Abstract
Background
African horse sickness (AHS) is an equine disease endemic to Senegal. The African horse sickness virus (AHSV) is transmitted to the mammalian hosts by midges of the Culicoides Latreille genus. During the last epizootic outbreak of AHS in Senegal in 2007, 1,169 horses died from this disease entailing an estimated cost of 1.4 million euros. In spite of the serious animal health and economic implications of AHS, very little is known about determinants involved in transmission such as contact between horses and the Culicoides species suspected of being its vectors.
Methods
The monthly variation in host/vector contact was determined in the Niayes area, Senegal, an area which was severely affected by the 2007 outbreak of AHS. A horse-baited trap and two suction light traps (OVI type) were set up at each of five sites for three consecutive nights every month for one year.
Results
Of 254,338 Culicoides midges collected 209,543 (82.4%) were female and 44,795 (17.6%) male. Nineteen of the 41 species collected were new distribution records for Senegal. This increased the number of described Culicoides species found in Senegal to 53. Only 19 species, of the 41 species found in light trap, were collected in the horse-baited trap (23,669 specimens) largely dominated by Culicoides oxystoma (22,300 specimens, i.e. 94.2%) followed by Culicoides imicola (482 specimens, i.e. 2.0%) and Culicoides kingi (446 specimens, i.e. 1.9%).
Conclusions
Culicoides oxystoma should be considered as a potential vector of AHSV in the Niayes area of Senegal due to its abundance on horses and its role in the transmission of other Culicoides-borne viruses.
Similar content being viewed by others
Background
Midges in the genus Culicoides Latreille are small biting dipterans (1 to 4 mm in length) that belong to the Ceratopogonidae family. Culicoides present a worldwide distribution. Some 1,250 species have been recorded [1], some of which are vectors of viral and parasitic (both protozoan and nematode) pathogens. Their impact is mainly on animal health: in particular, the transmission of virus of two epizootic diseases in horses and ruminants, respectively African horse sickness (AHS) and bluetongue (BT) [2].
AHS is an arboviral disease endemic to sub-Saharan Africa. In the north it is present up to the latitude that links Senegal to Ethiopia. South Africa presents the most southerly distribution. Nine antigenically serotypes are recognized [3]. African horse sickness virus (AHSV) has been responsible for epizootic outbreaks outside of its endemic area in regions previously free of the disease, namely in the Middle East and Asia between 1959 and 1960 [4], in the Maghreb and the Iberian peninsula between 1965–1966 [4], on the Iberian peninsula between 1987 and 1990 [4], in Yemen in 1997 [5], and in the Cape Verde Islands in 1999 [5].
AHS has devastating consequences on susceptible horses with death rates in excess of 90% [6]. Disease control depends on vaccination, isolation or slaughter of infected animals, restrictions on movements of equines and vector control. Only attenuated viral strains are used for vaccination purposes with the drawbacks inherent to the use of live vaccines in addition to the teratogenic effects that prohibit their use on gestating females. Vector control requires the identification of all Culicoides species involved in the transmission. However, control against Culicoides is very limited in the field due to a lack of knowledge of their bio-ecology and efficient operational methods [7].
The most recent epizootic outbreak of AHS in Senegal in 2007 caused the death of 1,169 horses, with an estimated cost of 1.4 million Euros [8]. Riding centres in the Dakar region were very badly affected with a mortality rate of 8.6% [8]. Virological investigations identified serotype 2 and 7 [8,9] as the responsible viral strains, whereas only serotype 9 had been encountered in Senegal previously [10,11].
Up to 1994, 34 Culicoides species were recorded in Senegal [1,12-14]. Amongst these are Culicoides imicola Kieffer is a proven vector of AHSV in southern Africa [15,16]. However, similar as to BT in Europe, virus detection in field collected specimens coupled to oral susceptibility results in the laboratory [16-18] infers that susceptibility to AHSV infection is not restricted to a few species but that it is widespread in the genus Culicoides.
To identify all potential Culicoides vectors in Senegal, we carried out light trap and horse-baited trap collections in an area which experienced outbreaks of AHSV in 2007. Seasonal dynamics of Culicoides collected in light traps during these collections was described previously [19], updating the list of species known in Senegal from 34 to 45 [19,20]. In this work we focused on host-baited trap collections with the aim to (i) draw up an inventory of the Culicoides species relevant for animal health in the Niayes area of Senegal, (ii) identify Culicoides with a host preference for horses and are therefore potential vectors of AHSV and (iii) monitor the seasonal variations in this vector-host contact.
Methods
Study area
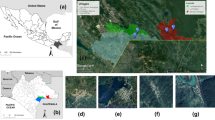
This study was conducted in the southern part of the Niayes area. The Niayes are depressions between dunes that are liable to flooding during the rainy season. They are located just inland of the coastline of Grande Côte, covering a strip 25 to 30 km wide that follows the coast for 180 km, stretching from Dakar to the mouth of the Senegal River. The climate is of the sub-Canarian tropical type with cool temperatures that vary within a narrow range [21]; the average highest monthly temperature is 27.5°C in Dakar and 28.l °C in Saint-Louis [22]. The presence of the ocean is conducive to a high relative moisture rate ranging from 15% to 90% depending on distance from the sea and time of year. The vegetation in the area is diversified, with a plant cover of less than 50%. Typical vegetation consists of large expanses of household vegetable species (cabbage, potato, tomato, carrot, onion, green beans, salad) and fruit trees (citrus, mango). Dairy and poultry farms are also found. In addition, several riding centres that accommodate exotic breeds of horses are located in this area. Rainfall rarely exceeds 500 mm/year in the south, close to Dakar, and 350 mm/year in the north, close to the Senegal River delta. There are two main seasons in Senegal: the rainy season (from July to October) and the dry season (from November to June), which can be subdivided into a cold dry season (from November to February) and a warm dry season (from March to June). Occult precipitation called the ‘heug’ or mango rains often come during the dry season, and particularly during the cold dry season (December, January and February).
Collection sites
The survey was carried out at five riding centres, described in Diarra et al. [20], in the southern part of the Niayes area close to Dakar and Thies (Figure 1). The geographical coordinates and numbers of animals for each site are given by Diarra et al. [20].
The reasons for choosing the southern part of the Niayes area and the five sampling sites were its history of repeated AHS outbreaks and its considerable economic potential for stock-breeding in conjunction with many modern farms with exotic breeds established there. Another consideration was the massive mortality among purebred horses during the last epizootic outbreak of AHS in 2007 [8].
Culicoides trap collections
To assess vector/host contact, Culicoides were collected using a horse-baited trap identical to the one used by Fall et al. for entomological investigations of mosquitoes’ vectors of West Nile fever [23,24] (Figure 2A). In parallel, two light traps of the OVI (Onderstepoort Veterinary Institute) type were operated to compare host-baited collections with this more widely used Culicoides trapping method [25,26] (Figure 2B). The horse-baited trap consists of net boxes (3.5 m × 2.5 m × 2.5 m, with mesh of 1.5 mm × 0.3 mm) with an open space of 15 cm from the ground allowing Culicoides to enter, to engorge or not on horse, and avoiding the escape of trapped midges. Both sampling methods were used at each of the five sites for three consecutive nights per month from July 2011 to June 2012 at Parc Hann, Mbao, Thies and Pout sites and from November 2011 to October 2012 at Niague.
The horse-baited trap and the light traps were activated simultaneously, operating from 6 p.m. to 8 a.m. based on the dusk/dawn and night-time activity of most Culicoides species. At every site, one of the light traps was placed within 5 m from the door of a loose box containing a horse, while the second was positioned outside the line of vision of the loose box more than 10 m away from the horses. Both light traps were placed at a height of 1.5 to 2 m and sheltered from the wind and any source of artificial light. The horse-baited trap was positioned more than 10 m away from the closest horse housed in a loose box. The three traps were fairly distant from one another (>10 m) so as to minimize possible interactions [27].
The Culicoides specimens collected in the light traps were recovered in the morning, strained to separate the insects from the soapy water and transferred to an appropriate labelled jar. Samples were preserved in 90% alcohol and stored in the dark until identified.
As for the horse-baited traps, the animals were fed and watered according to the usual procedures applied at the stable. We used the same five horses, approximately in the same size and weight, during the entire survey: four were females (the male was in Parc Hann) and four were chestnut (the fifth was cream-colored and located at Niague). The Culicoides were collected into a collection bottle with a mesh bottom early in the morning using an electric vacuum cleaner for between 10 and 15 minutes. The sampling site and date of capture were indicated on each bottle. These live specimens were killed by freezing (−20°C), sorted and identified the same day before being stored in 90% alcohol in a labelled bottle.
Identification of the Culicoides
Morphological identification of Culicoides species was conducted by examination of the wing pigmentation pattern using a stereomicroscope. For species that are difficult to identify, the specimens were dissected and slide-mounted in accordance with the Wirth and Marston technique for observation under a microscope [28]. Several identification keys were used depending on the species found and their subgenus or group [13,14,29-34]. A count of all the Culicoides identified was made by species, gender and, for females, by physiological status. They were stored in 90% alcohol. Samples comprising more than 3 ml of insects were subsampled according to the modified procedure based on Van Ark and Meiswinkel [35].
Statistical analyses
Monthly abundance of each Culicoides species was determined by taking the largest of the catches made on three consecutive nights in every trap. Maximum was preferred to mean because the number of specimens collected can drop very quickly when local weather conditions are sub-optimal. Hence, the maximum of several consecutive collections was considered as the best representation of abundance over a short time period [36]. Capture frequency is the ratio of the number of nights a species was present over the total number of capture nights per site. The engorgement rate for a species is the ratio of engorged females to the total number of females of this species. Engorgement rates were compared between species by means of the Kruskal Wallis test [37]. Monthly abundances were compared between capture methods by performing Pearson’s correlation test.
Diversity of Culicoides species by trap and by site was assessed using cumulative monthly abundances by Shannon index and the Simpson-Yule. Between-class (sites) and within-class (trap types) measures were obtained by a centred principal component analysis of the natural logs of the cumulative abundance percentages by species, site and trap type [38,39].
The statistical analyses were all performed with the R programming language at the significance level of 5% [40].
Ethical approval
This protocol was approved by the Scientific and Technological Advice board of ISRA, Senegal on 22–27 November 2010. The protocol strictly adhered to the usual conditions of animal welfare. Animals used as baits were not no more exposed to Culicoides bites than other animals of the private stables in the area. They also received veterinary care during the experiment.
Results
Overall diversity
In total, 254,338 specimens of the Culicoides genus, of which 209,543 females (82.4%) and 44,795 males (17.6%) were collected using the two sampling methods over the 515 nights of trapping: 181 and 178 for each light trap and 156 for the horse-baited trap (Table 1). Diarra et al. [20] listed 24 different species in light trap collections, but 398 specimens remained unsorted and grouped into Culicoides sp. After completing the identification, we finally identified at least in light trap and horse-baited trap collections, 19 of which are new distribution records in Senegal (11 from Diarra et al. [20] and 8 from this paper). Hence the list of Culicoides in Senegal now comprises 53 species (Table 2). The most abundant species captured were C. oxystoma (41.4% of overall captures), C. kingi (39.0%), C. imicola (10.0%), C. enderleini (3.9%) and C. nivosus (1.4%). The co-dominance of C. oxystoma and C. kingi is illustrated by a Shannon index of 1.39 (maximum value 3.7) and a Simpson-Yule index of 0.34.
Culicoides collected on horses
During the overall collection nights, a total of 23,669 specimens were captured in horse-baited traps (9.3% of overall captures) as against a total of 230,670 specimens in the light traps (accounting for 90.7% of overall captures). Only 19 of the 41 species found in light traps were found in horse-baited trap (Table 2). No species was found only in horse-baited trap.
Culicoides oxystoma was the dominant species on horse (22,302 individuals; 94.2% of overall captures). The three most abundant species, C. oxystoma, C. imicola and C. kingi, represented 98.2% of collections (23,239 individuals) and the eight most abundant, which included C. enderleini and C. bolitinos (Table 3), 99.5% of collections.
Overall, biting rates on horses were high at Mbao, medium at Hann, Niague and Thies and relatively low at Pout (Table 3). For C. oxystoma, the mean value at Mbao was 772 females/trap/night as compared to 15 or 30 females/trap/night at the Hann Pony Club and at Thies, and close to 0 at Niague and Pout. The highest attack rates on horses were observed in the second half of the rainy season (September and October) at Parc Hann, Mbao and Thies for C. oxystoma, C. imicola and C. kingi (Figure 3). At these three sites, the primary peak of attack rates was accompanied by a secondary peak during the hot dry season before the rains set in. These species had low abundances during the cold dry season (November to February). In contrast, at Niague, C. kingi appeared to be attracted by the horse all year round, and C. imicola during the dry seasons (Figure 3).
Dynamics of monthly abundances of females for the three main species (C. oxystoma, C. imicola and C. kingi) in the horse-baited trap by season at 5 sites of the Niayes area in Senegal. (RS = rainy season; CDS = cold dry season; HDS = hot dry season). NB: a log10 (n + 1) transformation was applied to the abundance data; the Pout site was not plotted due to the low abundances observed.
All engorged females found in the horse-baited traps belong to the Imicola, Schultzei and Milnei groups (Table 4), with an overall engorgement rate of 36.7%. Differences in engorgement rates between species were significant (Kruskal Wallis test, p = 0.03). The engorgement rates may be classified in three groups: the species with an engorgement rate of more than 75% that include C. bolitinos, a proven vector of AHSV in South Africa, the species whose engorgement rate ranges between 50 and 75% that include C. imicola the main vector of AHSV in Africa, and those whose engorgement rate is less than 50% that include the species in the Schultzei group: C. oxystoma, C. enderleini and C. kingi. Females of C. gambiae, C. leucostictus, C. murphyi, C. nevilli, C. nivosus and C. similis species which were rarely collected in the horse-baited trap were found not to be engorged.
Comparison with light trap collections
A total of 127,197 Culicoides was collected by the light trap located <5 m from the horses, whereas the light trap located >10 m from the horses collected 103,473 Culicoides. The total number of Culicoides by species was highly correlated between traps (r = 0.95, p < 0.001), the nine most abundant species (98% of the total collection for both traps) being the same. The three or four most abundant species (C. oxystoma and/or C. kingi, C. imicola and C. enderleini) presented the same rank at each site, with the only exception of Mbao where C. kingi and C. imicola were at the rank 2 and 3 in the light trap <5 m from the horses (12.8% and 9.2% of the total collection) and at the rank 3 and 2 in the light trap >10 m from the horses (15.2% and 24.1%). The differences of proportional representation were low between both light traps for the four most abundant species (mean = 4%, N = 20), with only three differences > 4%: the differences were 33% between collections of the light trap < 5 m and of the light trap > 10 m for C. oxystoma at Mbao, but −10% for this species at Hann, and −18% for C. kingi at Niague. Moreover the monthly abundances estimated with the light trap < 5 m and the light trap > 10 m were highly correlated (r = 0.73, p < 0.001). Considering thus that both light traps gave comparable results, this data was grouped for the remainder of the analyses, while maintaining the maximum abundance observed per species, site and month.
We compared horse-baited trap collections to light trap collections using two complementary approaches: 1) a principal component analysis (PCA) using between and within-classes decomposition to quantify relative influence of both sites and trap types on Culicoides diversity as a whole, and 2) correlations between horse-baited and light trap collections by species.
In a first step, centred PCA was performed on the natural logs of the percentages of cumulative abundance by species, site and type of trap. The four first axes represented 89.8% of total variance (Figure 4A respectively 41.3%, 63.2% and 78.6% for each of the first three axes). Between-class (between sites) variance explained 74.0% (as against 44.9% predicted by a permutation test, p < 0.01) of the PCA variance, while within-class (between trap types) accounted for 26.0%, indicating that the site effect was much more predominant in Culicoides diversity than the trap effect. This result was illustrated by the resemblance of Figure 4A (PCA) and Figure 4C (PCA with a maximisation of the variance between sites) with the opposition on axis 1 between the Niague site characterised by C. kingi and the other sites characterised by C. oxystoma, and on axis 2 between Parc Hann and Mbao sites characterised by C. austeni and C. milnei and Pout and Thies sites characterised by C. imicola and C. bolitinos. Figure 4D illustrated the opposition between Mbao characterised by both C. oxystoma and C. kingi, and Parc Hann and Pout characterised by C. enderleini and C. nivosus on axis 3. Indeed, in Parc Hann, C. oxystoma was dominant (66.7% of light trap catches) and was associated with C. enderleini (12.6%), in Mbao C. oxystoma was predominant (69.9%) in association with C. kingi (12.5%) and C. imicola (11.4%), in Niague the prevalent species was C. kingi (78.6%) in association with C. imicola (10.3%), in Pout C. oxystoma was dominant (49.3%) alongside C. imicola (18.6%) and C. enderleini (10.9%) and in Thies C. oxystoma was predominant (38.9%) followed by C. imicola (30.0%). Hence there was a west-to-east gradient of increasing proportional representation of C. imicola (from 3.2 to 30.0%) and of decreasing proportional representation of C. oxystoma (from 66.7 to 38.9%, with the exception of Niague where C. oxystoma was less represented [3.1%]). Within-class (between trap types) analysis showed differences in catch diversity as between light and baited traps (Figure 4B). This structure was mainly attributable to the species C. kingi and C. enderleini, which were chiefly captured by light traps (total cumulative monthly abundance is respectively 39,308 and 3,942 for the light trap as against 258 and 50 for the baited trap).
Diversity of the Culicoides captured by site (5 sites in the Niayes area of Senegal) and trap (light- and horse-baited trap) from July 2011 to October 2012. A: Centred principal component analysis (PCA) performed on the natural logs of the cumulative percentages of abundance by species, site and type of trap (axis 1/axis 2). B: PCA within-class analysis (axis 1/axis 2). C and D: PCA between-class analysis (axis 1/axis 2 and axis 2/axis 3).
In a second step, correlations were assessed between horse-baited and light trap collections. Monthly abundances of female C. oxystoma captured in the light traps were correlated with those collected on the horse bait (Figure 5) at the Parc Hann, Mbao and Thies sites, but not for the other two sites where hardly any of this species were captured on the horse. For those three sites, light trap captured more Culicoides by a factor of 1.57 with a log10 scale (log10(No. Culicoides on horse + 1) × 1.57 = log10(No. Culicoides in light trap + 1); R2 = 85.4; p < 0.001) than horse baited trap. Culicoides kingi was captured on the horse bait in Mbao and Niague alone. At these two sites (Figure 5), the light trap considerably collected more Culicoides by a factor of 3.82 with a log10 scale (R2 = 74.1; p < 0.001) than horse baited trap. Light trap catches of C. imicola were correlated with the horse-baited estimated abundances at the Mbao and Thies sites (Figure 5), where the light trap collections were greater by a factor of 2.41 with a log10 scale (R2 = 75.6; p < 0.001). There was no correlation between horse-baited and light trap collections for C. enderleini or C. nivosus.
Correlation of monthly abundances for females collected with light traps and those captured in horse-baited traps from July 2011 to October 2012 at 5 sites in the Niayes area of Senegal. The 95% prediction interval corresponds to interval in which future observations will fall, with a 95% probability, assuming that future observations have the same error variance as those used for fitting (observation data).
Discussion
The Culicoides inventory conducted during these horse-baited and light trap surveys in a relatively limited area in the southern part of the Niayes area provided an updated list of Culicoides for Senegal comprising 53 species and, more significantly, established the presence and abundance of C. oxystoma on horse-baited trap. This species’ distribution was previously confined to the Eastern and Australasian Regions [30,41] and was during this survey identified for the first time in the Afro-tropical Region [19]. In Senegal, there are now at least four species that belong to the Schultzei group: C. enderleini, C. kingi, C. nevilli, and C. oxystoma. The presence of a number of other species in the Imicola group such as C. bolitinos and C. miombo and in the Milnei group such as C. austeni, C. hortensis, C. milnei, C. quinquelineatus and C. wansoni was also recorded for the first time in Senegal (some of these species were already mentioned by Diarra et al. [20]). Twelve species previously captured in other bioclimatic areas of Senegal [12,14] were not detected during this survey.
Culicoides oxystoma was the most abundant species associated with horses in the Niayes area, which is known for past outbreaks of AHS [11], and where the latest AHS epizootic in Senegal in 2007 caused considerable losses [8,42]. This raises questions about its role in transmitting the AHSV in Senegal. Its competence against AHSV has never been determined. Culicoides oxystoma is known to be involved in the transmission of bovine arboviruses such as Akabane in Japan [43,44]. It is a suspected vector of epizootic haemorrhagic disease virus in Israel [45] and a potential vector of the bluetongue virus (BTV) in India [46].
To a lesser extent, horses were also attacked by C. imicola in Mbao, Niague and Thies, with C. kingi in Mbao and Niague, with C. enderleini in Mbao, with C. austeni in Parc Hann, and by C. bolitinos in Thies. Culicoides imicola is a proven vector of AHSV in South Africa [47] and probably in the Maghreb (Morocco) and the Iberian peninsula (Spain and Portugal) [48]. It is also the main vector of BTV in South Africa, in the Maghreb and in Southern Europe [49]. The role of C. kingi and of C. enderleini as vectors of AHSV is not clearly established. Field-collected specimens of C. kingi in Sudan have been found to be infected by epizootic haemorrhagic disease virus [50]. Culicoides enderleini is a potential vector for BTV in South Africa [47,51] and experimental infections conducted in the laboratory have shown that AHSV could be isolated in field specimens of this species 10 days after feeding on a virus-infected blood meal [52]. Culicoides bolitinos, the second proven vector for AHSV in South Africa [16], was rarely found at our sampling sites. It is difficult to conclude if this species may play a major role in the transmission of AHSV in Senegal because only a few sites were sampled. This species is mostly found in sub-tropical regions with a humid, cool climate like the coast and mountains of South Africa and locally closely linked with the presence of bovids. Indeed, the coprophilous larvae of C. bolitinos develop preferentially on cattle, buffalo and wildebeest dung [16]. However, molecular studies should be conducted on the rare individuals of C. bolitinos found in Senegal to ascertain their specific status in view of the many morphological variations observed on these specimens. It is interesting to note that C. bolitinos is morphologically similar to C. brevitarsis, a species whose coprophilous larvae also develop on cattle dung [53] and whose distribution in the Eastern and Australasian Regions is identical to that of C. oxystoma.
In tropical areas, high numbers of Culicoides are generally observed at the end of the rainy season, with significant decreases in population during the dry season, in connection with rainfall, relative moisture and temperature which impact both the productivity of larval habitats and the activity of adults [49,54]. The same trend was found in Senegal where the highest abundances of C. oxystoma were observed at the end of the rainy season (September and October) and, to a lesser extent, at the end of the dry season (June). These seasonal dynamics for the dominant species in horse-baited traps was highly correlated with that in light traps [20]. These two peaks of abundance coincided with the periods of emergence and expansion of cases of AHS during the 2007 outbreak [42].
The dynamics for the three most abundant species on horses differ according to the site. Between-site variations in the dynamics of C. oxystoma may depend on local ecological conditions, in particular the productivity of the available larval habitats [55]. Indeed, the Mbao and Hann sites are respectively located close to a river and a marsh whereas in Thies flooding of larval habitats and their productiveness may be more dependent on rainfall alone. Some of the species in the Schultzei group are regularly found along streams and drainage canals and in mud containing little organic matter [56]. The larvae of C. oxystoma can develop both in mud and sand. This may explain the overall abundance of C. oxystoma in the Niayes area (depressions between dunes that are liable to flooding in the rainy season). Studies out in India have shown that C. oxystoma is a euryhaline species that is present all year round in the Sagar Island estuary in India [57,58]. Culicoides kingi, a species typical of brackish environments [13], is the dominant species at the Niague site located close to Lake Retba (the pink lake) where salt is harvested. At this location, the species’ maximum abundance occurs at the end of the dry season (June) and diminishes with rain when the salt concentration in breeding sites may drop creating conditions that are less conducive to the development of this species. This phenomenon is documented for Anopheles melas (Diptera: Culicidae), a mosquito species in the Anopheles gambiae complex whose halophilic larvae develop on West African lagoons [59]. As for C. imicola, C. kingi is relatively abundant at Niague and Mbao with essentially year round activity but its larval ecology is poorly known and deserves investigation, particularly in Senegal.
The high engorgement rates of the specimens belonging to the Imicola group, including C. bolitinos and C. imicola, confirmed the trophic preference of these two species for horses [60,61] and the probable role of C. imicola in the transmission of AHSV in Senegal in relation to its abundance and vector status in South Africa [15,47]. However, the average below engorgement rates on horses of the main species in the Schultzei group (C. oxystoma, C. kingi and C. enderleini) may be offset by their abundances, in particular as regards C. oxystoma, if it is proven that these species are competent vectors of AHSV.
It is established that light trap collections do not assess biting rates on animals accurately [62,63]. Nevertheless, both methods gave the same picture of Culicoides diversity as a whole, as highlighted by the decomposition of PCA variance into between-class (sites) and within-class (traps). This PCA was carried out using the proportional representation rather than the abundance to avoid a “size effect” due to important difference of abundance between sites and between traps. Using abundance in PCA, data were structured mainly along a single axis, highlighting the opposition for C. kingi and C. enderleini between light traps with high abundance and horse-baited traps with low abundances. This structure driven by C. kingi and C. enderleini was also found in the within-classes analysis.
The horse-baited trap collected fewer species and is therefore more appropriate for investigating host/vector contact. The difference is reflected by the over-representation of species such as C. oxystoma (1.57 in a log10 scale), C. kingi (3.82 in a log10 scale) and C. imicola (2.41 in a log10 scale) in the light traps compared to the horse-baited traps. Determining such as correction factors is crucial to rescale wide-scale abundance maps, established with light traps as the most convenient trapping method, to probable biting rates which could be used in transmission models.
Conclusion
This study allows an update of list of Culicoides species of veterinary interest in Senegal (53 species to date). Culicoides oxystoma, and to a lesser extent C. kingi and C. enderleini, were furthermore identified as potential vectors of AHSV in the Niayes area of Senegal additionally to the vector C. imicola due to their abundance and aggressiveness on horses, as well as their role as vectors for transmitting other animal viral diseases such as Akabane and Epizootic haemorrhagic disease viruses. These preliminary results should however be corroborated by further studies, both in the field and in the laboratory, so as to better specify their bio-ecology, in particular their larval biotope, and their vector competence, and gain a better understanding of their role in the epidemiology of AHS in Senegal.
References
Borkent A. World species of biting midges (Diptera: Ceratopogonidae). British Colombia, Canada: RBC Museum; 2014.
Du Toit RM. The transmission of blue-tongue and horse sickness by Culicoides. Onderstepoort J Vet Sci Anim Ind. 1944;19:7–16.
Coetzer JAW, Erasmus BJ. African horse sickness. In: Coetzer JAW, Thomson GR, Tustin RC, editors. Infectious Disease of Livestock with Special Reference to Southern Africa. Cape Town: Oxford University Press; 1994. p. 460–75.
Mellor PS, Hamblin C. African horse sickness. Vet Res. 2004;35(4):445–66.
OIE. African Horse Sickness. 2009. p. 1–5.
Coetzer JAW, Guthrie AJ. African horse sickness. In: Coetzer JAW, Tustin RC, editors. Infectious Diseases of Livestock, 2nd. Southern Africa: Oxford University Press; 2004. p. 1231–46.
Carpenter S, Mellor PS, Torr SJ. Control techniques for Culicoides biting midges and their application in the U.K. and northwestern Palaearctic. Med Vet Entomol. 2008;22:175–87.
Akakpo AJ, Wombou Toukam CM, Mankor A, Ly C. Impact economique de l’epizootie de peste equine de 2007 au Senegal. Bull Anim Health Prod Afr. 2011;59:1–16.
MacLachlan NJ, Guthrie AJ. Re-emergence of bluetongue, African horse sickness, and other orbivirus diseases. Vet Res. 2010;41(6):35.
Bourdin P, Sarr J, Lejan C. Isolement et identification de la Peste équine africaine en zone sahélienne à partir de foyers récents. Bull Int Epiz. 1976;86:717–9.
Sarr J, Diop M, Cissoko S. La peste equine africaine au senegal :un nouveau foyer a type 9 dans la commune de Thies, ISRA VIROLOGIE. 1987.
Cornet M. Les Culicoides (Diptera: Ceratopogonidae) de l'Ouest Africain (1ere note). Cah ORSTOM Ser Entomol med Parasitol. 1969;7(4):341–64.
Cornet M, Brunhes J. Révision des espèces de Culicoides apparentées à C. shultzei (Enderleini, 1908) dans la région afro-tropicale (Diptera: Ceratopogonidae). Bull Soc Entomol Fr. 1994;92(2):149–64.
Cornet M, Chateau R. Les Culicoides de l’Ouest africain (2° note) Espèces apparentées à C. similis Carter, Ingrain et Macfie, 1920 (Diptera: Ceratopogonidae). Cah ORSTOM Ser Entomol med Parasitol. 1970;8(2):141–73.
Venter GJ, Graham SD, Hamblin C. African horse sickness epidemiology: vector competence of south african Culicoides species for virus serotypes 3, 5 and 8. Med Vet Entomol. 2000;14(3):245–50.
Meiswinkel R, Paweska JT. Evidence for a new field Culicoides vector of African horse sickness in South Africa. Prev Vet Med. 2003;60(3):243–53.
Venter GJ, Paweska JT. Virus recovery rates for wild-type and live-attenuated vaccine strains of African horse sickness virus serotype 7 in orally infected South African Culicoides species. Med Vet Entomol. 2007;21:377–83.
Meiswinkel R, Gomulski LM, Delécolle JC, Goffredo M, Gasperi G. The taxonomy of Culicoides vector complexes - unfinished business. Vet Ital. 2004;40(3):151–9.
Bakhoum MT, Fall M, Fall AG, Bellis GA, Gottlieb Y, Labuschagne K, et al. First record of Culicoides oxystoma Kieffer and diversity of species within the Schultzei group of Culicoides Latreille (Diptera: Ceratopogonidae) biting midges in Senegal. PLoS ONE. 2013;8(12):1–8.
Diarra M, Fall M, Fall AG, Diop A, Seck MT, Garros C, et al. Seasonal dynamics of Culicoides (Diptera: Ceratopogonidae) biting midges, potential vectors of African horse sickness and bluetongue viruses in the Niayes area of Senegal. Parasit Vectors. 2014;7:147.
Sagna P. Le climat. In: Paris JA, editor. Atlas du Sénégal. 5th ed. 2000. p. 16–9.
Faye O, Gaye O, Fontenille D, Hébrard G, Konate L, Sy N, et al. La sécheresse et la baisse du paludisme dans les Niayes du Sénégal. Cah Santé. 1995;5:299–305.
Fall AG, Diaite A, Lancelot R, Tran A, Soti V, Etter E, et al. Feeding behaviour of potential vectors of West Nile virus in Senegal. Parasit Vectors. 2011;99(4):1–7.
Fall AG, Diaite A, Seck MT, Bouyer J, Lefrançois T, Vachiery N, et al. West Nile virus transmission in sentinel chickens and potential mosquito vectors, Senegal river delta, 2008–2009. Int J Environ Res Public Health. 2013;10:4718–27.
Venter GJ, Paweska JT, Van Dijk AA, Mellor PS, Tabachnick WJ. Vector competence of Culicoides bolitinos and C. imicola (Diptera: Ceratopogonidae) for South African bluetongue virus serotypes 1, 3 and 4. Med Vet Entomol. 1998;12:378–85.
Goffredo M, Meiswinkel R. Entomological surveillance of bluetongue in Italy: methods of capture, catch analysis and identification of Culicoides biting midges. Vet Ital. 2004;40(3):260–5.
Venter GJ, Majatladi DM, Labuschagne K, Boikanyo SNB, Morey L. The attraction range of the Onderstepoort 220 V light trap for Culicoides biting midges as determined under South African field conditions. Vet Parasitol. 2012;190:222–9.
Wirth WW, Marston N. A method for mounting small insects on microscope slides in canada balsam. Ann Entomol Soc Am. 1968;61:783–4.
Cornet M, Nevill EM, Walker AR. Notes sur les Culicoides (Diptera: Ceratopogonidae), du groupe de C. milnei Auten,1909, en Afrique orientale et australe. Cah ORSTOM Ser Entomol med Parasitol. 1974;12(4):231–43.
Boorman J. Culicoides (Diptera: Ceratopogonidae) of the Arabian Peninsula with Notes on their médical and veterinary importance. Fauna Saudi Arabia. 1979;10:160–224.
Boorman J, Dipeolu OO. A taxonomic study of adult Nigerian Culicoides Latreille (Diptera: Ceratopogoaidae) species. Occ Publ Ent Soc Nigeria. 1979;22:1–121.
Meiswinkel R. Afrotropical Culicoides: a redescription of C. (Avaritia) imicola Kieffer, 1913 (Diptera: Ceratopogonidae) with description of the closely allied C. (Avaritia) bolitinos sp. Nov. reared from the dung of the african buffalo, blue wildebeest and cattle in South Africa. Onderstepoort J Vet Res. 1989;56:23–39.
Meiswinkel R. Afrotropical Culicoides: C. (Avaritia) miombo sp. nov., a widespread species closely allied to C. (A.) imicola Kieffer, 1913 (Diptera: Ceratopogonidae). Onderstepoort J Vet Res. 1991;58(3):155–70.
Glick JI. Culicoides biting midges (Diptera: Ceratopogonidae) of Kenya. J Med Entomol. 1990;27(2):85–195.
Van Ark H, Meiswinkel R. Subsampling of large light trap catches of Culicoides (Diptera: Ceratopogonidae). Onderstepoort J Vet Res. 1992;59:183–9.
Baylis M, El Hasnaoui H, Bouayoune H, Touti J, Mellor PS. The spatial and seasonal distribution of African horse sickness and its potential Culicoides vectors in Morocco. Med Vet Entomol. 1997;11(3):203–12.
Hollander M, Wolfe DA. Non parametric statistical inference. N Y: John Wiley & Sons, Inc; 1973.
Benzécri JP. Analyse de l’inertie intra-classe par l’analyse d’un tableau de correspondances. Cah Anal Donnees. 1983;8:351–8.
Dolédec S, Chessel D. Rythmes saisonniers et composantes stationnelles en milieu aquatique I- Description d’un plan d'observations complet par projection de variables. Acta Oecologica, Oecologia Generalis. 1987;8(3):403–26.
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. ISBN 3-900051-07-0 2005.
Wirth WW, Hubert AA. The Culicoides of southeast Asia (Diptera: Ceratopogonidae). Mem Am Entomol Inst. 1989;44:1–509.
Diouf ND, Etter E, Lo MM, Lo M, Akakpo AJ. Outbreaks of African horse sickness in Senegal and methods of control of the 2007 epidemic. Vet Record. 2013;172(6):152.
Kurogi H, Akiba K, Inaba Y, Matumoto M. Isolation of Akabane virus from the biting midge Culicoides oxystoma in Japan. Vet Microbiol. 1987;15(3):243–8.
Yanase T, Kato T, Kubo T, Yoshida K, Ohashi S, Yamakawa M, et al. Isolation of bovine arboviruses from Culicoides biting midges (Diptera: Ceratopogonidae) in southern Japan: 1985–2002. J Med Entomol. 2005;42(1):63–7.
Morag N, Saroya Y, Bravermann Y, Klement E, Gottlieb Y. Molecular identification, phylogenetic status and geographic distribution of Culicoides oxystoma (Diptera: Ceratopogonidae) in Israel. PLoS ONE. 2012;7(3):e33610.
Dadawala AI, Biswas SK, Rehman W, Chand K, De A, Mathapati BS, et al. Isolation of bluetongue virus serotype 1 from Culicoides vector captured in livestock farms and sequence analysis of the viral genome segment-2. Transbound Emerg Dis. 2012;59:361–8.
Venter GJ, Mellor PS, Paweska JT. Oral susceptibility of South African stock-associated Culicoides species to bluetongue virus. Med Vet Entomol. 2006;20:329–34.
Rawlings P, Pro MJ, Pena I, Ortega MDC. Spatial and seasonal distribution of Culicoides imicola in Iberia in relation to the transmission of African horse sickness virus. Med Vet Entomol. 1997;11:49–57.
Mellor PS, Boorman J, Baylis M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol. 2000;45:307–40.
Mellor PS, Osborne R, Jennings DM. Isolation of bluetongue and related viruses from Culicoides spp. in the Sudan. J Hyg. 1984;93(3):621–8.
Nevill EM, Venter GJ, Edwardes M. Potential Culicoides vectors of livestock orbiviruses. In: Walton TE, Osburn BI, editors. Bluetongue, African horse sickness, and related orbiviruses: Proceedings of the 2nd International Symposium. Boca Raton, Florida: CRC Press; 1992. p. 306–13.
Venter GJ, Labuschagne K, Hermanides KG, Boikanyo SNB, Majatladi DM, Morey L. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet Parasitol. 2009;166(3):299–307.
Yanase T, Matsumoto Y, Matsumori Y, Aizawa M, Hirata M, Kato T, et al. Molecular identification of field-collected Culicoides larvae in the southern part of Japan. J Med Entomol. 2013;50(5):1105–10.
Herniman KA, Boorman JP, Taylor WP. Bluetongue virus in a Nigerian dairy cattle herd. 1. Serological studies and correlation of virus activity to vector population. J Hyg. 1983;90(2):177–93.
Bishop AL, Mckenzie J, Sporh LJ, Barchia IM. Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) in different farm habitats. Aust J Zool. 1994;42:372–84.
Wirth WW, Dyce AL. The taxonomic status of the Culicoides vectors of bluetongue viruses. In: Barber TL, Jochim MM, Alan R, editors. Bluetongue and Related Orbiviruses. NY: Liss; 1985. p. 151–64.
Ray S, Choudhury A. Vertical distribution of a biting midge, Culicoides oxystoma (Diptera: Ceratopogonidae) during different seasons in the Hooghly Estuary, Sagar Island, India. Insect Sci Appl. 1988;9(3):329–33.
Poddar TK, Ray S, Choudhury A. Ecology of larval Culicoides oxystoma (Diptera: Ceratopogonidea) in the Hoogly Estuary, Sagar Island, India. Ann Entomol Soc Am. 1992;10(1):19–25.
Akogbeto M. Etude entomologique sur la transmission du paludisme côtier lagunaire : cas d’un village construit sur un lac d’eau saumâtre. Ann Soc Belge Med Trop. 1995;75:219–27.
Nevill EM, Anderson D. Host preferences of Culicoides midges (Diptera: Ceratopogonidae) in South Africa as determined by precipitin tests and light trap catches. Onderstepoort J Vet Res. 1972;39(3):147–52.
Meiswinkel R, Baylis M, Labuschagne K. Stabling and the protection of horses from Culicoides bolitinos (Diptera: Ceratopogonidae), a recently identified vector of African horse sickness. Bull Entomol Res. 2000;90:509–15.
Viennet E, Garros C, Lancelot R, Allene X, Gardes L, Rakotoarivony I, et al. Assessment of vector/host contact: comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of Orbiviruses. Parasites Vectors. 2011;4:119.
Scheffer EG, Venter GJ, Labuschagne K, Page PC, Mullens BA, MacLachlan NJ, et al. Comparison of two trapping methods for Culicoides biting midges and determination of African horse sickness virus prevalence in midge populations at Onderstepoort, South Africa. Vet Parasitol. 2012;185:265–73.
Acknowledgements
The authors want to thank both anonymous referees for their excellent suggestions. The authors are particularly grateful to Karien Labuschagne for her help on morphology, to all people who provided assistance in operating traps on several nights (technicians, animal keepers, shepherds) in Senegal and to Hélène Guis for manuscript reading. This study was funded partly from the EU FP7-HEALTH-2010-single-stage grant 261504 EDENext. This paper is catalogued by the EDENext Steering Committee as EDENext247 (http://www.edenext.eu). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TBt, AGF, MTS, TBn and JB designed and supervised the study. MF, AGF, IM performed sampling and global management of the entomological material. MF and IM performed the species identification with the help of CG, TB, XA, JCD, and IR. MTB, AMD, MD and MN participated in field and laboratory activities. MF, AGF, GG, TBt, LK, OF and TBn analysed the data and wrote the first draft of the manuscript. All authors revised and approved the final version of the manuscript.
Moussa Fall and Maryam Diarra contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fall, M., Diarra, M., Fall, A.G. et al. Culicoides (Diptera: Ceratopogonidae) midges, the vectors of African horse sickness virus – a host/vector contact study in the Niayes area of Senegal. Parasites Vectors 8, 39 (2015). https://doi.org/10.1186/s13071-014-0624-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-014-0624-1