Abstract
Background
Cystic echinococcosis (hydatid disease), caused by the tapeworm Echinococcus granulosus (class Cestoda; family Taeniidae), is a neglected tropical disease that results in morbidity and mortality in millions of humans, as well as in huge economic losses in the livestock industry globally. Proteins from the tetraspanin family in parasites have recently become regarded as crucial molecules in interaction with hosts in parasitism and are therefore suitable for the development of vaccines and diagnostic agents. However, no information is available to date on E. granulosus tetraspanin.
Methods
In this study, a uroplakin-I-like tetraspanin (Eg-TSP1) of E. granulosus was cloned and expressed in E. coli. The immunolocalization of Eg-TSP1 in different life stages of E. granulosus was determined using specific polyclonal antibody. The antibody and cytokine profiles of mice that immunized with recombinant Eg-TSP1 (rEg-TSP1) were measured for the immunogenicity analysis of this protein. Additionally, we use RNA interference method to explore the biological function of Eg-TSP1 in larva of E. granulosus.
Results
Immunofluorescence analysis showed that endogenous Eg-TSP1 mainly localized in the tegument of larvae and adults. Significantly elevated levels of antibodies IgG1 and IgG2a and of cytokines IFN-γ and IL-12 were observed in the sera of mice after immunization with rEg-TSP1, suggesting a typical T helper (Th)1-mediated immune response elicited by rEg-TSP1. On further probing the role of Eg-TSP1 in E. granulosus by RNA interference, we found that a thinner tegmental distal cytoplasm was induced in protoscoleces treated with siRNA-132 compared to controls.
Conclusions
This is the first report characterizing a tetraspanin from the tapeworm E. granulosus. Our results suggest that Eg-TSP1 is associated with biogenesis of the tegument and maintenance of structural integrity of E. granulosus and could therefore be a candidate intervention target for control of hydatid disease.
Similar content being viewed by others
Background
Tetraspanins (TSPs) are a superfamily of plasma membrane associated proteins, also known as the trans-membrane-4-superfamily (TM4SF), which can be classified into four major subfamilies, including: the CD family (CD9, CD81, CD 151, etc.); the CD63 family (CD63, TSPAN31, etc.); the uroplakin family (UPK 1A/1B); and the retinal degeneration slow (RDS) family (RDS-ROM) [1]. These proteins usually consist of four conserved transmembrane domains, cytoplasmic tails at the N- and C-termini, a small extracellular loop, and a large extracellular loop (LEL) containing a Cys-Cys-Gly motif plus 2–6 additional cysteines [2]. These LELs have been proven to play a central role in interactions with several molecules, including the LELs of other tetraspanin, which is known as the “tetraspanin web” [3]. Tetraspanins participate in a broad spectrum of cellular activities, such as cell differentiation, adhesion, motility, aggregation, cell signaling and sperm-egg fusion [4–6], and are involved in many pathological processes, including cancer metastasis and infections caused by pathogenic organisms [7–10].
In spite of the importance of tetraspanins, only a small number have been studied to date [11]. In parasites, recent studies show that tetraspanins are associated with the development, maturation and stability of the tegument of trematodes, and are involved in the immune evasion of schistosome [12–16]. Of note, some members of the tetraspanin family have been targeted as candidate vaccines against schistosomiasis [17–20], clonorchiasis [21], alveolar echinococcosis [22–25], and filariasis [26]. For example, inoculation with the LELs of two tetraspanins of S. mansoni (Sm-TSP-1 and Sm-TSP-2) was confirmed to significantly decrease the adult worm burdens and liver egg burdens in experimentally infected mice [20]. Moreover, a Taenia solium tetraspanin (T24) was found to be a potential diagnostic candidate, which exhibited excellent sensitivity and specificity in detecting cases of cysticercosis with two or more viable cysts [27].
Echinococcus granulosus is a causative pathogen of human and domestic animal hydatid disease, infects ~3 million people globally and causes annual losses of US$2 billion in livestock [28, 29]. E. granulosus has recently been reported to have more tetraspanin genes (30) than Caenorhabditis elegans (20) and other parasitic nematodes (≤20) [1, 30]. However, there are no further reports about the potential roles of tetraspanins in E. granulosus to date.
In this study, we characterized a tetraspanin, Eg-TSP1, from E. granulosus and investigated its locations in different life cycle stages. The immune mechanism in mice immunized with the recombinant LEL region of Eg-TSP1 (rEg-TSP1) was also evaluated by measuring the levels of IgG and IgG subclasses (IgG1 and IgG2a), and of cytokines interleukin (IL)-4, IL-10, IL-12 and interferon (IFN-γ). In addition, to further explore the potential role of Eg-TSP1 in E. granulosus, RNA interference (RNAi) was employed to suppress the endogenous gene expression in its larvae (protoscoleces, PSCs) in vitro. To our knowledge, this is the first report that focuses on the functional genomics of E. granulosus by means of RNAi, and the results will provide the foundation for future research into prevention and control of hydatid disease.
Methods
Ethics statement
This study was reviewed and approved by the National Institute of Animal Health Animal Care and Use Committee at Sichuan Agricultural University (Ya’an, China; approval no. 2013–028). All animal experiments were conducted in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (approved by the State Council of the People’s Republic of China).
Animals
Six- to eight-week-old female specific pathogen free (SPF) ICR mice were purchased from the Laboratory Animal Center of Sichuan University (Chengdu, China). A 15-week-old male New Zealand white rabbit and a 5-month-old dog of mixed breed were obtained from the Laboratory Animal Center of Sichuan Agricultural University.
Parasites
Cysts of E. granulosus were collected from an infected sheep at a local slaughterhouse in Xining, Qinghai Province, China. PSCs and cyst wall (including laminated layer and germinal layer) were separated under sterile conditions and washed in phosphate-buffered saline (PBS). Fresh PSCs were diluted to a concentration of 2,000 mL−1 and then cultured in complete RPMI 1640 medium (Hyclone) containing 10 % fetal calf serum (Hyclone), 100 U.mL−1 penicillin G and 100 μg.mL−1 streptomycin (Sigma) at 37 °C in an atmosphere containing 5 % CO2. Adult worms were collected from a dog 35 days post-infection with 20,000 PSCs.
Bioinformatic analysis
Genome-wide tetraspanins of E. granulosus were download from GeneDB (http://www.genedb.org/) according to the summary by Tsai et al. [30], and were aligned and employed for phylogenetic analysis by MEGA software (version 5.05). Full length E. granulosus TSP1 nucleotide sequence [GenBank: FJ384717] was downloaded from GeneDB [31] based on sequence homology with E. multilocularis TSP1 [GenBank: FJ384717]. Open Reading Frame Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and MEGA software were used to analyze the open reading frame (ORF) and deduce the amino acid sequence. To find other tetraspanins, the amino acid sequence of Eg-TSP1 was used for BLAST against the National Center for Biotechnology Information (NCBI) database. Multiple sequence alignment was performed using the DNAStar program (Madison WI, USA), and the aligned full-length amino acid sequences were employed to build a phylogenetic tree using Mrbayes version 3.1.2 [32]. The hypervariable region of the tetraspanins was compared using the Clustal W2 online service. The molecular weight and transmembrane regions were predicted using ProtParam (http://web.expasy.org/protparam/) and DAS Transmembrane Prediction server (http://www.sbc.su.se/~miklos/DAS/), respectively.
Cloning, expression and purification of the LEL region of Eg-TSP1
The total RNA of PSCs was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and then reverse transcribed into cDNA using the ThermoScript™ RT-PCR System for First-strand cDNA Synthesis Kit (Invitrogen). The nucleotide sequence encoding the LEL domain of Eg-TSP1 was amplified from cDNA using a sense primer (5′-CGCGGATCCCCTGATAACCTAAACAAAGC-3′) containing a BamHI site (underlined) and an antisense primer (5′-CCCAAGCTTGAGGGTTTTGTTCTCTGCCAA-3′) containing a HindIII site (underlined). The PCR products were ligated into the pET32a(+) plasmid (Novagen, Madison, WI, USA). The recombinant plasmid with correct sequence was transformed into Escherichia coli BL21 (DE3) cells (Invitrogen) and subsequently grown at 37 °C in Luria-Bertani broth containing 50 μg.mL−1 ampicillin. When the OD600nm value of bacteria reached 0.6, 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) was added for 5 h induction at 37 °C. The recombinant protein was harvested from E. coli lysates, followed by purification with a Ni2+ affinity column (Bio-Rad, USA) under denaturing conditions in 8 M urea. The purified protein was refolded by dialysis against PBS and concentrated using an Amicon Ultra-15 10,000 MWCO centrifugal filters (Millipore, USA), and then analyzed by SDS-PAGE.
Sera
Mouse anti-PSC sera was produced as described previously [33] and used for immunoblot analysis. Briefly, each mouse was inoculated intraperitoneally with 200 μL of a suspension containing 2,000 viable protoscoleces, and then serum was acquired 9 month later. For immunolocalization, a rabbit was immunized with a subcutaneous injection of 50 μg.mL−1 of rEg-TSP1 purified as described above mixed with Freund’s complete adjuvant (FCA; Sigma, St. Louis, MO, USA), followed by two booster immunizations (2 weeks apart) using the same route and dose in Freund’s incomplete adjuvant (FIA; Sigma). The rabbit was bled 2 weeks after the second booster immunization. The polyclonal antisera were collected, purified by Protein A affinity chromatography (Bio-Rad, USA), and stored at −80 °C until use.
Immunoblotting and immunolocalization
For immunoblotting, rEg-TSP1 was run on 12 % SDS-PAGE and then transferred onto a nitrocellulose membrane. After blocking, the membrane was incubated with mouse anti-PSC sera (1:200), followed by goat anti-mouse IgG (H+L) HRP conjugate (1:2,000) (Bio-Rad). Nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP; USA, Cleveland, OH) were used as substrates to develop the color reaction. The serum of non-infected mice was used as a control. The specificity and sensitivity of rabbit anti-rEg-TSP1 IgG were determined by western blotting against crude extracts from PSCs and rEg-TSP1 as described above.
To determine the location of Eg-TSP1, fresh PSCs, cyst wall (including the laminated layer and germinal layer), and adult worms were fixed in 4 % paraformaldehyde-phosphate buffer for 36 h and then embedded in paraffin after dehydration. Sections were deparaffinated and rehydrated, and subsequently treated with 0.01 M citrate buffer (pH 6.0) in a microwave at 700 W for 10 min for antigen exposure, followed by blocking with 3 % H2O2. Then these sections were washed and incubated with purified IgG fractions (diluted 1:200 in PBS) at 4 °C overnight. The sections were washed and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (H+L) (Bethyl Laboratories) at 37 °C for 1 h in darkness. After four washes with PBS, sections were examined under a fluorescence microscope (Nikon, Japan). Antibody from pre-immunized rabbit was used as negative control.
Immunization experiment
In the immunized group, eight mice were subcutaneously injected with rEg-TSP1 (25 μg per animal) mixed with FCA (Sigma), followed by three boosters separately performed on days 14, 28 and 42 using the same route and dose in FIA (Sigma). In the control group, eight mice were treated in the same way as immunized group with the replacement of rEg-TSP1 into PBS. Sera were collected for antibody analysis at days 0 (pre-immunization), 14, 28, 42 and 56. Two weeks after the final immunization, all mice were sacrificed and the spleens were removed aseptically for cytokine assays.
Antibody assays
The levels of antibodies including IgG, IgM, IgE, and IgG subclasses against rEg-TSP1 were measured by ELISA. In brief, 96-well plates were coated with rEg-TSP1 (2 μg.mL−1) using 100 μL reaction volume at 4 °C for 14–16 h. After three washes, the plates were blocked with PBS containing 5 % skimmed milk for 1 h at 37 °C. The mouse antisera (1: 200) was subsequently added and incubated for 1 h at 37 °C, followed by HRP-conjugated goat anti-mouse IgG (1:500), IgM (1:5,000), IgE (1:5,000), or IgG subclass (IgG1, IgG2a; 1:5,000) antibodies (Bethyl Laboratories). Antibody binding was detected at 37 °C with 100 μL of 3,3′,5,5′-tetramethylbenzidine (TMB; Tiangen, China) and the reaction was stopped by adding 100 μL of 2 M H2SO4. ELISA OD values were measured at 450 nm using a microplate reader (Thermo Scientific, USA). All samples were run in triplicate.
Cytokine assays
The cytokine profiles (IL-4, IL-10, IL-12 and IFN-γ) of immunized mice were measured using quantitative PCR (qPCR). The amplification primer pairs are shown in Additional file 1. For amplification, total RNA was isolated from mouse spleens, and first-strand cDNA was synthesized as described above. All real-time PCR amplifications were carried out in a MiniOpticon thermal cycler (Bio-Rad) in a total volume of 12.5 μL, containing 1 μL of cDNA, 6.25 μL of SYBR® Premix Ex Taq™ II (Takara, China), 0.5 μM of each primer, and nuclease free water. The amplification steps included initial denaturation at 95 °C for 30s, followed by 40 cycles of amplification, each including denaturation at 95 °C for 30 s, annealing at 55.0–59.7 °C for 30 s, and extension at 72 °C for 30 s. The reactions were performed in triplicate, with negative controls. Dissociation curves were analyzed for each sample to confirm the specific amplification. The relative gene expression levels were calculated using the comparative 2-ΔΔCt method [34] and normalized with the hypoxanthine phosphoribosyl transferase (HPRT) gene.
RNAi and qPCR
To further investigate the role of Eg-TSP1 protein in the larval stage of E. granulosus, we knocked down the expression level using small interfering RNA (siRNA). Three specific siRNAs were designed to target different CDS regions of Eg-TSP1 (Additional file 2) and were chemically synthesized by GenePharma Co., Ltd. (Shanghai, China); and an irrelevant siRNA, that was non-specific to any sequence of E. granulosus, was also synthesized as negative control. For delivery of siRNAs, PSCs were treated with 10 μL of transfection reagent (Engreen Biosystem, China) plus siRNA at a final concentration of 200 μM in 2.5 mL culture medium at days 0 and 2. Medium was changed daily.
To confirm the knockdown effect of RNAi in PSCs, the transcription level of Eg-TSP1 was determined by qPCR. Approximately 2,000 PSCs were collected for total RNA isolation and cDNA synthesis 24 h after the final siRNA delivery. The qPCR primer pairs of Eg-TSP1 and elongation factor 1 alpha (EF1α, an optimized housekeeping gene in Echinococcus spp. [35]) were given in Additional file 1. All amplifications were performed as described above, except for the annealing temperatures: 53.2 °C for Eg-TSP1 and 57.8 °C for EF1α. qPCR was conducted in triplicate and Eg-TSP1 transcript levels were calculated by 2-ΔΔCt analysis.
TEM analysis
For transmission electron microscope (TEM) analysis, PSCs were treated with Eg-TSP1-specific and irrelevant siRNAs for 4 days at 37 °C in an atmosphere containing 5 % CO2. Then, PSCs were fixed with 3 % glutaraldehyde in 0.1 M PBS for 48 h at 4 °C, followed by post-fixing using 2 % OsO4. Fixed PSCs were dehydrated in graded acetones (50–100 %) and embedded in Epon Resin (ProSciTech, Australia). Sections (60 nm) were mounted onto copper grids, stained with uranyl acetate and lead citrate, and observed using a TEM (Rigaku, Japan) operated at 75 kV. This experiment was performed in triplicate.
Statistical analysis
All data are presented as mean ± SD. Statistical analyses were performed with Student’s t-test for comparison between groups using the software package GraphPad Prism (www.graphpad.com).
Results
General characteristics of Eg-TSP1
The full-length 792 bp CDS of Eg-TSP1 encoded 263 residues including the four typical transmembrane regions (9–32 aa; 70–92 aa; 100–123 aa; and 236–255 aa), the small extracellular loop (33–69 aa), and the crucial LEL located in amino acids 124–235. Alignment of the hypervariable regions in the LEL of parasite and human tetraspanins showed different cysteine distributions (Fig. 1a). Most of the sequences contained four or six cysteine residues, while only Sj-TSP1 and Sj-TSP6 had ≥8 cysteine residues. Eg-TSP1 had a six-cysteine motif: CCG…DF…PXXCC…C…GC. Notably, the residues CCG, the first C of PXXCC, and GC in this motif were highly conserved.
Phylogenetic analysis and the cysteine pattern in Eg-TSP1. a The hypervariable region of tetraspanins was aligned and they were grouped according to their cysteine patterns. The conserved cysteine-motif is summarized and the canonical topologies of disulfide bonds are shown above the alignment. b A phylogenetic tree was constructed based on the amino acid sequences of parasite and human tetraspanins using the Bayesian method. Three major monophyletic subfamilies were detected, including the CD family, the CD63 family, and the Uroplakin family. Eg, E. granulosus; Em, E. multilocularis; Sm, S. mansoni; Sj, S. japonicum; Sh, S. haematobium; Ts, T. solium; Hs, Homo species. GenBank accession numbers: Em-TSP 1–7 [FJ384717, FJ384718, FJ384719, FJ384720, FJ384721, FJ384722 and FJ384716, respectively]; Sj-TSP 1–6 [AAW26928, AAW24822, AAW24863, AAP05954, AAW27174 and AAW26326, respectively]; Sm-TSP-1 [AF521093]; Sm-TSP-2 [AF521091]; Sm23 [M34453]; Sj23 [M63706]; Sh23 [U23771]; Sm25 [AF028730]; Sj25 [U77941]; Ts-T24 [AY211879] and Human-TSP1 [NP_005718.2]
On searching against the conserved domain database of NCBI using the amino acid sequence of Eg-TSP1, we found that there was high homology between Eg-TSP1 and uroplakin-I-like family (Accession: cd03156; E-value: 2.64e-06). Additionally, our Bayesian analysis (Fig. 1b) showed three distinct branches, including members of the CD family, uroplakins, and the CD63 family. E. granulosus Eg-TSP1 belonged to the same clade as Sj-TSP2/4/5, Em-TSP1 and Sm25. Further, all tetraspanins of E. granulosus could also be classified into three parts, and Eg-TSP1 (GeneDB ID: EgrG_000355800.1) together with a single paralogue (GeneDB ID: EgrG_000355900.1) (equal to LophDB Cluster ID: EGC00290, http://www.nematodes.org/NeglectedGenomes/Lopho/; and showed absence of ESTs in transcriptome data of PSCs [36]) consist of a independent branch belonged to uroplakin family (see Additional file 3).
Expression, purification and recognition of rEg-TSP1
A 330 bp fragment encoding the LEL of Eg-TSP1 was amplified from PSCs and this fragment was expressed in E. coli as a His6-tagged fusion protein with an expected size of ~32 kDa (Fig. 2). Minus a ~20 kDa epitope tag fusion peptide, rEg-TSP1 had an approximate molecular mass of 12 kDa, which was similar to the MW of the LEL predicted in silico. Immunoblotting using sera from mice experimentally infected with E. granulosus showed a single ~32 kDa band (Fig. 2), indicating that rEg-TSP1 can be recognized by the mouse specific IgG against E. granulosus.
Immune recognition of recombinant and native Eg-TSP1 by Western blot. Lane 1, SDS-PAGE of purified rEg-TSP1 after dialysis and concentration; Lane 2 and lane 3, immune recognition of rEg-TSP1 using sera from E. granulosus infected mice (Lane 2) and healthy control (Lane 3); Lane 4 and lane 5, western blot analysis using recombinant Eg-TSP1 (Lane 4) and crude extracts of protoscolexes (Lane 5) against the purified rabbit anti-rEg-TSP1 IgG. A single band was detected in each lane with molecular weight of ~32 kDa for recombinant Eg-TSP1 (partial sequence plus ~20 kDa fusion peptide) and ~29 kDa for native Eg-TSP1, respectively
Localization of Eg-TSP1 in different life cycle stages of E. granulosus
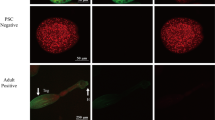
The localizations of native Eg-TSP1 in PSCs (Fig. 3a, b), cyst wall germinal layer (Fig. 3c, d), and adult worms (Fig. 3e, f) were determined by immunofluorescence using specific polyclonal antibodies against rEg-TSP1 (Fig. 2). The Eg-TSP1 was located only in the tegument and invaginated sucker of PSCs. No signals were detected in the germinal layer of cyst walls, or in the non-cell structural laminated layer (data not shown).
Immunolocalization of Eg-TSP1 in the larval and adult stages of E. granulosus. Eg-TSP1 in the protoscolexes (a, b), germinal layer (c, d) and adult (e, f) was immunofluorescently labeled using specific anti-rEg-TSP1 IgG (a, c and e), or control pre-immune serum (b, d and f), followed by FITC-conjugated anti-rabbit IgG. Fluorescence-labeled regions are marked with arrows. Teg: tegument; H: hooks
Antibody profiles
To evaluate the levels of specific IgG, IgM, IgE and IgG subclass (including IgG1 and IgG2a) antibodies to rEg-TSP1, sera from eight immunized mice were measured at 0, 14, 28, 42 and 56 days after first vaccination. Compared with the PBS control group, significant IgG responses were observed after the first immunization in the group immunized with rEg-TSP1, and the peak was detected 56 days post-vaccination (Fig. 4a). We did not find any significant differences in rEg-TSP1-specific IgM and IgE levels at any time points we studied (data not shown). To evaluate the IgG isotype profiles induced by rEg-TSP1, specific IgG1 and IgG2a antibody levels were measured. Both IgG1 and IgG2a levels were significantly increased at 14, 28, 42 and 56 days post-immunization compared to the controls. The IgG2a level was lower than the IgG1 level at all time points but, interestingly, the IgG1/IgG2a ratio declined at days 28, 42, and 56 due to a fast increase in IgG2a (Fig. 4b), suggesting a Th1-type response tendency.
Serum antibody profiles induced by rEg-TSP1 in mice. Eight mice per group were subcutaneously vaccinated four times with rEg-TSP1 or PBS mixed with FCA/FIA at 14 day intervals and serum samples were collected via the tail vein for ELISA tests after each vaccination. a Serum IgG levels of mice vaccinated with rEg-TSP1. Vaccination times are marked with arrows. b Serum IgG subclass levels of mice vaccinated with rEg-TSP1. The ratio of IgG1/IgG2a were given above the bars. Asterisks indicate statistically significant differences between the rEg-TSP1 and control groups (P < 0.0001). NS represent not significant. Error bars represent SD
Cytokine response induced by rEg-TSP1
Mouse spleens were used for the detection of cytokine expression profiles 56 days after vaccination with rEg-TSP1 or PBS. From the qPCR data (Fig. 5), we clearly found that the levels of IFN-γ and IL-12 (cytokines of Th1 cell response) were highly elevated, while IL-4 (a typical cytokine of Th2 immune response) showed no difference compared to the control group (P > 0.05). These observations suggested a Th1 immune response induced by rEg-TSP1 vaccination. However, a high level of IL-10 (a Th2 cytokine) was also detected (Fig. 5).
Relative expression levels of cytokines determined in mice post-immunization with rEg-TSP1 or PBS. Spleens were isolated at day 56 from mice subcutaneously immunized with rEg-TSP1 or PBS. Total RNAs were extracted and then cDNAs were synthesized for qRT-PCR analysis. *P < 0.001. Boxes represent the interquartile range, i.e. the middle 50 %, of observations. The dotted line represents the median gene expression. Whiskers represent the minimum and maximum observations
SiRNA mediated knockdown of Eg-TSP1 transcript
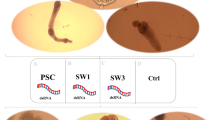
SiRNAs (siRNA-132, −480 and −540) were introduced into PSCs to mediate gene knockdown using two transfections, and caused 64.30 % (P = 0.0001), 46.03 % (P = 0.0028) and 44.93 % (P < 0.0001) decreases in mRNA transcripts compared to the negative control group, respectively (Fig. 6). The results showed that siRNA-132 was the most efficient siRNA and that the effect of inhibition by siRNA was sequence-dependent.
Suppression of Eg-TSP1 transcripts in E. granulosus protoscoleces using different siRNAs. siRNAs were transferred twice to E. granulosus protoscoleces by soaking. An irrelevant siRNA was used as the control and three Eg-TSP1-specific siRNAs (siRNA-132, −480 and −540, respectively) were the experimental samples. Eg-TSP1 transcript levels were measured by qRT-PCR and normalized using Eg-EF1α transcripts. Data are presented as the mean ± SD of triplicate experiments. Statistically significant differences between the control group and the Eg-TSP1-specific siRNA groups were determined by Student’s t-test (* P < 0.01; ** P < 0.0001)
Structural changes in parasites caused by RNAi
To investigate the effect of inhibition of Eg-TSP1 expression in E. granulosus in vitro, PSCs that were treated with the most efficient siRNA (siRNA-132) or irrelevant siRNA were observed by TEM. The tegument structure of PSCs that were exposed to Eg-TSP1-specific siRNA was significantly different from that of the control group soaked in the irrelevant siRNA (Fig. 7). The tegumental distal cytoplasm of PSCs treated with siRNA-132 was much thinner than that of the controls, with less vesicles inside.
Ultrastructure of the tegument of E. granulosus protoscoleces treated with Eg-TSP1 siRNA. g: glycocalyx; v: vesicle; dc: distal cytoplasm; C: parenchyma cell. a The distal cytoplasm in the somal regions of protoscoleces treated with siRNA-132 is observed to be thinner than that in (b) protoscoleces treated with irrelevant siRNA. Analysis was by transmission electron microscopy (magnifications 10000×)
Discussion
Tetraspanins are transmembrane proteins widely distributed in eukaryotic organisms [37], involved in many cell biological processes [38, 39], and that interact with immune molecules from hosts [40], especially specific molecules such as the major histocompatibility complex (MHC) II [41, 42]. These interactions tend to downregulate host immune responses so that parasites can mask their nonself status to escape host detection and successfully establish infection in the host. Thus, targeting tetraspanins via various methods such as monoclonal antibodies, soluble large-loop proteins or RNAi technology, should be therapeutically valuable [43]. Recent studies on parasite tetraspanins as vaccine candidates have made great progress and given this protein family an important status. However, there were no experimental data about tetraspanins in the zoonotic platyhelminth pathogen, E. granulosus. In this study, we first characterized a uroplakin-I-like tetraspanin on the surface of E. granulosus and found that it plays a crucial role in the tegument formation of this parasite.
In this study, the amino acid sequence of Eg-TSP1 showed the classical “CCG” pattern of tetraspanins and shared high similarity with uroplakin-I-like Sj-TSP2/4/5 [44], suggesting that Eg-TSP1 is another uroplakin-I-like tetraspanin in platyhelminths and enriches the spectrum of this protein family. Uroplakins Ia and Ib (special tetraspanins) specifically bind with their associated (non-tetraspanin) uroplakins II and IIIa, and together form two dimensional crystals of 16 nm particles, known as urothelial plaques, which cover almost the entire apical surface of the mammalian bladder urothelium, modulating the urothelial permeability barrier [45, 46], and are involved in urinary tract infection [47]. However, E. granulosus is a tapeworm without a bladder and urinary tract; thus, we can speculate that mammals and cestodes belong to distinct animal lineages and their tetraspanins diverged with the divergence of the groups, coming to be expressed in different tissues and played different roles [46]. Hence, further exploration of the structure and function of Eg-TSP1 was required.
We confirmed the localization of Eg-TSP1 in the larval and adult stages of E. granulosus by immunofluorescence, and the results showed that Eg-TSP1 was mostly present in the tegument. As mentioned previously, E. granulosus is a cestode devoid of a gut, and the tegument with microvilli or microtriches constitutes the principal site of absorption of nutrients and elimination of waste materials, similar to the gut of animals turned inside out [48]. Thus, many proteins located on the tegument of E. granulosus should be contributed to parasite nutrition. Given the primary function of uroplakin in mammals is to regulate cell permeability, we can speculate that Eg-TSP1 may have a role in managing flow of materials in and out of the parasite. However, in this study, we have not excluded the possibility that Eg-TSP1 is expressed in the ducts of the excretory system of the parasites, so further detailed analysis should be done. We did not detect the expression of Eg-TSP1 on the germinal layer of cyst walls, in contrast to its homologue TSP1 in E. multilocularis [23]. However, this result was supported by transcriptome data for E. granulosus [36], where reads of Eg-TSP1 (LophDB Cluster ID: EGC00446; http://www.nematodes.org/NeglectedGenomes/Lopho/) in the cyst wall were also not detectable. Considering that rEg-TSP1 could be recognized by the mouse-anti-PSCs serum, it seems like that Eg-TSP1 interact with host immune system in the context of secondary infection, when E. granulosus cysts were broken.
Dang et al. [23] evaluated protective effects of seven E. multilocularis tetraspanins on the murine-alveolar echinococcosis model; the recombinant LEL of Em-TSP1 induced the highest cyst lesion reduction rate (87.9 %). However, a lower cyst lesion reduction rate was detected when using another immunization strategy [24]. Here, we measured the levels of antibodies in mice immunized with rEg-TSP1 and found an increasing rEg-TSP1-specific IgG level after the second immunization. Moreover, analysis of the IgG1/IgG2a ratio showed a tendency towards a protective Th1 type of immune response induced by rEg-TSP1. This was confirmed by the significantly upregulated levels of splenic IL-12 and IFN-γ and an unchanged level of IL-4 in the cytokine profile. Of note, IL-12 is a key cytokine in the development of Th1 immunity and can facilitate Th1 response by enhancing the secretion of IFN-γ by T cells and NK cells, but plays an antagonist role in the production of IL-4. Furthermore, IFN-γ is important in protective immune response against parasite infections since it enhances the secretion of IgG2a from B lymphocytes [49], thus resulting in a decrease of the IgG1/IgG2a ratio and triggering the Th1 response. In the case of cystic echinococcosis, IFN-γ was involved in activation of macrophages for production of nitric oxide synthase that can induce host defense against E. granulosus [50]. In addition, a high level of IL-10 is a hallmark of chronic E. granulosus infection [51]. Here, we detected high-level IL-10 production in mice after immunization with rEg-TSP1, so we hypothesize that this cytokine might be involved in the regulation of Th2 response and the prevention of a highly polarized Th1 response in which hosts might tolerate the parasite infection [52, 53]. Further confirmation of the protective effect of rEg-TSP1 may require a challenge experiment in the mouse model.
RNAi has been widely used to knockdown genes in nematodes and trematodes (reviewed by Maule et al. [54] and Geldhof et al. [55], respectively), but there are few reports of RNAi in cestodes except Moniezia expansa [56] and E. multilocularis [57, 58]. Mizukami et al. [57] successfully knocked down E. multilocularis PSC 14-3-3 and elp gene expressions to 21.8 % and 35.5 %, respectively, and demonstrated that the gene knockdown happened only on siRNA delivery by electroporation and not by soaking in siRNA with transfection reagent. However, in this study, PSCs of E. granulosus soaked with Eg-TSP1-specific siRNA in transfection reagent showed a statistically significant decrease in Eg-TSP1 gene expression that led to malformation of the worm tegument, compared to samples treated with an irrelevant siRNA. These results suggested a role for Eg-TSP1 in biogenesis of the tegumental distal cytoplasm and maintenance of structural integrity. Importantly, they also demonstrate that soaking is a viable approach, with fewer cell lesions than electroporation, when transferring siRNA to PSCs of E. granulosus. The use of RNAi in functional genomic analysis of E. granulosus will facilitate the decoding of functions of many important genes and contribute to prevention and control of human and domestic animal hydatid disease [59].
Conclusion
In conclusion, more and more attention is being paid to the proteins of the tetraspanin superfamily because of their wide distribution and crucial biological roles in various organisms. In this study, a uroplakin like Eg-TSP1 in E. granulosus was characterized and exhibited induction of a notable Th1 type immune response in a mouse model. Endogenous Eg-TSP1 was mainly expressed on the tegument of E. granulosus and was involved in maintenance of tegument structural integrity. These results suggest that Eg-TSP1 could be used as an intervention target for therapy, prevention and control of hydatid disease.
References
Garcia-España A, Chung PJ, Sarkar IN, Stiner E, Sun TT, DeSalle R. Appearance of new tetraspanin genes during vertebrate evolution. Genomics. 2008;91:326–34.
Seigneuret M, Conjeaud H, Zhang H, Kong X. Structural bases for tetraspanin functions. Tetraspanins. Springer; 2013. pp. 1–29.
Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–48.
Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci. 2003;28:106–12.
Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422.
Higginbottom A, Takahashi Y, Bolling L, Coonrod SA, White JM, Partridge LJ, et al. Structural requirements for the inhibitory action of the CD9 large extracellular domain in sperm/oocyte binding and fusion. Biochem Biophys Res Commun. 2003;311:208–14.
Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2008;9:40–55.
Gordón-Alonso M, Yañez-Mó M, Barreiro O, Álvarez S, Muñoz-Fernández MÁ, Valenzuela-Fernández A, et al. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol. 2006;177:5129–37.
Yalaoui S, Zougbédé S, Charrin S, Silvie O, Arduise C, Farhati K, et al. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathog. 2008;4:e1000010.
Silvie O, Charrin S, Billard M, Franetich JF, Clark KL, van Gemert GJ, et al. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci. 2006;119:1992–2002.
Moribe H, Mekada E. Tetraspanins in lower eukaryotes. Tetraspanins. Springer; 2013. pp. 187–201.
Tran MH, Freitas TC, Cooper L, Gaze S, Gatton ML, Jones MK, et al. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog. 2010;6:e1000840.
Piratae S, Tesana S, Jones MK, Brindley PJ, Loukas A, Lovas E, et al. Molecular characterization of a tetraspanin from the human liver fluke, Opisthorchis viverrini. PLoS Negl Trop Dis. 2012;6:e1939.
Walker AJ. Insights into the functional biology of schistosomes. Parasit Vectors. 2011;4:203.
Cai P, Bu L, Wang J, Wang Z, Zhong X, Wang H. Molecular characterization of Schistosoma japonicum tegument protein tetraspanin-2: sequence variation and possible implications for immune evasion. Biochem Biophys Res Commun. 2008;372:197–202.
McWilliam HE, Driguez P, Piedrafita D, McManus DP, Meeusen EN. Discovery of novel Schistosoma japonicum antigens using a targeted protein microarray approach. Parasit Vectors. 2014;7:290.
Zhang W, Li J, Duke M, Jones MK, Kuang L, Zhang J, et al. Inconsistent protective efficacy and marked polymorphism limits the value of Schistosoma japonicum tetraspanin-2 as a vaccine target. PLoS Negl Trop Dis. 2011;5:e1166.
Cardoso FC, Macedo GC, Gava E, Kitten GT, Mati VL, de Melo AL, et al. Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. PLoS Negl Trop Dis. 2008;2:e308.
Jiang N, Cai P, Yin J, Hao L, Lu H, Wang X, et al. Characterization of antibody responses to the Sj23 antigen of Schistosoma japonicum after infection and immunization. Acta Trop. 2010;116:9–14.
Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–40.
Kim TY, Chung EJ, Sohn WM, Hong SH, Yong TS. Molecular characterization of Clonorchis sinensis tetraspanin 2 extracellular loop 2. Parasitol Res. 2012;110:707–11.
Dang Z, Watanabe J, Kajino K, Oku Y, Matsumoto J, Yagi K, et al. Molecular cloning and characterization of a T24-like protein in Echinococcus multilocularis. Mol Biochem Parasitol. 2009;168:117–9.
Dang Z, Yagi K, Oku Y, Kouguchi H, Kajino K, Watanabe J, et al. Evaluation of Echinococcus multilocularis tetraspanins as vaccine candidates against primary alveolar echinococcosis. Vaccine. 2009;27:7339–45.
Dang Z, Yagi K, Oku Y, Kouguchi H, Kajino K, Matsumoto J, et al. A pilot study on developing mucosal vaccine against alveolar echinococcosis (AE) using recombinant tetraspanin 3: vaccine efficacy and immunology. PLoS Negl Trop Dis. 2012;6:e1570.
Dang Z, Feng J, Yagi K, Sugimoto C, Li W, Oku Y. Mucosal adjuvanticity of Fibronectin-Binding Peptide (FBP) fused with Echinococcus multilocularis tetraspanin 3: systemic and local antibody responses. PLoS Negl Trop Dis. 2012;6:e1842.
Dakshinamoorthy G, Munirathinam G, Stoicescu K, Reddy MV, Kalyanasundaram R. Large extracellular loop of tetraspanin as a potential vaccine candidate for filariasis. PLoS ONE. 2013;8:e77394.
Hancock K, Pattabhi S, Whitfield FW, Yushak ML, Lane WS, Garcia HH, et al. Characterization and cloning of T24, a Taenia solium antigen diagnostic for cysticercosis. Mol Biochem Parasitol. 2006;147:109–17.
Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7:385–94.
Torgerson PR, Macpherson CN. The socioeconomic burden of parasitic zoonoses: global trends. Vet Parasitol. 2011;182:79–95.
Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sanchez-Flores A, Brooks KL, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63.
Zheng H, Zhang W, Zhang L, Zhang Z, Li J, Lu G, et al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat Genet. 2013;45:1168–75.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42.
Dematteis S, Pirotto F, Marques J, Nieto A, Örn A, Baz A. Modulation of the cellular immune response by a carbohydrate rich fraction from Echinococcus granulosus protoscoleces in infected or immunized Balb/c mice. Parasite Immunol. 2001;23:1–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–8.
Espínola SM, Ferreira HB, Zaha A. Validation of suitable reference genes for expression normalization in Echinococcus spp. larval stages. PLoS ONE. 2014;9:e102228.
Parkinson J, Wasmuth JD, Salinas G, Bizarro CV, Sanford C, Berriman M, et al. A transcriptomic analysis of Echinococcus granulosus larval stages: implications for parasite biology and host adaptation. PLoS Negl Trop Dis. 2012;6:e1897.
Huang S, Yuan S, Dong M, Su J, Yu C, Shen Y, et al. The phylogenetic analysis of tetraspanins projects the evolution of cell–cell interactions from unicellular to multicellular organisms. Genomics. 2005;86:674–84.
Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11.
Tarrant JM, Robb L, van Spriel AB, Wright MD. Tetraspanins: molecular organisers of the leukocyte surface. Trends Immunol. 2003;24:610–7.
Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–63.
Szöllósi J, Horejsí V, Bene L, Angelisová P, Damjanovich S. Supramolecular complexes of MHC class I, MHC class II, CD20, and tetraspan molecules (CD53, CD81, and CD82) at the surface of a B cell line JY. J Immunol. 1996;157:2939–46.
Kropshofer H, Spindeldreher S, Röhn T, Platania N, Grygar C, Daniel N, et al. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat Immunol. 2001;3:61–8.
Hemler ME. Targeting of tetraspanin proteins - potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–58.
Jiang Y, Xu X, Qing X, Pan W. Identification and characterization of six novel tetraspanins from Schistosoma japonicum. Parasit Vectors. 2011;4:1–11.
Hu CCA, Liang FX, Zhou G, Tu L, Tang CHA, Zhou J, et al. Assembly of urothelial plaques: tetraspanin function in membrane protein trafficking. Mol Biol Cell. 2005;16:3937–50.
Sun T, Kreibich G, Pellicer A, Kong X, Wu X. Uroplakins as unique tetraspanin networks. Tetraspanins. Springer; 2013. pp. 299–320.
Wang H, Min G, Glockshuber R, Sun TT, Kong XP. Uropathogenic E. coli adhesin-induced host cell receptor conformational changes: implications in transmembrane signaling transduction. J Mol Biol. 2009;392:352–61.
Smyth JD, McManus DP. The Physiology and Biochemistry of Cestodes. Cambridge University Press; 2007.
Bossie A, Vitetta ES. IFN-γ enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-γ in class switching. Cell Immunol. 1991;135:95–104.
Amri M, Aissa SA, Belguendouz H, Mezioug D, Touil-Boukoffa C. In vitro antihydatic action of IFN-γ is dependent on the nitric oxide pathway. J Interferon Cytokine Res. 2007;27:781–8.
Vuitton DA. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite? Acta Trop. 2003;85:119–32.
Baz A, Ettlin GM, Dematteis S. Complexity and function of cytokine responses in experimental infection by Echinococcus granulosus. Immunobiology. 2006;211:3–9.
Zhang W, Ross AG, McManus DP. Mechanisms of immunity in hydatid disease: implications for vaccine development. J Immunol. 2008;181:6679–85.
Maule AG, McVeigh P, Dalzell JJ, Atkinson L, Mousley A, Marks NJ. An eye on RNAi in nematode parasites. Trends Parasitol. 2011;27:505–13.
Geldhof P, Visser A, Clark D, Saunders G, Britton C, Gilleard J, et al. RNA interference in parasitic helminths: current situation, potential pitfalls and future prospects. Parasitology. 2007;134:609–19.
Pierson L, Mousley A, Devine L, Marks NJ, Day TA, Maule AG. RNA interference in a cestode reveals specific silencing of selected highly expressed gene transcripts. Int J Parasitol. 2010;40:605–15.
Mizukami C, Spiliotis M, Gottstein B, Yagi K, Katakura K, Oku Y. Gene silencing in Echinococcus multilocularis protoscoleces using RNA interference. Parasitol Int. 2010;59:647–52.
Spiliotis M, Mizukami C, Oku Y, Kiss F, Brehm K, Gottstein B. Echinococcus multilocularis primary cells: improved isolation, small-scale cultivation and RNA interference. Mol Biochem Parasitol. 2010;174:83–7.
Hagen J, Lee E, Fairlie W, Kalinna B. Functional genomics approaches in parasitic helminths. Parasite Immunol. 2012;34:163–82.
Acknowledgments
We thank Tianyu Liu (Faculty of Veterinary Medicine, Ghent University) for his crucial advice in our manuscript. We also thank Yinan Liang and Min Yan (Sichuan Agricultural University) for their assistance in animal experiment, and Jiahai Wang (Sichuan Agricultural University) for thoughtful comments. This study was supported by grants from the Science and Technology Projects from General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (No. 2009IK019; http://www.aqsiq.gov.cn/), and the Key Technology R&D Program of sichuan, China (No.2015NZ0041; http://www.scst.gov.cn/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DH, XS and GY conceived and designed the study. DH, XS and YX performed the experiments, analysed the data and drafted the manuscript. XZ, NW, and YZ helped in animal experiments and immunofluorescence analysis. XG, TW and XP helped in study design, study implementation and manuscript revision. All authors read and approved the final manuscript.
Dandan Hu and Xingju Song contributed equally to this work.
Additional files
Additional file 1: Table S1.
Real-time PCR primers involved in this study.
Additional file 2: Table S2.
Sequences of siRNAs.
Additional file 3: Figure S1.
Genome-wide phylogenetic analysis of E. granulosus tetraspanins. All tetraspanins from the genome of E. granulosus were employed to construct a phylogenetic tree using neighbor-joining method by MEGA software (version 5.05). The name of each tetraspanin is the accessing numbers of the gene in GeneDB (http://www.genedb.org/). The tetraspanins could be classified into 3 distinct branches represent the CD63 family, CD family and Uroplakin family, respectively. This phylogenetic tree was rooted by the Human-TSP1 (GenBank ID: NP_005718.2). Note: A total of 30 tetraspanin genes were reported in E. granulosus genome, but we can not find “EgrG_001021800.1” throughout the whole genome database.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hu, D., Song, X., Xie, Y. et al. Molecular insights into a tetraspanin in the hydatid tapeworm Echinococcus granulosus . Parasites Vectors 8, 311 (2015). https://doi.org/10.1186/s13071-015-0926-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-015-0926-y