea indicate that these organisms may be transmitted by vectors other than Rhipicephalus sanguineus because this tick species has rarely been found in Korea. This is the first nationwide survey for canine haemotropic mycoplasma infections in Korea.
Conclusions
This study showed that the risk of exposure to major vector-borne diseases in dogs is quite high throughout all areas of South Korean Peninsula. Since achieving full elimination of many pathogens causing CVBDs from infected animals is often impossible even when they are clinically cured, dogs once exposed to CVBDs can remain as lifetime reservoirs of disease for both other animals and humans in the close vicinity, and should therefore be treated with preventative medications to minimise the risk of pathogen transmission by the competent vectors.
Similar content being viewed by others
Background
Previously, we reported results of a serological survey for Dirofilaria immitis, Anaplasma phagocytophilum, Ehrlichia canis and Borrelia burgdorferi infections in rural hunting and urban shelter dogs mainly from south-western regions of the Republic of Korea [1] in which the highest prevalence observed was for D. immitis (22.3%), followed by A. phagocytophilum (18.8%), E. canis (6.1%) and the lowest prevalence was for B. burgdorferi (2.2%) among 229 hunting dogs. In contrast, stray dogs found within the city limits of Gwangju showed seropositivity only to D. immitis (14.6%) and none of the 692 dogs responded positive for A. phagocytophilum, E. canis or B. burgdorferi antibodies.
Although our previous survey in dogs showed that they are exposed to multiple vector-borne pathogens, perception for a nation-wide canine vector-borne disease (CVBD) occurrence has been missing. Haemotropic mycoplasmas, for instance, are a group of bacteria that parasitize the surface of erythrocytes in a wide range of mammal species including dogs which are mainly infected with Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum”. Infections are usually chronic and subclinical in immunocompetent dogs but may lead to clinical signs related to haemolytic anaemia following splenectomy, immunosuppression or concurrent infections [2]. Although the natural mode of transmission of feline and canine haemoplasmas has not been definitely elucidated, blood transfusions and blood-sucking arthropods such as ticks have been implicated to be involved in the transmission of haemoplasmas in dogs and cats [3–5]. Since dogs in Korea are expected to be frequently exposed to arthropod infestation during their outdoor activities, they are also vulnerable to the infection with haemotropic mycoplasmas.
This article reports results of a serological survey on selected vector-borne diseases in dogs from five major provinces of the South Korean Peninsula. Also, a molecular survey on arthropod-borne pathogens such as “Ca. M. haematoparvum”, M. haemocanis, Babesia spp., Hepatozoon spp., Bartonella spp., Leishmania spp., Neorickettsia spp. and Rickettsia spp. were included in the survey.
Methods
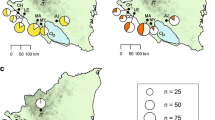
From January of 2012 to December of 2016, blood samples were collected from 532 dogs from all over the South Korean Peninsula which included 100 dogs from Chungcheong, 35 dogs from Gangwon, 88 dogs from Gyeonggi, 123 dogs from Gyeongsang and 186 dogs from Jeolla provincial areas (Fig. 1). All dogs included in this study were strictly outdoor dogs at an average of 3.2 years of age with slightly higher number of male dogs (300/532, 56.4%). The majority of dogs were of mixed-bred (48.1%) followed by Korean native Jindo (17.7%), laikas (14.3%), hounds (7.0%), pointers (4.9%) and terriers (2.4%).
Blood samples were collected from the cephalic or lateral saphenous veins of dogs and were stored in EDTA tubes. Blood samples were tested within 30 min after collection using a commercial ELISA assay kit (SNAP® 4Dx®; IDEXX Laboratories, Inc., Westbrook, ME, USA) which detected D. immitis antigen, and antibodies specific to Anaplasma spp. [A. phagocytophilum and A. platys, synthetic peptide from the major surface protein (p44/MSP2)], Ehrlichia spp. (E. canis and E. ewingii, P30 and P30-1 outer membrane proteins), and B. burgdorferi (C6 peptide).
Of 532 dogs in the survey, blood samples from 440 dogs were also tested by real-time PCR assays which included a canine haemotropic Mycoplasma test (RealPCR test code #2632, IDEXX Laboratories, Inc., West Sacramento, CA, USA) and a Tick/Vector canine comprehensive panel with Lab 4Dx (test code #2871, IDEXX Laboratories, Inc.). Hydrolysis probe based real-time PCR assays were designed and validated according to published protocols [6]. Pathogens of interest included in the study were A. phagocytophilum (msp2 [p44], GenBank accession number of target gene: DQ519570), A. platys (groEL, heat-shock protein, AY848753), Babesia spp. (ssrRNA, AF271082), Bartonella spp. (citrate synthase gene, AJ439406), Mycoplasma haemocanis (ribosomal RNA ssrRNA, AF197337), “Ca. Mycoplasma haematoparvum” (ribosomal RNA ssrRNA, AY383241), Ehrlichia canis (disulfide oxidoreductase, dsb, gene, AF403710), E. ewingii (dsb gene, AY428950), E. chaffeensis (dsb gene, AF403711), Hepatozoon canis (ssrRNA, AF176835), H. americanum (ssrRNA, AF176836), Leishmania spp. (glycoprotein gp63, YO8156), Neorickettsia risticii (ribosomal RNA 16S RNA, AF184082), and Rickettsia rickettsii (GroEL heat-shock protein, AJ293326). Samples positive by PCR for Babesia spp. were submitted for species-specific real-time PCR testing, including B. canis (heat-shock protein, hsp 70, AB248735), B. canis vogeli (hsp 70, EF527401), B. canis rossi (hsp 70, AB248738), B. felis (ITS-2, AY965742), B. gibsoni (hsp 70, AB248731), and B. conradae (ITS-2, AY965742 [7]. In addition, for confirmatory sequencing purposes, two outside primers beyond the synthetic positive control were designed, normally leading to a 300 to 500 bp long PCR product. These re-sequencing outside primers were used to confirm the analytical specificity of the real-time PCR tests and to resolve discrepant results.
Real-time PCR was performed as single plex reactions with six quality controls, including quantitative PCR-positive controls (synthetic DNA, Integrated DNA Technologies IDT, Coralville, IA, USA), PCR-negative controls (RNase-free PCR-grade water, Fisher Scientific, Waltham, MA, USA), negative extraction controls (lysis solution only), quantitative DNA internal sample quality control targeting the host 18S rRNA gene complex, an internal positive control spiked into the lysis solution, and an environmental contamination monitoring control (swab-based laboratory monitoring). Real-time PCR tests were validated analytically and clinically according to industry standard protocols (Applied Biosystems, User Bulletin #3 [8]), in order to obtain fully standardised real-time PCR tests. Following the industry standard allowed for the design and validation of standardized real-time PCR tests which could be carried out in parallel as a panel. For the analytical validation, each assay was evaluated for six validation criteria including amplification efficiency, linearity, reproducibility intra-run, reproducibility inter-run, 2 square value, and signal-to-noise ratio of the fluorescent signal.
Briefly, 90 μl of blood was lysed in a guanidinium thiocyanate-based lysis solution, incubated for 10 min and extracted using Whatman filters on a Corbett X-Tractor platform (Qiagen, Valencia, CA, USA). Nucleic acids were eluted into 150 μl of PCR-grade nuclease-free water (Fisher Scientific, Fremont, CA, USA) and 5 μl amplified in subsequent single-plex real-time PCR reactions. Analysis was performed on a Roche LightCycler 480 (Roche Applied Science, Indianapolis, USA) and raw data analysed using the 2nd derivative maximum method to generate crossing points (CP).
A test of independence for significance of the relationship between categorical variables (gender, age, and geographic regions) for both serological and molecular studies was made via Pearson’s Chi-square test and Fisher’s exact test for expected counts under five using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Questionnaires were delivered to owners of the 440 dogs for which both 4Dx and RealPCR assays were tested. The questionnaire was designed in order to obtain information as to whether their dogs were periodically medicated with heartworm preventive compounds and parasiticides.
Results
The serological prevalence of D. immitis, Anaplasma spp., Ehrlichia spp. and B. burgdorferi in 532 outdoor dogs from Korea is shown in Table 1. The number of dogs serologically positive to any of the four pathogens surveyed in this study was 216 (40.6%). The number of dogs with single, dual, triple or quadruple seropositivity was 188 (35.3%), 26 (4.9%), 0 (0.0%) and 2 (0.4%), respectively. The highest prevalence was observed for D. immitis (134, 25.2%), followed by Anaplasma spp. (83, 15.6%), Ehrlichia spp. (25, 4.7%); the lowest prevalence was recorded for B. burgdorferi (6, 1.1%).
Seropositive dogs to any of the four pathogens was higher in male dogs (22.7%) than in female dogs (17.9%; χ 2 = 22.7720, P < 0.001), and dogs of above 2 years were significantly more exposed to these pathogens than younger dogs (34, 6%; χ 2 = 6.3525, P < 0.012). Geographically, dogs from Gangwon and Jeolla provincial areas were significantly more exposed to any of the four selected vector-borne pathogens than the rest of the regions of South Korea (χ 2 = 10.7582 and 3.7655, respectively; P = 0.001 and 0.052, respectively). Mixed breed dogs had lower prevalence for D. immitis infection than other breeds (15.2 vs 34.4%; χ 2 = 25.9441, P < 0.001) whereas Jindo dogs had higher prevalence for D. immitis than other breeds (57.4 vs 18.3%; χ 2 = 63.0665, P < 0.001). Similarly, mixed breed dogs had lower in prevalence for any four of the pathogens detected by SNAP 4Dx test (32.4 vs 48.2%; χ 2 = 13.6896, P < 0.001) whereas Jindo dogs had higher prevalence than other breeds (58.5 vs 36.8%; χ 2 = 15.1844, P < 0.001).
Results of the molecular prevalence study of 440 dogs for 10 selected vector-borne pathogens by real-time PCR are shown in Table 2. Although most dogs (62.3%) were positive to at least one of the 10 pathogens, the most commonly identified were “Ca. M. haematoparvum” (43.2%) and Mycoplasma haemocanis (38.2%), the prevalence of which were significantly higher among male dogs (χ 2 = 20.2113 and 11.2841, respectively, both P < 0.001). The number of dogs positive to both “Ca. M. haematoparvum” and M. haemocanis was 95 (21.6%), while the number of dogs positive to any of the two mycoplasmas was 263 (59.8%). Compared to other breeds, higher prevalence rates were observed in mixed-breed dogs for both “Ca. M. haematoparvum” (54.7 vs 27.2%) and Mycoplasma haemocanis (41.4 vs 33.7%) infections, but significant difference was only observed for “Ca. M. haematoparvum” (χ 2 = 33.0302, P < 0.001 vs χ 2 = 2.6965, P < 0.1006).
Of those 440 dogs for which both real-time PCR and SNAP 4Dx tests were performed, 83 dogs were serologically positive to Anaplasma spp. while only 10 dogs (2.3%) were real-time PCR-positive; the species was identified as A. phagocytophilum. The number of dogs positive by both serological and real-time PCR to Anaplasma spp. was 6 (1.4%) while the number of dogs positive to Anaplasma spp. by either serological or real-time PCR methods was 87 (19.8%). None of the 25 dogs serologically positive to Ehrlichia spp. were positive by real-time PCR method. Dogs positive to Babesia gibsoni (5.2%), A. phagocytophilum (2.3%) and Hepatozoon canis (0.2%) were few, and no dogs were positive to Bartonella spp., Ehrlichia spp., Leishmania spp., Neorickettsia spp. or Rickettsia spp.
Of those 440 dogs for which both real-time PCR and SNAP 4Dx tests were performed, the number of dogs on heartworm preventive medication, as responded by their owners, was 348 (79.1%). The number of dogs with heartworm medication but still positive to D. immitis infection was 60 (13.6%). However, the mean number of months that these dogs were on heartworm medication was only 6.5 months. Owners of 292 dogs (66.4%) responded that they medicated their dogs with both heartworm preventives and yearly parasiticides. Owners of 332 dogs (75.5%) responded that they visit veterinary clinics at an average of 2.02 times per year.
Discussion
Previously, we did a similar serological survey from December of 2007 to August of 2009 for D. immitis, A. phagocytophilum, E. canis and B. burgdorferi infections in rural hunting and urban shelter dogs from southwestern regions of South Korea using IDEXX SNAP 4Dx kit [1]. Considering that the 532 dogs in this study were all outdoor dogs and from all over South Korea, the results showed CVBD patterns similar to those in hunting dogs from the south-western regions of South Korea.
It was surprising that 60 out of 440 dogs (13.6%) were on heartworm preventive medication but still seropositive to D. immitis. As 348 dog owners responded that their dogs were on heartworm preventive medication, 17.2% of these dogs were not protected by the preventive medication. Although the Korean Peninsula has a temperate climate with four distinct seasons (hot and humid summer; cold and dry winter; and mild and pleasant spring and fall), adults of some species of mosquitoes are active throughout the year including freezing winter months [9, 10]. Therefore, our result clearly show that all year round heartworm preventive medication is required for a successful prevention of dogs from heartworm disease in Korea. Considering that the mean number of months these dogs were on heartworm preventive medication was only 6.5 while 75.5% of dog owners visited their veterinary clinics at an average of 2.02 times per year, it appears that education for both veterinary practitioners and dog owners for a strict compliance to year round heartworm preventive medication is critical for a successful prevention of heartworm disease in Korea.
Compared to other vector-borne pathogens surveyed in this study, dogs were highly exposed to the two major haemotropic mycoplasmas of dogs, “Ca. M. haematoparvum” (43.2%) and M. haemocanis (38.2%). Since all dogs investigated in this study were clinically healthy, the two haemotropic mycoplasmas affecting dogs from Korea may cause chronic or asymptomatic infections, as is the case in other countries unless dogs are either splenectomised or immune-compromised [11–13]. Since previous reports indicate that canine haemoplasma infection has been associated with immune-mediated haemolytic anaemia (IMHA) [14, 15], the potential clinical significance of this finding needs to be further investigated in the future in relation to the causation of IMHA by haemoplasmas in dogs in South Korea. Significantly higher infection rates of the two mycoplasmas among male dogs in this study coincide with previous reports on the prevalence of canine hemotropic mycoplasma infections in Mediterranean countries [16]. This is the first nation-wide survey for canine haemotropic mycoplasma infections in Korea
Although the natural mode of transmission of feline and canine haemoplasmas has not been definitely elucidated, blood transfusion, blood-sucking arthropods and more recently mange mites may be involved [11]. Among tick species, Rhipicephalus sanguineus and Ixodes spp. have been indicated as the major vectors for canine haemotropic mycoplasmas, but different transmission patterns may be present in Korea because very low population of Rhipicephalus and Ixodes spp. infests dogs in Korea [17]. Instead, majority of ticks found in dogs from Korea is either Haemaphysalis longicornis or H. flava which indicates that the principle arthropod vectors transmitting canine haematrophic mycoplasmas in Korea may be different from those in other countries. Since the risk of exposure to canine haemoplasmosis among outdoor dogs from Korea have been shown to be high, both pet owners and veterinary practitioners should be alerted in Korea.
Our study indicates that considerably high number of dogs were also exposed to B. gibsoni infection (5.2%). Unlike other countries where dogs are exposed to more than one species of Babesia, B. gibsoni is the only known causative agent for canine babesiosis in Korea [18]. As in other countries, high infection rates among pit bull terriers were also reported in Korea [19].
Of those 440 dogs for which both real-time PCR and SNAP 4Dx tests were performed, discrepancy was observed between the exposure rates of Anaplasma spp. by serological assay (18.9%) and by real-time PCR (2.3%). A previous report from Germany indicates that no significant differences were observed concerning the rates of seropositivity or PCR-positivity in dogs with and without clinical signs of canine granulocytic anaplasmosis [20]. However, if a survey is performed in an area endemic for anaplasmosis seropositivity rates outnumber PCR-positivity. For example, in a study of 731 dogs from Minnesota, USA, the serological survey using the IDEXX SNAP 4Dx test as in our study among both healthy and clinically anaplasmosis-suspected dogs showed high seropositive response to A. phagocytophilum (67.4 and 53.7% respectively, 55.4% combined), while only clinically anaplasmosis-suspected dogs were highly positive by the PCR assay (37.3%). Among the healthy group of dogs, however, only 7 of 222 dogs (3.2%) were PCR-positive to A. phaocytophilum [21].
One dog from Gyeonggi Province showed positive to Hepatozoon canis by real-time PCR. Although four free-ranging Korean leopard cats (Prionailurus bengalensis) have been previously reported to contain schizonts of H. felis in the heart muscle, there has been no report of H. canis infection among dogs from Korea. The dog in our study was an 18 month-old female mixed-breed dog lactating a litter of four puppies. Since this was the first indication of H. canis infection in dogs in Korea, attempts were made to trace back the dog for a morphological identification of the organism, but in vain because the dog was killed by a car accident before the revisit.
Conclusions
This study clearly shows that outdoor dogs from all over the South Korea are frequently exposed to pathogens causing major CVBDs. We also report for the first time that haemotropic mycoplasma infections with M. haemocanis and “Ca. M. haematoparvum” are especially high among these dogs. Since achieving full elimination of the pathogens from the animal is often impossible even when achieving a ‘clinical cure’, treated dogs can remain as reservoirs of disease for dogs or other animals and humans in the close vicinity, and should therefore be treated with preventative compounds to minimise the risk of pathogen transmission by the competent vectors.
References
Lim S, Irwin PJ, Lee S, Oh M, Ahn K, Myung B, Shin S. Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasit Vectors. 2010;3:32.
Messick JB. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet Clin Path. 2004;33:2–13.
Willi B, Boretti FS, Baumgartner C, Tasker S, Wenger B, Cattori V, et al. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J Clin Microbiol. 2006;44:961–9.
Gary AT, Richmond HL, Tasker S, Hackett TB, Lappin MR. Survival of Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’in blood of cats used for transfusions. J Feline Med Surg. 2006;8:321–6.
Seneviratna P, Ariyadasa S. Transmission of Haemobartonella canis by the dog tick, Rhipicephalus sanguineus. Res Vet Sci. 1973;14:112.
Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5‘-3‘exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A. 1991;88:7276–80.
Cannon S, Levy J, Kirk S, Crawford P, Leutenegger C, Shuster J, et al. Infectious diseases in dogs rescued during dogfighting investigations. Vet J. 2016;211:64–9.
Windsor RC, Johnson LR, Sykes JE, Drazenovich TL, Leutenegger CM, Cock HE. Molecular detection of microbes in nasal tissue of dogs with idiopathic lymphoplasmacytic rhinitis. J Vet Intern Med. 2006;20:250–6.
Sohn SR. Seasonal prevalence of the Culex pipiens group (Diptera: Culicidae) larvae occurring in a puddle in the basement of an apartment building. Entomol Res. 2007;37:95–9.
Lee D-K. Occurrence of Culex pipiens (Diptera, Culicidae) and effect of vent net sets for mosquito control at septic tanks in south-eastern area of the Korean peninsula. Korean J Appl Entomol. 2006;45:51–7.
Willi B, Novacco M, Meli M, Wolf-Jäckel G, Boretti F, Wengi N, et al. Haemotropic mycoplasmas of cats and dogs: transmission, diagnosis, prevalence and importance in Europe. Schweiz Arch Tierheilkd. 2010;152:237.
Wengi N, Willi B, Boretti FS, Cattori V, Riond B, Meli ML, et al. Real-time PCR-based prevalence study, infection follow-up and molecular characterization of canine hemotropic mycoplasmas. Vet Microbiol. 2008;126:132–41.
Sykes JE, Bailiff NL, Ball LM, Foreman O, George JW, Fry MM. Identification of a novel hemotropic mycoplasma in a splenectomized dog with hemic neoplasia. J Am Vet Med Assoc. 2004;224:1946–51.
Bundza A, Lumsden J, McSherry B, Valli V, Jazen E. Haemobartonellosis in a dog in association with Coombs‘ positive anemia. Can Vet J. 1976;17:267.
Bellamy J, MacWilliams P, Searcy G. Cold-agglutinin hemolytic anemia and Haemobartonella canis infection in a dog. J Am Vet Med Assoc. 1978;173:397.
Novacco M, Meli ML, Gentilini F, Marsilio F, Ceci C, Pennisi MG, et al. Prevalence and geographical distribution of canine hemotropic mycoplasma infections in Mediterranean countries and analysis of risk factors for infection. Vet Microbiol. 2010;142:276–84.
Choe HC, Fudge M, Sames WJ, Robbins RG, Lee IY, Chevalier NA, et al. Tick surveillance of dogs in the Republic of Korea. Syst Appl Acarol. 2011;16:215–22.
Song K, Kim D, Hayasaki M. The PCR-based detection of Babesia gibsoni infection in dogs (German shepherds) reared in South Korea. Ann Trop Med Parasitol. 2004;98:149–53.
Lee M-J, Yu D-H, Yoon J-S, Li Y-H, Lee J-H, Chae J-S, et al. Epidemiologic and clinical surveys in dogs infected with Babesia gibsoni in South Korea. Vector Borne Zoonotic Dis. 2009;9:681–6.
Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. 2011;91:71–6.
Beall MJ, Chandrashekar R, Eberts MD, Cyr KE, Diniz PPV, Mainville C, et al. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis. 2008;8:455–64.
Acknowledgements
This paper has been sponsored by Bayer Animal Health in the framework of the 12th CVBD World Forum Symposium.
Funding
This study was supported by the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (Grant No. 315016-3-C00).
Availability of data and materials
The data used in the present study are available from the corresponding author on reasonable request.
Authors’ contributions
GHS and SSS conceived the paper and wrote the manuscript. KSA and JHA contributed to the field trips, KSA, HJK and CL to laboratory studies. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
This study was approved by the Animal Research Ethics Committee of Chonnam National University as complying with the Korean legislation for the animal protection (Low No. 13023, articles 8 and 9).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Suh, GH., Ahn, KS., Ahn, JH. et al. Serological and molecular prevalence of canine vector-borne diseases (CVBDs) in Korea. Parasites Vectors 10, 146 (2017). https://doi.org/10.1186/s13071-017-2076-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2076-x