Abstract
Background
Species of the Anopheles minimus complex are considered to be the primary vectors of malaria in South and Southeast Asia. Two species of the complex, Anopheles minimus and Anopheles harrisoni, occur in Thailand. They are sympatric and difficult to accurately distinguish based on morphological characters. The aim of this study was to investigate the potential of antennal sensory organs to distinguish these two species. Additionally, we investigated their ability to mate in cages of different sizes, as well as the possible mechanism(s) that evokes stenogamous behavior.
Methods
Large sensilla coeloconica present on the antennae of females of An. minimus and An. harrisoni were counted under a conventional light microscope and various types of antennal sensilla were examined under a scanning electron microscope (SEM). Determinations of mating ability were carried out in 20 and 30 cm3 cages with a density resting surface (DRS) of 7.2. The insemination rate, frequency of clasper (gonocoxopodite) movement of the male genitalia during induced copulation and duration of mating of the two species were compared.
Results
The mean numbers of large sensilla coeloconica on antennal flagellomeres 1–8 and the mean number of large sensilla coeloconica on each flagellum in An. minimus (26.25) and An. harrisoni (31.98) were significantly different. Females of both species bear five types of antennal sensilla: chaetica, trichodea, basiconica, coeloconica and ampullacea. Marked differences in the structure of the large sensilla coeloconica were observed between the two species. Furthermore, only An. minimus could copulate naturally in the small cages. The frequency of clasper movement in the stenogamous An. minimus was significantly higher than in An. harrisoni, but there was no difference in the duration of mating.
Conclusions
To our knowledge, this study is the first to examine and discover the usefulness of large sensilla coeloconica on the antennae of females and the frequency of clasper movement in males for distinguishing the sibling species An. minimus and An. harrisoni. The discovery provides an effective and relatively inexpensive method for their identification. Additionally, the greater frequency of clasper movement of An. minimus might influence its ability to mate in small spaces.
Similar content being viewed by others
Background
Malaria is a life-threatening disease caused by plasmodial protozoa that are transmitted to people through the bites of infected female Anopheles mosquitoes. About 3.2 billion people are at risk of malaria [1]. In 2015, there were roughly 214 million malaria cases and an estimated 438,000 deaths due to the disease. Malaria is one of the most important parasitic diseases in Thailand and has long been one of the major causes of morbidity and mortality, especially along the international borders with Cambodia, Myanmar and Malaysia. To date, there are 472 formally recognized species of Anopheles worldwide [2]. Seventy-five formally named species occur in Thailand [3–5]. Some species of the An. dirus complex (An. baimaii and An. dirus), An. minimus complex (An. minimus) and the An. maculatus group (An. maculatus) are recognized as primary malaria vectors in the country.
The An. minimus complex (henceforth the Minimus Complex) belongs to the Minimus Subgroup within the Funestus Group of the Myzomyia Series [2, 6]. The Minimus Complex includes three species, An. minimus (formerly minimus species A) [7, 8], An. harrisoni (formerly minimus species C) [7, 9] and An. yaeyamaensis (formerly minimus species E) [10]. The last species is only known to occur on the Yaeyama and Miyako Islands located at the southern end of the Ryukyu Archipelago of Japan. Anopheles minimus occurs throughout Thailand whereas An. harrisoni is confined to western and northern areas, including Tak and Chiang Mai Provinces [3]. Anopheles minimus was incriminated as a primary vector of malaria in Thailand, whereas the vector status of An. harrisoni has not yet been determined. Anopheles minimus is mainly anthropophilic, endophagic and exophilic, whereas An. harrisoni has shown a greater tendency toward zoophily, exophagy and exophily [11, 12]. In addition, it has been shown that An. minimus can copulate successfully in 30 cm3 cages (stenogamous colony) [13–15].
Anopheles minimus has often been misidentified because the adult females exhibit overlapping morphological characters with other members of the Funestus Group, particularly An. aconitus, An. pampanai and An. varuna [14]. Sucharit et al. [16] noted that the absence of a humoral pale spot (HP) on the costa of the wing could distinguish An. minimus from An. harrisoni. However, the findings of Green et al. [7], Sharpe [17], Chen et al. [18], Sungvornyothrin et al. [19] and Cuong et al. [20] showed that presence of a humeral pale spot was not reliable for distinguishing the two species. Subsequently, isoenzymes (esterase and octanol dehydrogenase) were used to distinguish the two species in an area of sympatry [7], but this identification method requires fresh or frozen specimens; thus, molecular methods have been used to identify them. Allele-specific polymerase chain reaction (AS-PCR) is frequently used to distinguish the two species from other species of the Funestus Group [21, 22]. Analyses based on mitochondrial DNA fragments of An. minimus, such as the cytochrome c oxidase subunits 1 (cox1) and 2 (cox2), have been used for molecular identification, as well as for phylogenetic and population genetics studies, of species of the Minimus Subgroup [20, 23–25].
Researchers have attempted to find effective methods for the identification of Anopheles vectors based on morphological attributes, e.g. light microscopy of antennal sensilla [26] and scanning electron microscopy (SEM) of eggs and antennal sensilla [27–30]. However, no studies of the antennal sensilla of members of the Minimus Complex have been conducted. Hence, the purpose of this study was to examine and compare the antennal sensilla in females of An. minimus and An. harrisoni using light and scanning electron microscopy. The various types of sensilla borne on the antennae of the two species are described herein. In addition, we investigated the mating behavior of An. minimus (stenogamous) and An. harrisoni (eurygamous) by comparing the insemination rates, frequency of clasper (gonocoxopodite) movement of the male genitalia during induced copulation and duration of copulation of the two species.
Methods
Mosquitoes and species identification
Free mating (stenogamous) An. minimus were obtained from the Office of Disease Prevention and Control No. 1, Department of Disease Control, Ministry of Public Health, Chiang Mai Province, Thailand. Specimens of An. harrisoni were reared from wild-caught larvae collected in Ban Pu Tuey (village), Sai Yok District, Kanchanaburi Province, Thailand. Specimens and reared F1 progeny from individual females (see below) were identified to species using morphological keys for the Anopheles in Thailand [3]. Following morphological identification, molecular identifications were performed using the AS-PCR assay based on ITS2 rDNA sequences [22]. Genomic DNA was extracted from individual adult females using the DNeasy® Blood and Tissue Kit (Qiagen, Hilden, Germany) and isolated DNA was subjected to sequential PCR procedures. The ITS2 region was amplified using the universal forward primer ITS2A (5′-TGT GAA CTG CAG GAC ACA T-3′) and the specific reverse primers MIA (5′-CCC GTG CGA CTT GAC GA-3′ for An. minimus) and MIC (5′-GTT CAT TCA GCA ACA TCA GT-3′ for An. harrisoni). PCR was carried out using 25 μl volumes containing 0.5 U of Taq DNA polymerase, 1× Taq buffer, 2.0 mM of MgCl2, 0.2 mM of each dNTP, 0.25 μM of each primer and 1 μl of the extracted DNA. The amplification profile comprised initial denaturation at 94 °C for 2 min, 30 cycles at 94 °C for 30 s, 45 °C for 30 s and 72 °C for 40 s and a final extension at 72 °C for 5 min. The amplified products were electrophoresed on 1.5% agarose gel. In addition, PCR products were sequenced using the BigDye® Terminator Cycle Sequencing Kit and analyzed with the 3130 genetic analyzer for species confirmation.
Mosquito rearing
Larvae of An. minimus and An. harrisoni were reared to adults using the procedure described by Choochote and Saeung [31]. Mosquitoes were colonized and maintained continuously for several generations at 27 ± 2 °C, 70–80% relative humidity and with illumination from a combination of natural daylight from a glass window and fluorescent lighting provided for approximately 12 h a day in an insectary of the Department of Parasitology, Chiang Mai University. Five days after emergence, meals of bovine blood acquired from a slaughterhouse were provided with an artificial membrane feeding method [31, 32].
Light microscopy
Large sensilla coeloconica (peg organs in pits) on antennal flagella were observed using an Olympus BX53 compound microscope. These sensilla were counted and compared in 36 h post-emerged females. Females from each species were immersed in 10% potassium hydroxide and left for 30–45 min in a hot oven. After clearing, they were washed with 80% ethanol, their antennae were removed using an insect needle and the two antennae of each female were mounted together in Hoyer’s medium on a microscope slide. The large sensilla coeloconica borne on the left and right flagellum of 30 females of each species were counted (n = 60 flagella/species).
Scanning electron microscopy
Thirty heads of 4- to 5-day-old females of each species were excised under a stereomicroscope and rinsed three times in phosphate buffer (pH 7.4) to remove surface debris. The heads were then dehydrated through an ethanol series of 35, 70, 80% (10 min, two changes) and 95% (15 min, two changes), followed by absolute ethanol (10 min, two changes). Following dehydration, they were dried in a critical point dryer. The antennae were carefully dissected from the head capsule under a stereomicroscope, as described by Hempolchom et al. [30]. Antennae were mounted on aluminum stubs with double-sided carbon adhesive tape and sputter-coated with gold. Sensilla were observed and photographed in a JEOL-JSM6610LV scanning electron microscope (JEOL, Japan).
Mating of adult mosquitoes
Adult F9 mosquitoes of An. minimus and An. harrisoni were used to observe their mating ability in cages of two sizes [33]. Density resting surface (DRS) of the cages was calculated by dividing the vertical resting surface area (RS) (cm2) by the mosquito population density (D) [34]. Comparison of mating ability in 20 and 30 cm3 cages with DRS of 7.2 was carried out using various ratios of female/male mosquitoes. At a DRS of 7.2, female/male (total) cohorts of 89/133 (222) and 200/300 (500) were introduced into the 20 and 30 cm3 cages, respectively, where they remained for 1 week. Solutions of 10% sucrose and 5% multivitamin syrup were provided as nutrients [31]. Subsequently, mean insemination rate was calculated from dissection of 200 spermathecae (duplicate experiments, 100 spermathecae/experiment). Intact spermathecae were placed on microscope slides and examined for movement of the long thread-like spermatozoa under 100× magnification using the compound microscope mentioned above. Spermathecae with detected movement of spermatozoa were ruptured by placing a coverslip on the microscope slide, and the surrounding field was scanned for spermatozoa. The relative percentage of the volume of spermathecae containing spermatozoa was graded as 0 (fairly transparent or uninseminated spermatheca), 1+ (25% of area of coverslip with spermatozoa), 2+ (50% of area of coverslip with spermatozoa), 3+ (75% of area of coverslip with spermatozoa) and 4+ (100% of area − spermatheca full of spermatozoa) [26].
Frequency of clasper movement and mating time
The procedures described by Wijit et al. [26] were used for measuring clasper movement and the duration of mating. The frequency of clasper movement during induced copulation and the duration of mating were determined using 5-day-old females and males of each species (n = 30/species). During induced copulation, females are grasped initially by the claspers of males and remain coupled for a period of time before sperm transfer, with movement of their claspers until separation. Two persons participated in this experiment. The frequency of clasper movement was observed and counted under a stereomicroscope by one person while the other person, the timekeeper, measured the duration of mating with the aid of an electric watch.
Statistical analysis
The Chi-square test was used to determined insemination rates. The numbers of large sensilla coeloconica on female antennae, and frequency of clasper movement during induced copulation and mating times, were assessed by Student’s t-test. All data were analyzed using SPSS v. 20.0 for Windows (Chicago, SPSS Inc.). Statistical significance was set at P < 0.05.
Results
Molecular identification
The AS-PCR successfully confirmed the morphological identification of An. minimus and An. harrisoni. PCR species-specific products were 310 bp and 180 bp for An. minimus and An. harrisoni, respectively (Fig. 1).
General morphology of the antennae of females
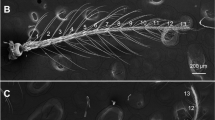
The antennae of females of An. minimus and An. harrisoni are morphologically similar (Fig. 2). The antennae of mosquitoes consist of a basal scape, a pedicel and a long terminal flagellum. The scape (Sc) is collar-shaped and hidden behind the pedicel (Pe), which is a bulbous cup shaped-segment containing Johnston’s organ and provides the attachment of the flagellum. Each flagellum consists of 13 flagellomeres. The surface of the scape, pedicel and first flagellomere is covered densely with aculeae (ac) (Fig. 3a, b). The number of aculeae decreases from the proximal to the distal end of each of flagellomeres 1–5.
Scanning electron micrographs of flagellomere 1 and the tip of flagellomere 13 (representative of both An. minimus and An. harrisoni). a Scape (Sc), pedicel (Pe) and first flagellomere (I) densely covered with aculeae. b Higher magnification of sensillum ampullaceum (sa) surrounded by aculeae (ac). c Large sensilla chaetica (lch) at the base and small sensilla coeloconica (sco) at the tip of flagellomere 13. d Higher magnification of sensilla coeloconica (sco) at the tip of flagellomere 13
Characterization of antennal sensilla
Like most dipterans, four main types of sensilla occur on the antennae of females of An. minimus and An. harrisoni, including sensilla chaetica, sensilla trichodea, sensilla basiconica (grooved pegs) and sensilla coeloconica. A fifth type, sensilla ampullacea, occurs on the first flagellomere.
Sensilla chaetica (ch) are long, thick-walled setae set in sturdy sockets (alveoli) and are putative mechanoreceptors [35]. There are large and small subtypes of sensilla chaetica. The shape and distribution of these sensilla are similar in the two species. The large sensilla chaetica (lch) are arranged mostly in a whorl of about six sensilla at the bases of flagellomeres 2–13 (Figs. 2, 3c, 4a). The small sensilla chaetica (sch) usually occur on the distal ends of flagellomeres 2–13 (Fig. 4a). They are interspersed with minute aculeae and their numbers decrease slightly from the proximal to the distal ends of the flagellomeres in both species. Both subtypes also occur on the ventral surface of the first flagellomere and are often interspersed with aculeae (Fig. 3a).
Scanning electron micrographs showing the various types of sensilla borne on the antennae of females of An. minimus and An. harrisoni. a Large sensilla chaetica (lch) with raised sockets; small sensilla chaetica (sch); and a sensillum basiconicum (grooved peg) (sb). b Long sharp sensilla trichodea (ltc); short sharp sensilla trichodea (stc); a blunt-tipped sensillum trichodeum (btc); and a large sensillum coeloconicum (lco)
Sensilla trichodea (tc), like sensilla chaetica, are structurally setiform or hair-like sensory structures but they do not arise from a basal alveolus and are olfactory organs [35]. They are the most abundant sensilla on flagellomeres 2–13 of both species. Three subtypes of sensilla trichodea are present: long sharp (acuminate) trichodea (ltc), short sharp (acuminate) trichodea (stc) and blunt-tipped trichodea (btc). Sharp trichodea have a smooth surface (Fig. 4b). The number of long sharp trichodea increases from the proximal to the distal ends of flagellomeres in both species. Short sharp trichodea are fewer in number than the long sharp trichodea on the flagellum of both species (Fig. 4b). Blunt-tipped trichodea, unlike the long sharp trichodea, are not tapered to a point and are more or less of equal diameter throughout their length (Fig. 4b). These sensilla are also apparently more uniform in length than the sharp trichodea. Blunt-tipped sensilla trichodea occur in fewer numbers than the sharp trichodea in both species. However, they do not occur on the first flagellomere.
Sensilla basiconica (sb) are curved peg-like sensilla with approximately 10–12 longitudinal grooves on their surfaces. They arise from slightly raised alveoli (Figs. 4a, 5). Sensilla basiconica are similarly slender, tapered and apically pointed in both An. minimus (Fig. 5a) and An. harrisoni (Fig. 5b). They are morphological olfactory sensilla [35].
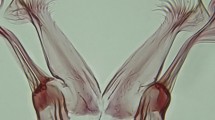
Sensilla coeloconica (co) are small, thick-walled sensilla that are commonly called pitted pegs because they are borne in a cup-like depression of the antennal wall. The pegs may or may not protrude through the circular openings at the surface of the cuticle. The surface of sensilla coeloconica is grooved lengthwise similar to sensilla basiconica, but the grooves are deeper. They have been putatively classified as hygro- and thermoreceptors [35]. The large sensilla coeloconica (lco) differ on the antennal flagella of An. minimus and An. harrisoni as follows. Those of An. minimus are characterized by short somewhat conical pegs that do not reach the cuticular opening of the pit (Fig. 6a, b). In contrast, the pegs of An. harrisoni are much longer and protrude beyond the external rim of the pit (Fig. 6c, d). The distributions of sensilla coeloconica on the antennae of both species based on SEM observation were consistent with the distributions observed using a compound light microscope (Table 1). Small sensilla coeloconica (sco) have the peg set on the bottom of a pit with a small cuticular opening, hence the peg is not visible. This type of sensillum was found on the tip of the distal (13th) antennal flagellomere (Fig. 3c, d).
Sensilla ampullacea (sa) are small peg-like organs that are not readily visible because they are borne in pits with narrow or slit-like openings. This type of sensillum occurs on the first antennal flagellomere, surrounded by aculeae (Fig. 3a, b). Sensilla ampullacea are putative hygro- and thermoreceptors [35].
Number of large sensilla coeloconica on antennae of females
The number of large sensilla coeloconica per antennal flagellomere ranged from 0–7 and 0–9 in An. minimus and An. harrisoni, respectively. Remarkably, no large sensilla coeloconica were found on flagellomeres 9–13 of either species. The mean number of large sensilla coeloconica on each of flagellomeres 1–8 of the two species was significantly different (Table 1). Likewise, the mean number of large sensilla coeloconica per flagellum of An. minimus (26.25) and An. harrisoni (31.98) was significantly different (t (118) = 7.685, P < 0.0001). No surface distribution bias (dorsal and ventral) was observed in either species.
Mating of mosquitoes in cages of different sizes at DRS 7.2
The mean insemination rates of An. minimus were 63 and 55.5 in 20 and 30 cm3 cages, respectively. No spermatozoa were found in the spermathecae of An. harrisoni placed in cages of these sizes (Table 2). More than 80% of inseminated An. minimus females had high sperm density (3+ and 4+) in their spermathecae in cages of both sizes (data not shown).
Clasper movement and duration of mating
The frequency of clasper movement in males of the stenogamous An. minimus was significantly higher (t (118) = -2.984, P = 0.030) than in males of An. harrisoni. However, the duration of mating of the two species was not significantly different (t (118) = -0.889, P = 0.370) (Table 3).
Discussion
Precise species identification of malaria vectors is essential for an understanding of epidemiological patterns of disease transmission and for designing appropriate strategies for vector control. However, it has been impossible to accurately distinguish the two sympatric sibling species, An. minimus and An. harrisoni, based on larval, pupal and adult morphological characters. During the past couple of decades, several alternative, relatively inexpensive and reliable methods have been reported for mosquito identification. For example, scanning electron microscopy (SEM) revealed various novel characteristics, such as antennal sensilla [36], and features of the cibarial armature [37], eggs [29] and wings [38, 39], which are purportedly useful for identifying species. Somboon et al. [37] reported differences in the cibarial armature of An. flavirostris, An. minimus and An. yaeyamaensis using SEM. However, the antennal sensilla of the two sibling species of the Minimus Complex that occur in Thailand have not been studied before now. The results of the present study show that An. minimus and An. harrisoni can be distinguished by differences of their antennal sensilla, specifically the form and numbers of sensilla coeloconica on antennal flagellomeres 1–8 (Table 1, Fig. 6).
The antennae of adult mosquitoes bear numerous sensilla, which usually contain one to three receptor neurons (ORNs). In the present study, five types of sensilla with similar distributions were observed on the antennae of females of An. minimus and An. harrisoni. The same morphological types of sensilla were recognized by Pitts and Zwiebel [36] and Hempolchom et al. [30] in their studies of Anopheles mosquitoes, and by Hill et al. [35] in their study of Culex quinquefasciatus. Hempolchom et al. [30] constructed a key based on differences in antennal sensilla to reliably distinguish eight species of the An. hyrcanus group in Thailand.
It is important to note that the difference in the form of the large sensilla coeloconica present on the antennae of An. minimus and An. harrisoni (as seen using SEM and noted above) can be used to distinguish and identify them. Additionally, the mean number of large sensilla coeloconica on each flagellum of An. minimus (26.25) is significantly fewer than in An. harrisoni (31.98). In comparison, the mean number of these sensilla on the antennae of these species is greater than on the antennae of An. gambiae (21.6) and An. quadriannulatus (29.0) [34] but less than those on the antennae of An. pursati (27.85), An. nitidus (28.73), An. nigerrimus (33.10), An. sinensis (36.47) and An. paraliae (37.55) [26].
Wijit et al. [26] documented that at least 20 Anopheles species are known to be able to successfully copulate in small cages. To shed light on the mechanism(s) that influences the mating behavior of An. minimus (stenogamous) and An. harrisoni (eurygamous), we compared the insemination rates, the frequency of clasper movement in males during induced copulation and the duration of mating of these species. Unlike An. minimus, it appears that females and males of An. harrisoni could not copulate in small cages based on the absence of sperm in the spermathecae of females (zero insemination rate). This observation agrees with that of Wijit et al. [26] who reported the highest insemination rates (70–97%) in stenogamous An. peditaeniatus in different cage sizes at DRS of 3.6 and 7.2 whereas the eurygamous An. crawfordi had the lowest rate (0–4%). The frequency of clasper movement of the stenogamous An. minimus was significantly greater than that in the eurygamous An. harrisoni; however, the duration of mating of the two species was not significantly different. These results sharply contrast with the finding of Wijit et al. [26], who found that the frequency of clasper movement in the stenogamous An. peditaeniatus was lower than that in the seven eurygamous species listed above. Likewise, Sucharit and Choochote [36], working with members of the An. dirus complex, found that stenogamous An. cracens has a lower frequency of clasper movement and a shorter period of copulation than An. dirus.
Conclusions
Using light and scanning electron microscopy we found that the form and number of large sensilla coeloconica on antennae could distinguish females of An. minimus and An. harrisoni. The mean number of large sensilla coeloconica on antennal flagellomeres 1–8 (approximately 30 in An. minimus and greater than 30 in An. harrisoni) should foster the identification of these species. We expect that this discovery will be useful for epidemiological studies in localities where the two species occur in sympatry. It is possible that the faster clasper movement observed in males of An. minimus might be an important component of the mechanism that controls the ability of this species to mate in small spaces. The results of the study provide a better understanding of the mating behaviour of both this species and the closely related An. harrisoni.
Abbreviations
- ac:
-
Aculeae
- AS-PCR:
-
Allele-specific polymerase chain reaction
- btc:
-
Blunt-tipped sensilla trichodea
- D:
-
Mosquito population density
- DRS:
-
Density resting surface
- lch:
-
Large sensilla chaetica
- lco:
-
Large sensilla coeloconica
- ltc:
-
Long sharp sensilla trichodea
- Pe:
-
Pedicel
- RS:
-
Vertical resting surface area
- sa:
-
Sensilla ampullacea
- sb:
-
Sensilla basiconica
- Sc:
-
Scape
- sch:
-
Small sensilla chaetica
- sco:
-
Small sensilla coeloconica
- SEM:
-
Scanning electron microscopy
- stc:
-
Short sharp sensilla trichodea
References
World Health Organization. World Health Organization World Malaria Report 2015. Geneva: World Health Organization; 2015.
Harbach RE. Anopheles classification, Mosquito Taxonomic Inventory. Available at: http://mosquito-taxonomic-inventory.info/node/11358 Accessed 10 Sept 2016.
Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37:1–128.
Somboon P, Thongwat D, Harbach RE. Anopheles (Cellia) rampae n. sp., alias chromosomal form K of the Oriental Maculatus Group (Diptera: Culicidae) in Southeast Asia. Zootaxa. 2011;2810:47–55.
Taai K, Harbach RE. Systematics of the Anopheles barbirostris species complex (Diptera: Culicidae: Anophelinae) in Thailand. Zool J Linn Soc. 2015;174:244–64.
Harbach RE. The classification of genus Anopheles (Diptera: Culicidae): a working hypothesis of phylogenetic relationships. Bull Entomol Res. 2004;94:537–53.
Green CA, Gass RF, Munstermann LE, Baimai V. Population-genetic evidence for two species in Anopheles minimus in Thailand. Med Vet Entomol. 1990;4:25–34.
Harbach RE, Parkin E, Bin C, Butlin RK. Anopheles (Cellia) minimus Theobald (Diptera: Culicidae): neotype designation, characterization, and systematics. Proc Entomol Soc Wash. 2006;108:198–209.
Harbach RE, Garros C, Duc Manh N, Manguin S. Formal taxonomy of species C of Anopheles minimus sibling species complex (Diptera: Culicidae). Zootaxa. 2007;1654:41–54.
Somboon P, Rory A, Tsuda Y, Takagi M, Harbach RE. Systematics of Anopheles (Cellia) yaeyamaensis sp. n., alias species E of the An. minimus complex in southeastern Asia (Diptera: Culicidae). Zootaxa. 2010;2651:43–51.
Saeung A. Anopheles (Diptera: Culicidae) species complex in Thailand: Identification, distribution, bionomics and malaria-vector importance. Int J Parasitol Res. 2012;4:75–82.
Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89.
Wilkinson RN, Gould DJ, Boonyakanist A. Laboratory colonization of Anopheles minimus Theobald. Mosq News. 1974;34:29–32.
Harrison BA. Medical entomology studies - XIII. The Myzomyia Series of Anopheles (Cellia) in Thailand, with emphasis on intra-interspecific variations (Diptera: Culicidae). Contrib Am Entomol Inst. 1980;117:1–195.
Somboon P, Suwonkerd W. Establishment of a stenogamous colony of Anopheles minimus species A. Ann Trop Med Parasitol. 1997;91:673–6.
Sucharit S, Komalamisra N, Leemingsawat S, Apiwathnasorn C, Thongrungkiat S. Population genetic studies on the Anopheles minimus complex in Thailand. Southeast Asian J Trop Med Public Health. 1988;19:717–23.
Sharpe RG. The status of cryptic species with Anopheles minimus. PhD. Thesis. Leeds: University of Leeds; 1997.
Chen B, Harbach RE, Butlin RK. Molecular and morphological studies on the Anopheles minimus group of mosquitoes in southern China: taxonomic review, distribution and malaria vector status. Med Vet Entomol. 2002;16:253–65.
Sungvornyothin S, Garros C, Chareonviryaphap T, Manguin S. How reliable is the humeral pale spot for identification of cryptic species of the Minimus Complex? J Am Mosq Control Assoc. 2006;22:185–91.
Cuong do M, Van NT, Tao le Q, Chau TL, Anh le N, Thanh NX, et al. Identification of Anopheles minimus complex and related species in Vietnam. Southeast Asian J Trop Med Public Health. 2008;39:827–31.
Sharpe RG, Hims MM, Harbach RE, Butlin RK. PCR-based methods for identification of species of the Anopheles minimus group: allele-specific amplification and single-strand conformation polymorphism. Med Vet Entomol. 1999;13:265–73.
Garros C, Koekemoer LL, Coetzee M, Coosemans M, Manguin S. A single multiplex assay to identify major malaria vectors within the African Anopheles funestus and the Oriental An. minimus groups. Am J Trop Med Hyg. 2004;70:583–90.
Garros C, Harbach RE, Manguin S. Systematics and biogeographical implications of the phylogenetic relationships between members of the Funestus and Minimus Groups of Anopheles (Diptera: Culicidae). J Med Entomol. 2005;42:7–18.
Garros C, Harbach RE, Manguin S. Morphological assessment and molecular phylogenetics of the Funestus and Minimus Groups of Anopheles (Cellia). J Med Entomol. 2005;42:522–36.
Chen B, Pedro PM, Harbach RE, Somboon P, Walton C, Butlin RK. Mitochondrial DNA variation in the malaria vector Anopheles minimus across China, Thailand and Vietnam: evolutionary hypothesis, population structure and population history. Heredity. 2011;106:241–52.
Wijit A, Taai K, Dedkhad W, Hempolchom C, Thongsahuan S, Srisuka W, et al. Comparative studies on the stenogamous and eurygamous behavior of eight Anopheles species of the Hyrcanus Group (Diptera: Culicidae) in Thailand. Insects. 2016;26:7(2).
Junkum A, Jitpakdi A, Komalamisra N, Jariyapan N, Somboon P, Bates PA, et al. Comparative morphometry and morphology of Anopheles aconitus Form B and C eggs under scanning electron microscope. Rev Inst Med Trop Sao Paulo. 2004;46:257–62.
Sallum MAM, Foster PG, Santos CLSD, Flores DC, Motoki MT, Bergo ES. Resurrection of two species from synonymy of Anopheles (Nyssorhynchus) strodei Root, and characterization of a distinct morphological form from the Strodei Complex (Diptera: Culicidae). J Med Entomol. 2010;47:504–26.
Saeung A, Hempolchom C, Yasanga T, Otsuka Y, Thongsahuan S, Srisuka W, et al. Scanning electron microscopy of Anopheles hyrcanus group (Diptera: Culicidae) eggs in Thailand and an ultrastructural key for species identification. Parasitol Res. 2014;113:973–81.
Hempolchom C, Yasanga T, Wijit A, Taai K, Dedkhad W, Srisuka W, et al. Scanning electron microscopy of antennal sensilla of the eight Anopheles species of the Hyrcanus Group (Diptera: Culicidae) in Thailand. Parasitol Res. 2017;116:143–53.
Choochote W, Saeung A. Systematic techniques for the recognition of Anopheles species complexes. In: Manguin S, editor. Anopheles mosquitoes - New insights into malaria vectors. In Tech. 2013. p. 57–79.
Saeung A, Choochote W. Development of a facile system for mass production of Brugia malayi in a small-space laboratory. Parasitol Res. 2013;112:3259–65.
Sucharit S, Choochote W. Comparative studies on the morphometry of male genitalia and frequency of clasper movements during induced copulation of Anopheles balabacensis (Perlis Form) and Anopheles dirus (Bangkok Colony Strain). Mosq Syst. 1983;15:90–7.
Gerberg EJ, Ward RA, Barnard DR. Manual for mosquito rearing and experimental techniques. Bulletin No. 5 (revised) Lake Charles. Louisiana: Am. Mosq. Control Assoc. (AMCA). Inc; 1994. p. 1–98.
Hill SR, Hansson BS, Ignell R. Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem Senses. 2009;34:231–52.
Pitts RJ, Zwiebel LJ. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar J. 2006;5:26.
Somboon P, Walton C, Sharpe RG, Higa Y, Tuno N, Tsuda Y, et al. Evidence for a new sibling species of Anopheles minimus from the Ryukyu Archipelago, Japan. J Am Mosq Control Assoc. 2001;17:98–113.
Börstler J, Lühken R, Rudolf M, Steinke S, Melaun C, Becker S, et al. The use of morphometric wing characters to discriminate female Culex pipiens and Culex torrentium. J Vector Ecol. 2014;39:204–12.
Wilke AB, Christe Rde O, Multini LC, Vidal PO, Wilk-da-Silva R, De Carvalho GC, et al. Morphometric wing characters as a tool for mosquito identification. PLoS One. 2016;11:e0161643.
Acknowledgments
We are grateful to Dr. Wannapa Suwonkerd and Professor Theeraphap Chareonviriyaphap, who kindly provided An. minimus and An. harrisoni larvae, respectively, used to establish the colonies that produced the mosquitoes used in the study.
Funding
The Chiang Mai University (CMU), Thailand for providing the Post-Doctoral Fellowship 2016 to KT (contract number 09/2016). AS was supported by the Anandamahidol Foundation Scholarship, the Thailand Research Fund (TRF) and the Office of the Higher Education Commission (OHEC) through the Research Grant for New Scholar (grant MRG5980101) and the TRF Senior Research Scholar (grant RTA5880001).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Authors’ contributions
KT and AS designed the study, analyzed the data, interpreted the findings and wrote the first draft of the manuscript. KT, KA, WS and AS participated in mosquito rearing and experimental testing. KA performed AS-PCR identification. YO carried out the sequencing analysis. RH inputted morphological information and assisted in writing the draft and final manuscripts. TY assisted with taking the photomicrographs. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Taai, K., Harbach, R.E., Aupalee, K. et al. An effective method for the identification and separation of Anopheles minimus, the primary malaria vector in Thailand, and its sister species Anopheles harrisoni, with a comparison of their mating behaviors. Parasites Vectors 10, 97 (2017). https://doi.org/10.1186/s13071-017-2035-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2035-6