Abstract
Background
The increasing spread of insecticide resistance in malaria vectors has been well documented across sub-Saharan Africa countries. The influence of irrigation on increasing vector resistance is poorly understood, and is critical to successful and ethical implementation of food security policies. This study investigated the insecticide resistance status of An. gambiae (s.l.) mosquitoes collected from the irrigated rice area of Okyereko, a village containing about 42 hectares of irrigated field within an irrigation project plan in the Central Region of Ghana. Large amounts of insecticides, herbicides and fertilizers are commonly used in the area to boost the annual production of the rice.
Methods
Mosquito larvae were collected and adults were assayed from the F1 progeny. The resistance status, allele and genotype were characterized using WHO susceptibility testing and PCR methods respectively.
Results
The An. gambiae (s.l.) populations from Okyereko are highly resistant to DDT and pyrethroid insecticides, with possible involvement of metabolic mechanisms including the elevation of P450 and GST enzyme as well as P-gp activity. The population was mostly composed of An. coluzzii specimens (more than 96 %) with kdr and ace-1 frequencies of 0.9 and 0.2 %, respectively.
Conclusion
This study brings additional information on insecticide resistance and the characterization of An. gambiae (s.l.) mosquitoes from Okyereko, which can be helpful in decision making for vector control programmes in the region.
Similar content being viewed by others
Background
The spread of insecticide resistance is threatening the continued efficacy of current malaria control tools and is exacerbated by the use of insecticides in controlling both agricultural pests and vectors that affect public health [1–5]. The Republic of Ghana is not exempt from this trend [6–8]. Pyrethroid resistance in malaria vectors is increasingly reported from different parts of Africa, and has been associated with selection pressure resulting from the scaling up of insecticide treated materials such as Long Lasting Insecticidal Nets (LLINs) and indoor residual house spraying (IRS) [9, 10], as well as the application of agricultural pesticides [1, 3, 11].
The dominant mosquito species responsible for the transmission of malaria parasites in Africa includes An. gambiae Giles (s.s.) and An. coluzzii Coetzee & Wilkerson (formerly An. gambiae S and M molecular forms, respectively), An. arabiensis Patton and An. funestus Giles (s.s.), all of which are widespread throughout tropical and subtropical Africa. Anopheles arabiensis prefers drier habitats and An. coluzzii is restricted to West-Central Africa [12, 13]. The adult behaviors and larval biology of these species are different, which impact the efficacy and/or suitability of control measures. Ecological differentiation indicates that An. coluzzii preferentially exploits immature sites that exist across seasons and are more associated with human activities, such as those created by irrigation, rice cultivation and urbanization [14–16] whilst An. gambiae (s.s.) immatures occupy rain-dependent pools and temporary puddles [17]. Adult female of An. gambiae (s.s.), An. coluzzii and An. funestus (s.s.) are mostly anthropophilic and prefer resting inside human habitations, while An. arabiensis will feed on either humans or cattle, and rest indoors or outdoors [18].
Malaria is considered to be one of the major contributors to poverty and the estimated annual cost to economies of the African continent ranges from under 0.5 % to almost 9 % of GDP [19]. Population growth in many malaria endemic countries has led to policies advocating local food production to enable self-sufficiency in order to reduce the importation of food and to positively impact the gross national product in these countries [20–22]. The Government of Ghana recently recommended increasing local rice production through the development and/or reorganization of irrigation projects to reduce the importation of rice [23]. Though the main objective of this initiative is to cultivate and produce local rice for consumption, collateral effects are likely to be linked to the increasing resistance already detected in An. gambiae (s.l.) in these areas [24–26]. In fact, the development of irrigation schemes in sub-Saharan Africa has been blamed for the increase of malaria risk through the creation of favorable breeding sites for malaria vector mosquitoes [27–29]. Moreover, there is considerable use of herbicides, insecticides and fertilizers in irrigated rice projects; hence multiple annual rice production cycles are expected to have a dramatic impact on the insecticide resistance of malaria vectors breeding in these zones, and potentially elsewhere in the country [30, 31]. Several studies have already been conducted in Okyereko and surroundings, showing the stability of An. coluzzii and An. gambiae (s.s.) density throughout the whole year and its impact in terms of malaria transmission and lymphatic filariasis [32–34]. This study was carried out to investigate and to characterize the resistance mechanisms involved in the insecticide resistance of the An. coluzzii and An. gambiae (s.s.) from Okyereko.
Methods
Study site
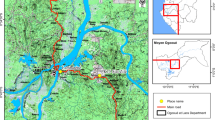
Okyereko is a village located about 50 km west of Accra, the capital city of Ghana (05°24’57.68”N, 00°35’53.99”W) (Fig. 1). The climate is coastal savanna vegetation with an annual average rainfall of 750 mm. The Okyereko irrigation project consists of an earthen dam with a catchment area of about 1,685 km2. The reservoir is fed by the tributary of the River Ayensu. Two canals on the left and right banks of the tributary convey water to the irrigable area. Eighty-one of the 125 hectares available have been developed, including 42 ha irrigated by the project.
Mosquito collections
Mosquito larvae were collected from water pools around the rice irrigated fields, brought to the laboratory and reared to adult stage in the insectary of Vestergaard-Noguchi Memorial Institute for Medical Research Vector Labs, Legon, in Accra. Larvae were reared in plastic trays (27 × 16 × 6.5 cm) containing 2 L of de-ionized water at a density of approximately 150/L, and fed with a mixture of finely ground tropical fish flakes. Pupae were transferred to 0.27 m3 screened cages and emerged adults were provided with 10 % glucose solution [35]. For consistency, all tests were conducted using the progeny of the F1 generation. The susceptible laboratory strain An. gambiae (s.s.) Kisumu was used as a control for all the tests that were conducted.
WHO susceptibility tests
Mortality and knock-down resulting from tarsal contact with insecticide impregnated papers using diagnostic doses were measured using WHO susceptibility test kits [36] against An. gambiae (s.l.) females. Batches of 20–25 non-blood fed females, aged 3–5 days, were exposed to insecticide impregnated papers for 1 h for all the insecticides and 2 h specifically for fenitrothion. The numbers of mosquitoes knocked down were recorded every 10 min for 60 min for DDT and pyrethroids and after the exposure time for the other classes of insecticides. Thereafter, the mosquitoes were transferred into observation tubes, provided with pads of cotton wool soaked in 10 % glucose solution and mortality was recorded after 24 h.
Additional synergist assays were similarly performed as described above. Batches of 20–25 non blood fed females aged 3–5 days were also pre-exposed to each synergist impregnated paper for 1 h and transferred to the insecticide impregnated paper tubes for another holding period of 1 h. The number of mosquitoes knocked down and the mortality were also recorded as described above. The insecticides tested were run along with the synergist assay as positive controls. Tests with either silicone oil or olive oil impregnated papers were run in parallel and served as negative controls. The tests were performed at 25 °C ±2 °C and 70 % ± 10 % humidity.
Mosquitoes were first assayed using WHO discriminating dosages of seven insecticides belonging to four different chemical classes: deltamethrin 0.05 % and permethrin 0.75 % (pyrethroids), DDT 4 % (organochlorine), bendiocarb 0.1 % and propoxur 0.1 % (carbamates), and fenitrothion 1 % and malathion 5 % (organophosphates). Additional synergist tests were performed using Piperonyl butoxide (PBO) 5 %, Verapamil 0.1 % and S,S,S-tributylphosphorotrithioate (DEF) 0.1 %. PBO, DEF and Verapamil are known to be inhibitors of cytochrome P450s and GSTs, carboxylesterases and P-glycoprotein efflux pumps (P-gps) respectively [37–39].
Following scoring of their mortality or survival status after 24 h, all the specimens tested were preserved at −20 °C for molecular analysis. Two hundred mosquitoes from the WHO susceptibility test kept at −20 °C were randomly sampled per insecticide, sorted according to their status for DNA extraction. PCR was performed for species identification and detection of resistance mechanisms.
Identification of sibling species in the An. gambiae complex
Genomic DNA was extracted from each mosquito using a slightly modified protocol of Collins et al. [40]. A single mosquito was homogenized in a 1.5 ml Eppendorf tube containing 200 μl of CTAB buffer (100 mM Tris HCL, pH 8.0, 10 mM EDTA, 1.4 M NaCl, 2 % Cetyl Trimethyl Ammonium Bromide) and incubated at 65 °C for 5 min. Chloroform (200 μl) was added and mixed by inversion of the tube. After centrifugation at 12000 rpm at room temperature for 5 min, the aqueous phase (upper layer) of the solution was pipetted into a fresh 1.5 ml tube; 200 μl of isopropyl alcohol was added, mixed by inversion and then centrifuged at 12000 rpm for 15 min. Afterwards, the supernatant was discarded and the DNA pellet formed at the bottom of tubes was washed with 70 % ethanol, dried and reconstituted in 20 μl of DNAse-free water.
Mosquito specimens were identified to the species level using the PCR method described by Scott et al. [41] and further characterized into molecular forms using the SINE PCR method of Santolamazza et al. [42]. The SINE PCR was performed using the primers; F6.1a (TCGCCTTAGACCTTGCGTTA) and R6.1b (CGCTTCAAGAATTCGAGATAC) in 25 μl PCR reaction mix containing 4 μl of 1/40th dilution of genomic DNA extracted from a single mosquito, 1 μl of 10 μM each of F6.1a and R6.1b primers, 6.5 μl of nuclease-free water and 12.5 μl of GoTaq (Promega).
Detection of the kdr and ace-1 mutations by diagnostic PCR assays
The PCR test for the detection of kdr mutations was carried out as described by Martinez-Torres et al. [43, 44] and confirmed by Real Time PCR following the protocol of Bass et al. [45]. A volume of 25 μl PCR reaction consisting of 12.5 μl of GoTaq, 4.5 μl of DNase-free water, 4 μl of 1/40th dilution of DNA template and 1 μl each of 20 μM of primers AGD1 (ATAGATTCCCCGACCATG); AGD2 (AGACAAGGATGATGAACC), AGD3 (AATTTGCATTACTTACGACA) and AGD4 (CTGTAGTGATAGGAAATTTA) was prepared.
A PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) diagnostic test was used to detect the presence of the G119S mutation in the ace-1 gene as described by Weill et al. [46]. A mixture of 25 μl PCR reaction was prepared and this consisted of 1 μl each of 10 μM Primers EX3AGdir (GATCGTGGACACCGTGTTCG) and EX3AGrev (AGGATGGCCCGCTGGAACAG), 12.5 μl of GoTaq, 9 μl of DNase-free water and 1.5 μl of 1/40 dilution of DNA template. An enzymatic digestion step was followed after the PCR reaction. A 20 μl restriction enzyme reaction mixture was prepared, consisting of 2 μl of Enzymatic Buffer B 10X, 0.2 μl of Acetylated BSA at 10 μg/μl and 0.5 μl of 10 U/μl restriction enzyme Alu I (Promega), 12.3 μl of DNase-free water and 5 μl of PCR products. The mixture was incubated at 37 °C for 4 h in a thermocycler.
The resulting restriction fragments as well as all the other PCR were run on 2 % agarose gels stained with ethidium bromide and visualized under UV light.
Statistical analysis
The susceptibility status of the colony against each insecticide was determined following WHO criteria [47]. The population is considered resistant when mortality after 24 h is in the range of 0–90 %, suspicion of resistance is recorded mortality is between 91 and 97 %, and populations are scored as susceptible when 98–100 % of the specimens are killed.
Abbott’s formula was applied when the mortality of the controls was between 5 and 10 % [48].
The kdr and ace-1 frequencies were calculated using the Hardy Weinberg formula [49] and compared with each population using the z-test for proportions with XLSTAT software.
Results
Resistance status
An average of 80 An. gambiae (s.s.) Kisumu strain female mosquitoes from colony were assayed for each of the seven insecticides tested and control. Anopheles gambiae (s.s.) Kisumu strain was found to be fully susceptible to all of them by yielding 100 % mortality after 24 h of observation and therefore confirmed the effectiveness of the insecticide-impregnated papers.
A total of 157 female An. gambiae (s.l.) from Okyereko were assayed for deltamethrin, 132 for permethrin and an average of 100 mosquitoes for all other insecticides. Mortality observed with DDT and pyrethroid insecticides was very low, ranging from 0 % with DDT to 18.4 and 34.1 % with permethrin and deltamethrin respectively. Higher mortalities were observed with the organophosphates with 86 % mortality for fenitrothion and 100 % with malathion. The observed mortalities using carbamates were 64 % for bendiocarb and 57 % for propoxur (Figs. 2 and 3).
Mortality with pyrethroids was slightly improved with pre exposure to synergists. PBO showed the highest improvement with mortality increasing by more than 2.5 fold for deltamethrin and permethrin. DEF + permethrin and Verapamil + permethrin did not show any significant mortality increment compared to permethrin alone (P > 0.05). A significantly higher increase in mortality was observed with PBO + deltamethrin than with PBO + permethrin, as well with DEF and Verapamil to synergize both pyrethroid insecticides. DEF and Verapamil yielded 2 and 1.7 fold increases in mortality rates with deltamethrin, respectively. No increase in mortality was observed with pre exposure to synergists and DDT (Fig. 3).
Identification of An. gambiae complex species and characterization of the resistance mechanisms
Two hundred mosquitoes were identified as An. gambiae (s.l.) of which 193 were An. coluzzii, representing 96.5 % and the remaining seven were identified as An. gambiae (s.s.) Allele and genotype frequency results at the kdr and ace-1 loci of An. gambiae (s.l.) (Table 1), showed a high prevalence of the kdr L1014F mutation in the An. gambiae populations at Okyereko at a frequency of almost 90 %. Most of the mosquitoes tested were either homozygotes (RR) or heterozygotes (RS). Only four homozygote susceptible (SS) mosquitoes were found among the analyzed samples. Secondly, both forms showed similar frequency of 0.9, though the number of An. coluzzii was far larger than the number of An. gambiae (s.s.).
The ace-1 detected in both An. coluzzii and An. gambiae (s.s.) were at a very low frequency. Only few heterozygote RS genotypes were observed giving a frequency of 2 % ace-1 mutation. Moreover, the frequency of ace-1 observed among both species was not significantly different (p = 0.433) with 0.2 and 0.3 % in An. coluzzii and An. gambiae (s.s.), respectively.
The presence of the kdr and ace-1 allele was also compared between the dead and the surviving mosquitoes after the WHO susceptibility testing. No significant difference in the kdr distribution between the two groups was observed (P = 0.699). In contrast, there was a significantly higher rate of RS genotypes among the mosquitoes that survived than among the dead specimens (P < 0.0001) for the ace-1 mutation (Table 2). The frequency of the ace-1 mutation observed was 0.25 for the surviving mosquitoes and significantly higher than that recorded in the dead mosquitoes (0.11) (P < 0.009).
Discussion
More than 96 % of the mosquitoes collected from Okyereko rice field area were identified as An. coluzzii, similar to species ratios reported in previous studies [32, 34]. This agrees with previous observations suggesting that An. coluzzii is more adapted to breed in irrigated fields, in contrast to An. gambiae (s.s.), which is described as a species of humid forested areas and temporary pools [50, 51].
The long-term use of insecticides in agriculture and households has been implicated in the increasing insecticide resistance of insect vectors and particularly the malaria vector An. gambiae (s.l.) [1, 5, 9, 52–55]. This study demonstrated that mosquito populations from Okyereko are resistant to pyrethroid, organochlorine and carbamate classes of insecticides, as well as an organophosphate commonly used in vector control programmes. Insecticide resistance mechanisms of An. coluzzii and An. gambiae (s.s.) from Okyereko involved high levels of kdr L1014F mutation, mainly expressed in resistance to DDT and pyrethroids, with 0 % mortality against DDT and less than 35 % for permethrin and deltamethrin. The use of PBO, in addition to either permethrin or deltamethrin increased the mortality rates by 2× and 3× respectively, indicating the likely involvement of oxidases and potentially esterase-based metabolic resistance mechanisms. Although oxidases may be involved in the detoxification of almost all insecticides, they are known to be mostly associated with pyrethroid resistance [38]. Furthermore, there are increasing reports of oxidases being involved in the metabolism of carbamates [56], which likely contributed to the higher carbamate resistance level detected than organophosphate. No effects of synergists were found with DDT, which is likely affected by the kdr mutation and possibly Glutathione-S-Transferase (GST) mechanisms, although increased GST activity was not verified in this study [57, 58]. The DDT mortality observed were similar to the extremely high resistance reported by Chouaibou et al. [59], in An. gambiae (s.l.) collected from rice irrigation fields of Tiassalé in Côte d’Ivoire.
These results also show a significant increase in mortality to deltamethrin after a pre-exposure to verapamil, suggesting a potential role of P-gps in the resistance mechanisms to this pyrethroid [37]. Several studies have demonstrated that pesticides in mammalian cells were partially regulated through the action of P-gps [60, 61]. These proteins belong to the superfamily of ATP-Binding Cassette (ABC transporters), which pump molecules out of cells by an ATP-dependent mechanism [62] and have been associated with resistance to a number of drugs [63]. The role of P-gps in defense against temephos and diflubenzuron insecticides in Aedes caspius (Pallas) has been demonstrated using Verapamil [64], so similar mechanisms in other species of mosquitoes such as An. gambiae (s.l.) are not unlikely.
The use of DEF, inhibitor of carboxylesterase activity yielded similar results as Verapamil for all the insecticides that were synergized. Significant improvement was noted with deltamethrin and permethrin. Carboxylesterase is involved in the detoxification of various chemicals, including pesticides. Inhibition of carboxylesterase by DEF has resulted in the potentiation of organophosphate pesticides such as Malathion that contain a carboxylic ester group [39]. Nevertheless, this trend wasn’t tested in this study due to the susceptibility level already expressed by the population, showing 100 and 86 % mortality against malathion and fenitrothion respectively.
Both An. coluzzii and An. gambiae (s.s.) expressed high pyrethroid-resistance with the involvement of the kdr L1014F mutation at almost the same frequency. No correlation was observed between the phenotypic status of the mosquitoes after the susceptibility test and the genotype of the specimens [65]. In contrast, the ace-1 G119S mutation was observed in a very low proportion among the dead mosquitoes. Only a few heterozygous mosquitoes were found after analysis (Freq ace-1 = 0.1). A correlation between the life status of the mosquitoes after WHO tube testing and the ace-1 mutation genotype was noted, with most of the dead mosquitoes found to be susceptible, and the majority of heterozygous RS found among those surviving. The correlation of the life status of mosquitoes has been well described with the carbamate insecticides where 75 % of heterozygous resistant (RS) were alive and 77 % of the dead mosquitoes were homozygous susceptible (SS). A similar trend was also observed using An. gambiae (s.l.) mosquitoes from Tiassalé in Côte d’Ivoire by Edi et al. [66], where the ace-1 mutation was mostly expressed among the mosquitoes surviving from the WHO susceptibility test. Overall, the insecticide resistance level of the mosquitoes from Okyereko could lead to a failure of some vector control measures in the area. It is well demonstrated that the ineffectiveness of LLINs impregnated with pyrethroid insecticides was mostly linked to the high kdr allele frequency of An. gambiae (s.l.) [9, 67].
Conclusion
This study brings additional information on the resistant status of the An. coluzzii and An. gambiae (s.s.) from Okyereko. The implications of this resistance profile for malaria control are considerable and should be taken into account when considering insecticide choices in the vector control programmes in the region. A policy for controlling insecticide use in irrigated fields would likely help slowdown the development of insecticide resistance in these areas.
References
Akogbeto MC, Djouaka R, Noukpo H. Use of agricultural insecticides in Benin. Bull Soc Pathol Exot. 2005;98:400–5.
Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189.
Diabate A, Baldet T, Chandre F, Akoobeto M, Guiguemde TR, Darriet F, Brengues C, Guillet P, Hemingway J, Small GJ, Hougard JM. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–22.
Djogbenou L. Vector control methods against malaria and vector resistance to insecticides in Africa. Med Trop. 2009;69:160–4.
Yadouleton AW, Padonou G, Asidi A, Moiroux N, Bio-Banganna S, Corbel V, N'Guessan R, Gbenou D, Yacoubou I, Gazard K, Akogbeto MC. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83.
Adasi K, Hemingway J. Susceptibility to three pyrethroids and detection of knockdown resistance mutation in Ghanaian Anopheles gambiae sensu stricto. J Vector Ecol. 2008;33:255–62.
Muller P, Donnelly MJ, Ranson H. Transcription profiling of a recently colonised pyrethroid resistant Anopheles gambiae strain from Ghana. BMC Genomics. 2007;8:36.
Yawson AE, McCall PJ, Wilson MD, Donnelly MJ. Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med Vet Entomol. 2004;18:372–7.
N’Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:199–206.
Padonou GG, Sezonlin M, Osse R, Aizoun N, Oke-Agbo F, Oussou O, Gbedjissi G, Akogbeto M. Impact of three years of large scale Indoor Residual Spraying (IRS) and Insecticide Treated Nets (ITNs) interventions on insecticide resistance in Anopheles gambiae s.l. in Benin. Parasit Vectors. 2012;5:72.
Akogbeto MC, Djouaka RF, Kinde-Gazard DA. Screening of pesticide residues in soil and water samples from agricultural settings. Malar J. 2006;5:22.
Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. ZOOTAXA. 2013;3619(3):246–74. 29.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117.
Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IH, Ose K, Fotsing JM, Sagnon N, Fontenille D, et al. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 2009;9:16.
Lehmann T, Diabate A. The molecular forms of Anopheles gambiae: a phenotypic perspective. Infect Genet Evol. 2008;8:737–46.
Simard F, Ayala D, Kamdem GC, Pombi M, Etouna J, Ose K, Fotsing JM, Fontenille D, Besansky NJ, Costantini C. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecol. 2009;9:17.
Della Torre A, Tu Z, Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem Mol Biol. 2005;35:755–69.
Knox TB, Juma EO, Ochomo EO, Pates Jamet H, Ndungo L, Chege P, Bayoh NM, N'Guessan R, Christian RN, Hunt RH, Coetzee M. An online tool for mapping insecticide resistance in major Anopheles vectors of human malaria parasites and review of resistance status for the Afrotropical region. Parasit Vectors. 2014;7:76.
Okorosobo TOF, Mwabu G, Orem JN, Kirigia JM. Economic burden of malaria in six countries of Africa. Eur J Bus Manage. 2011;3:42–62. 20.
Pant C. Vector borne diseases of man and their socio-economic impact. Insect Sci Applic. 1987;8:655–64.
Kaliyaperumal Karunamoorthi SS. Insecticide resistance in insect vectors of disease with special reference to mosquitoes: a potential threat to global public health. Health Scope. 2013;2(1):4–18. 15.
Service MW. Agricultural development and arthropod-borne diseases: a review. Rev Saúde públ, S Paulo. 1991;25:165–78.
GSSP. Ghana Strategy Support Program. GSSP Working Paper 2011, No. 0027
Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Akogbeto M, Hougard JM, Rowland M. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–16.
Dabire KR, Diabate A, Djogbenou L, Ouari A, N’Guessan R, Ouedraogo JB, Hougard JM, Chandre F, Baldet T. Dynamics of multiple insecticide resistance in the malaria vector Anopheles gambiae in a rice growing area in South-Western Burkina Faso. Malar J. 2008;7:188.
Djogbenou L, Labbe P, Chandre F, Pasteur N, Weill M. Ace-1 duplication in Anopheles gambiae: a challenge for malaria control. Malar J. 2009;8:70.
Boelee E KF, vander Hoek W (Eds). Malaria in irrigated agriculture: papers and abstracts for the SIMA special seminar at the ICID 18th International congress on irrigation and drainage, Montreal. Colombo. International Water Management Institute; IWMI Working Paper. 2002, 47.
Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes AM, Yohannes M, Teklehaimanot HD, Lindsay SW, Byass P. Incidence of malaria among children living near dams in northern Ethiopia: community based incidence survey. BMJ. 1999;319:663–6.
Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Med Vet Entomol. 2001;15:1–11.
Nkya TE, Akhouayri I, Kisinza W, David JP. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43:407–16.
Philbert A, Lyantagaye SL, Nkwengulila G. Review of agricultural pesticides use and the selection for resistance to insecticides in malaria vectors. Advances in Entomology. 2014;2:120–8.
Charlwood JD, Tomas EV, Egyir-Yawson A, Kampango AA, Pitts RJ. Feeding frequency and survival of Anopheles gambiae in a rice-growing area in Ghana. Med Vet Entomol. 2012;26:263–70.
Dzodzomenyo M, Dunyo SK, Ahorlu CK, Coker WZ, Appawu MA, Pedersen EM, Simonsen PE. Bancroftian filariasis in an irrigation project community in southern Ghana. Trop Med Int Health. 1999;4:13–8.
Okoye PN, Wilson MD, Boakye DA, Brown CA. Impact of the okyereko irrigation project in Ghana on the risk of human malaria infection by Anopheles species (Diptera: Culicidae). African Entomology. 2005;13(2):249–53.
Clements AN. The biology of mosquitoes: Development, nutrition and reproduction. London: Chapman and Hall; 1992.
WHO. Tests Procedures for insecticide resistance monitoring in malaria vec-tors, bioefficacy and persistence of insecticides on treated surfaces. In: Report of the WHO Informal Consultation, Geneva. Geneva: WHO/MAL/98.12, World Health Organization; 1998. p. 43.
Buss DS, McCaffery AR, Callaghan A. Evidence for p-glycoprotein modification of insecticide toxicity in mosquitoes of the Culex pipiens complex. Med Vet Entomol. 2002;16:218–22.
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91.
Murphy SD, Cheever KL, Chow SYK, Brewster M. Organophosphate insecticide potentiation by carboxylesterase inhibitors. Proc Eur Soc Toxicol Predict Chronic Toxicol. 1976;17:292–300.
Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987;37:37–41.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, Della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163.
Martinez-Torres D, Chevillon C, Brun-Barale A, Bergé JB, Pasteur N, Pauron D. Voltage-dependent Na + channels in pyrethroid-resistant Culex pipiens L. mosquitoes. Pestic Sci. 1999;55:1012–20.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Bass C, Williamson MS, Wilding CS, Donnelly MJ, Field LM. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malar J. 2007;6:155.
Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, Raymond M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13:1–7.
WHO. Test procedures for insecticide resistance monitoring in malaria vectors mosquitoes, vol. 40. Geneva: World Health Organization; 2013.
Abbott WS. A method of computing the effectiveness of an insecticide. J Am Mosq Control Assoc. 1925;3:302–3.
Rousset F, Raymond M. Testing heterozygote excess and deficiency. Genetics. 1995;140:1413–9.
Della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol Biol. 2001;10:9–18.
Wondji C, Simard F, Fontenille D. Evidence for genetic differentiation between the molecular forms M and S within the Forest chromosomal form of Anopheles gambiae in an area of sympatry. Insect Mol Biol. 2002;11:11–9.
Dabire RK, Diabate A, Baldet T, Pare-Toe L, Guiguemde RT, Ouedraogo JB, Skovmand O. Personal protection of long lasting insecticide-treated nets in areas of Anopheles gambiae s.s. resistance to pyrethroids. Malar J. 2006;5:12.
Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63:628–33.
Okumu FO, Mbeyela E, Lingamba G, Moore J, Ntamatungiro AJ, Kavishe DR, Kenward MG, Turner E, Lorenz LM, Moore SJ. Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Parasit Vectors. 2013;6:46.
Rivero A, Vezilier J, Weill M, Read AF, Gandon S. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog. 2010;6:e1001000.
David JP, Ismail HM, Chandor-Proust A, Paine MJ. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120429.
Mitchell SN, Rigden DJ, Dowd AJ, Lu F, Wilding CS, Weetman D, Dadzie S, Jenkins AM, Regna K, Boko P, et al. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS One. 2014;9:e92662.
Riveron JM, Yunta C, Ibrahim SS, Djouaka R, Irving H, Menze BD, Ismail HM, Hemingway J, Ranson H, Albert A, Wondji CS. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014;15:R27.
Chouaibou MS, Chabi J, Bingham GV, Knox TB, N’Dri L, Kesse NB, Bonfoh B, Jamet HV. Increase in susceptibility to insecticides with aging of wild Anopheles gambiae mosquitoes from Cote d’Ivoire. BMC Infect Dis. 2012;12:214.
Bain LJ, LeBlanc GA. Interaction of structurally diverse pesticides with the human MDR1 gene product P-glycoprotein. Toxicol Appl Pharmacol. 1996;141:288–98.
Bain LJ, McLachlan JB, LeBlanc GA. Structure-activity relationships for xenobiotic transport substrates and inhibitory ligands of P-glycoprotein. Environ Health Perspect. 1997;105:812–8.
Germann UA, Chambers TC. Molecular analysis of the multidrug transporter, P-glycoprotein. Cytotechnology. 1998;27:31–60.
Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–57.
Porretta D, Gargani M, Bellini R, Medici A, Punelli F, Urbanelli S. Defence mechanisms against insecticides temephos and diflubenzuron in the mosquito Aedes caspius: the P-glycoprotein efflux pumps. Med Vet Entomol. 2008;22:48–54.
Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WC. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 2009;25:213–9.
Edi CV, Djogbenou L, Jenkins AM, Regna K, Muskavitch MA, Poupardin R, Jones CM, Essandoh J, Ketoh GK, Paine MJ, et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10:e1004236.
Toe KH, Jones CM, N’Fale S, Ismail HM, Dabire RK, Ranson H. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis. 2014;20:1691–6.
Acknowledgements
This work was financially supported by the GEIS (Global Emerging Infections Surveillance) fund and Vestergaard. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Work was funded by Congressional Special Interest, work unit # E1431.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
JC was an MPhil candidate at Kwame Nkrumah University of Science and Technology and is employed by Noguchi Memorial Institute for Medical Research through funding from Vestergaard. HPJ is employed by Vestergaard.
Authors’ contributions
JC and JWD designed, implemented and coordinated the study. JC analyzed, interpreted data and drafted the manuscript. JWD, PKB, HPJ, SKD and MDW revised the manuscript. AKD, DO, AA and AI carried out all the laboratory experiments and field specimen collections. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chabi, J., Baidoo, P.K., Datsomor, A.K. et al. Insecticide susceptibility of natural populations of Anopheles coluzzii and Anopheles gambiae (sensu stricto) from Okyereko irrigation site, Ghana, West Africa. Parasites Vectors 9, 182 (2016). https://doi.org/10.1186/s13071-016-1462-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1462-0