Abstract
Background
Small molecule antagonists of mosquito dopamine receptors (DARs) are under investigation as a new class of vector-selective insecticides. Antagonists that inhibit the D1-like DARs AaDOP2 and CqDOP2 from the mosquitoes Aedes aegypti L. and Culex quinquefasciatus Say, respectively, also cause larval mortality in bioassays. Here, we report on the orthologous DAR, AgDOP2, from the malaria mosquito Anopheles gambiae Giles that was cloned and pharmacologically characterized in HEK293 cells. Larval bioassays were then conducted to examine the potential of DAR antagonist insecticides against Anopheles vectors.
Findings
Previous in vitro cAMP accumulation assays demonstrated Gαs coupling for AaDOP2 and CqDOP2 and dose-dependent inhibition by DAR antagonists. We observed a negligible response of AgDOP2 in the cAMP assay, which prompted an investigation of alternative coupling for mosquito DARs. In an in vitro IP-One Gαq second messenger assay of calcium signaling, dopamine stimulation increased IP1 accumulation in AaDOP2-, CqDOP2- and AgDOP2-expressing cells, and DAR antagonists inhibited IP1 signaling in a dose-dependent manner. In larval bioassays, DAR antagonists caused considerable mortality of An. gambiae larvae within 24 h post-exposure.
Conclusions
In vitro data reveal pleiotropic coupling of AaDOP2 and CqDOP2 to Gαq and Gαs. In contrast, AgDOP2 appeared to selectively couple to Gαq signaling. In vitro antagonist studies revealed general conservation in pharmacology between mosquito DARs. In vivo data suggest potential for DAR antagonist insecticides against An. gambiae. Sequence conservation among the DOP2 receptors from 15 Anopheles species indicates utility of antagonists to control residual malaria transmission. AgDOP2 Gαq-dependent signaling could be exploited for An. gambiae control via pathway specific antagonists.
Similar content being viewed by others
Background
Control of malaria transmitted by species of Anopheles mosquitoes is largely achieved via long lasting insecticide treated nets and indoor residual sprays. New insecticidal chemistries are needed to protect against mosquitoes that are resistant to existing insecticides. Furthermore, to achieve malaria eradication or elimination, new insecticides are required to disrupt outdoor “residual” transmission by exophilic, day biting mosquitoes [1]. Recently, the Innovative Vector Control Consortium (IVCC; http://www.ivcc.com) issued a call for three new insecticides with novel modes of action by 2023 to control malaria mosquitoes [2]. New products must be mosquito-selective and effective against the many species of Anopheles that transmit malaria (see [3]).
Small molecule antagonists of mosquito D1-like dopamine receptors (DARs) show promise as a new class of insecticides against the mosquito vectors Aedes aegypti and Culex quinquefasciatus [4–7]. Several antagonists are potent inhibitors of the Ae. aegypti AaDOP2 and C. quinquefasciatus CqDOP2 DARs in vitro. These chemistries are >100-fold more selective for the mosquito DARs versus the human receptor, hD1, and are highly toxic to mosquito larvae. Further, studies have shown that invertebrate DOP2 receptors are both phylogenetically and pharmacologically distinct from mammalian D1-like receptors [8], a significant rationale for targeting of these receptors for insecticides.
Here, building on our previous work for AaDOP2 and CqDOP2, we extend DAR analyses to the Anopheles system. The orthologous DAR AgDOP2 was identified from the genome of Anopheles gambiae, the mosquito vector of malaria in sub-Saharan Africa, cloned, and pharmacologically characterized. AgDOP2 was expected to exhibit D1-like pharmacology based on its relation to other invertebrate dopamine receptors. We present molecular and pharmacological characterization of AgDOP2, as well as larval bioassays that support the potential for developing DAR antagonists to control mosquito vectors of malaria and other devastating human and animal pathogens.
Findings
Discovery and molecular characterization of DOP2 DARs from Anopheles species
The AgDOP2 gene [GenBank: KU948225] was identified from the Anopheles gambiae genome assembly available at VectorBase (https://www.vectorbase.org/) and manual annotation was performed as described by [4]. The conceptual AgDOP2 protein sequence was aligned with AaDOP2 and CqDOP2 using ClustalW [9] (Additional file 1: Figure S1 and Table S1). Residues required for receptor activity and associated with the transmembrane (TM) domains were generally conserved, with greatest divergence observed in the N-terminal region and the intracellular loop 3 (IL3). Of note, the IL3, a region typically associated with coupling to G proteins, is 21 residues longer in An. gambiae as compared to Cx. quinquefasciatus and Ae. aegypti. Gene expression of AgDOP2 in An. gambiae developmental stages and sexes was confirmed by RT-PCR, suggesting this receptor, like AaDOP2 and CqDOP2, is constitutively expressed throughout the mosquito life-cycle, and is likely associated with essential neurological processes as in other invertebrates [10]. DOP2 sequences from an additional 14 Anopheles species [11] were identified by tBLASTn searches against the GenBank Whole Genome Shotgun Contigs (WGS) database and manual annotation. Alignments revealed between 78.0 and 99.6 % identity of these sequences to AgDOP2 (Additional file 1: Figure S2).
In vitro Pharmacology of AgDOP2
For functional characterization, AgDOP2 was synthesized by Genscript (Piscataway, NJ, USA), cloned into the expression vector pcDNA3.1+ (Invitrogen, Carlsbad, CA) and a stable cell line expressing the receptor in Human Embryonic Kidney (HEK)-293 cells was generated as previously described [4, 6] by plating cells in a 10 cm dish and transfecting with 15 μl Lipofectamine2000 and 3 μg of plasmid. The pharmacology of AgDOP2 was evaluated in comparison to that of AaDOP2, CqDOP2 and hD1. On the basis of its relationship to other invertebrate dopamine receptors [6] (Additional file 1: Figure S1), AgDOP2 was predicted to couple Gαs, a guanine nucleotide binding protein that stimulates adenylyl cyclase activity following receptor activation. However, as the receptor showed no significant response to dopamine in cAMP accumulation assays (See Additional file 1: Figure S3), alternative coupling was investigated using the Cisbio IP-One HTRF accumulation assay (Cisbio, Bedford, MA, USA) that measures receptor activation of Gαq and subsequent stimulation of phospholipase C leading to accumulation of downstream inositol monophosphate (IP1). Assays and analyses were performed as in previous studies for cAMP [5, 6] with the exception that cryopreserved cells were plated in 1X Stimulation Buffer (10 mM HEPES; 1 mM CaCl2, 0.5 mM MgCl2, 5.5 mM D-Glucose, 4.2 mM KCl, 146 mM NaCl, 50 mM LiCl) and incubated at 37 °C, 5 % CO2, and 90 % humidity for 2 h. Drugs were diluted to appropriate concentration in 1X Stimulation Buffer containing 0.02 % ascorbic acid, and added to cells to then incubate for 1 h at 37 °C. Ligand stimulation of cells was arrested by addition of 3 μL/well d2 labelled IP1 and 3 μL/well Cryptate labelled anti-IP1 (diluted 1:5 in lysis buffer). Following incubation for 1 h at room temperature, plates were read on the Synergy 4 (BioTek Instruments, Winooski, VT, USA).
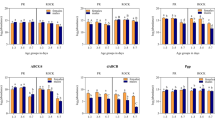
Increases in intracellular IP1 for each receptor were first measured as concentration response stimulation to dopamine (Fig. 1; Table 1). EC50 values revealed that AaDOP2 (1.3 μM ± 0.4) and CqDOP2 (0.7 μM ± 0.2) responded robustly to dopamine stimulation while AgDOP2 (4.7 μM ± 0.4) proved 3 and 7 fold less sensitive. These data suggest mosquito receptors can couple via Gαq in an HEK293 background and reveal a lack of Gαs-coupling for AgDOP2 in vitro. As expected, no increase in IP1 accumulation was observed when cells expressing hD1 were treated with dopamine, demonstrating that Gαq coupling does not reflect a general phenomenon for DARs expressed in the in vitro system employed here. Pleiotropic coupling to Gαs and Gαq has been reported for a D1-like DAR from the tick, Ixodes scapularis and the honey bee, Apis mellifera [12, 13]. Similar studies with the Drosophila melanogaster D1-like receptor, DopR99B, also implicate multiple second messenger systems [14] and the involvement of Gαq, Gαi/o- and Gβγ-coupling [15]. While hD1 couples only via Gαs, other human G protein coupled receptors (GPCRs) can signal via multiple G proteins [16, 17]. Further studies are required to confirm pleiotropic coupling of mosquito DARs in an insect cell background and in vivo, as well as to explore potential divergence between the signaling mechanisms of invertebrate and mammalian DARs. Apparent dependence of AgDOP2 on Gαq-coupling in vitro was an unexpected finding that may enable the identification of residues determining G protein interactions and development of products that selectively disrupt Gαq-mediated signaling of DOP2 in mosquitoes.
In vitro pharmacological characterization of AgDOP2 using IP1 accumulation assay and comparison to AaDOP2, CqDOP2 and hD1. Cryopreserved cells were plated in 384 well plates (20,000 cells/well), and receptor responses were analyzed for dopamine (upper left panel), or an EC90 concentration of dopamine in the presence of the indicated antagonists measured as IP1 accumulation. Data were analyzed using GraphPad prism v.6 software
The mosquito DARs exhibited similar profiles in response to DAR antagonists (Fig. 1; Table 1), suggesting a general conservation in receptor pharmacology. A suitable signal window was produced for these antagonist studies by stimulating the receptor-expressing HEK cells with an EC90 concentration of dopamine (10 μM for AaDOP2 and CqDOP2 and 100 μM for AgDOP2). Of the antagonists analyzed, amitriptyline, amperozide, chlorprothixene and methiothepin showed a higher potency at both AaDOP2 (4–35 fold) and CqDOP2 (3–40 fold), than at AgDOP2. Asenapine followed by SCH23390, a standard pharmacological probe used in previous investigations [4–6], proved the most potent for AgDOP2. Alternatively, butaclamol demonstrated slightly higher potency for AaDOP2 and AgDOP2, than CqDOP2.
Toxicity of DOP2 antagonists to Anopheles gambiae larvae
As in previous work with Aedes and Culex [5], we observed a correlation between in vitro and in vivo results in the Anopheles system. The in vivo activity of select antagonists was tested in L3 An. gambiae larvae, using concentration response assays conducted at 26 °C as described by [6] (note: SCH23390 was not included as this chemistry had no toxicity to Aedes and Culex larvae). Larvae of the KISUMU1 strain obtained through the MR4 (MRA catalog number MRA-762, KISUMU1 F34 strain, established by Dr. G. Davidson, donated by Vincent Corbel) were reared on a 12 h day/night cycle at 75 % RH at 28 °C in 25 × 40 cm plastic pans (400 larvae per pan) on a diet of ground flake fish food. Antagonists were selected based on demonstrated toxicity to L3 larvae of Ae. aegypti and C. quinquefasciatus [6]. DAR antagonists caused mortality of An. gambiae larvae 24 h post exposure (Fig. 2; Table 2). Methiothepin, asenapine and chlorprothixene were among the most toxic compounds at 72 h as compared to amitriptyline (LC50 = 151 μM), the chemistry employed as positive control in Ae. aegypti and Cu. quinquefasciatus bioassays [4, 5]. Amitriptyline was also identified by [18] as toxic to An. gambiae larvae and adults. Methiothepin and chlorprothixene were the most rapidly toxic to An. gambiae, presumably due to physico-chemical properties that affect absorption as discussed by [6]. Asenapine caused negligible toxicity at 24 h but toxicity was observed by 48 h. Chlorprothixene caused mortality (LC50 = 163 μM) initially, although most survivors remained viable for several days. The high sequence conservation between the DOP2 receptors of 14 Anopheles spp. from sub-Saharan Africa, south-east Asia and Latin America suggests the DAR antagonists identified may be broadly active at the DOP2 receptors of malaria vector species, including those that contribute significantly to residual malaria transmission. Genome assemblies for multiple Anopheles species [11] and populations [19] offer the opportunity to expand comparative molecular and pharmacological studies of DAR targets across the subfamily Anophelinae.
Conclusions
We present evidence of pleiotropic coupling via Gαs and Gαq among the mosquito DARs, AaDOP2 and CqDOP2. In contrast, AgDOP2 appeared to selectively couple to Gαq signaling in vitro. The heterologous expression studies also revealed general conservation in pharmacology between mosquito DARs including their relatively similar responses to DAR antagonists. Asenapine was the most potent and selective AgDOP2 antagonist in vitro and caused mortality of An. gambiae larvae. This and other antagonists offer “probes” for further pharmacological investigations. While physiochemical properties such as low lipophilicity and the presence of a charged amine group at physiological pH may limit the application of these chemistries as insecticidal leads, they never the less offer an important starting point for discovery of derivatives effective against Anopheles mosquitoes. Sequence conservation among the DOP2 DARs of 14 Anopheles species suggests potential to develop products to control residual transmission of malaria by multiple vectors. The discovery of an additional signaling pathway for mosquito DARs may offer opportunities to disrupt dopaminergic physiology of these vectors with new chemistries likely active through complex mechanisms.
Abbreviations
- AaDOP2:
-
Aedes aegypti dopamine receptor 2
- AgDOP2:
-
Anopheles gambiae dopamine receptor 2
- cAMP:
-
cyclic adenosine monophosphate
- CqDOP2:
-
Culex quinquefasciatus dopamine receptor 2
- DAR:
-
dopamine receptor
- EC:
-
effective concentration
- EL:
-
extracellular loop
- Gαs :
-
G protein subunit that stimulates adenylyl cyclase
- Gαq :
-
G protein subunit that stimulates phospholipase C
- GPCR:
-
G protein-coupled receptor
- HEK:
-
human embryonic kidney cells
- hD1:
-
human D1-like dopamine receptor
- HTRF:
-
homogenous time resolved fluorescence
- IC50 :
-
inhibitory concentration
- IL:
-
intracellular loop
- IP1:
-
inositol monophosphate
- IRS:
-
indoor residual spray
- LC50 :
-
lethal concentration
- LLIN:
-
long lasting insecticide treated net
- MoA:
-
mode of action
- PLC:
-
phospholipase C
- TM:
-
transmembrane domain
References
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330–52.
Hemingway J. The role of vector control in stopping the transmission of malaria: threats and opportunities. Phil Trans R Soc Lond B. 2014;369:20130431.
Sinka ME, Bangs MJ, Manguuin S, Rubio-Palis Y, Chareonviriyaphao T, Coetsee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69–80.
Meyer JM, Ejendal KFK, Avramova LV, Garland-Kuntz EE, Giraldo-Calderon GI, Brust TF, et al. A “genome-to-lead” approach for insecticide discovery: pharmacological characterization and screening of Aedes aegypti D1-like dopamine receptors. PLoS Negl Trop Dis. 2012;6:e1478.
Conley JM, Meyer JM, Nuss AB, Doyle TB, Savinov S, Hill CA, et al. In vitro and in vivo evaluation of AaDOP2 receptor antagonists reveals antidepressants and antipsychotics as novel lead molecules for yellow-fever mosquito control. J Pharmacol Exp Ther. 2015;352:53–60.
Nuss AB, Ejendal KFK, Doyle TB, Meyer JM, Lang EG, Watts VJ, et al. Dopamine receptor antagonists as new mode-of-action insecticide leads for control of Aedes and Culex mosquito vectors. PLoS Negl Trop Dis. 2015;9:e0003515.
Hill CA, Meyer JM, Ejendal KFK, Echeverry DF, Lang EG, Avramova LV, et al. Re-invigorating the insecticide discovery pipeline for vector control: GPCRs as targets for the identification of next gen insecticides. Pestic Biochem Physiol. 2013;106:141–8.
Mustard JA, Beggs KT, Mercer AR. Molecular biology of the invertebrate dopamine receptors. Arch Insect Biochem Physiol. 2005;59:103–17.
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–500.
Mustard JA, Pham PM, Smith BH. Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J Insect Physiol. 2010;56:422–30.
Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, et al. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science. 2015;347:1258522.
Šimo L, Koči J, Žitňan D, Park Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS One. 2011;6:e16158.
Beggs KT, Hamilton IS, Kurshan PT, Mustard JA, Mercer AR. Characterization of a D3-like dopamine receptor (AmDOP3) in honey bee Apis mellifera. Insect Biochem Mol Biol. 2005;35:873–82.
Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, et al. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci. 1996;16:3925–33.
Reale V, Hannan F, Hall LM, Evans PD. Agonist-specific coupling of a cloned Drosophila melanogaster D1-like dopamine receptor to multiple second messenger pathways by synthetic agonists. J Neurosci. 1997;17:6545–53.
Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta 2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91.
Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, et al. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–40.
Fuchs S, Rende E, Crisanti A, Nolan T. Disruption of aminergic signaling reveals novel compounds with distinct inhibitory effects on mosquito reproduction, locomotor function and survival. Sci Rep. 2014;4:5526.
Ag1000G: Anopheles gambiae 1000 Genomes. http://www.malariagen.net/projects/vector/ag1000g (year). Accessed 28 June 2015.
Acknowledgements
This work was supported by a U.S. Department of Defense, Deployed War Fighter Project award, W911QY and a Purdue University AgSEED award to CAH and VJW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CAH, JMM, ABN and VJW conceived of and designed the study. JMM and ABN performed receptor identification and annotation, ABN performed sequence alignments and in vivo studies, and TD and KFKE performed in vitro studies. TD, KFKE, CAH, JMM, ABN and VJW analyzed the data. TD, KFKE, CAH, ABN and VJW wrote the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1:
Genomic and pharmacologic assessment of mosquito dopamine receptors. (DOCX 1422 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hill, C.A., Doyle, T., Nuss, A.B. et al. Comparative pharmacological characterization of D1-like dopamine receptors from Anopheles gambiae, Aedes aegypti and Culex quinquefasciatus suggests pleiotropic signaling in mosquito vector lineages. Parasites Vectors 9, 192 (2016). https://doi.org/10.1186/s13071-016-1477-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1477-6