Abstract
Background
In times of global climate change, the conversion and capturing of inorganic CO2 have gained increased attention because of its great potential as sustainable feedstock in the production of biofuels and biochemicals. CO2 is not only the substrate for the production of value-added chemicals in CO2-based bioprocesses, it can also be directly hydrated to formic acid, a so-called liquid organic hydrogen carrier (LOHC), by chemical and biological catalysts. Recently, a new group of enzymes were discovered in the two acetogenic bacteria Acetobacterium woodii and Thermoanaerobacter kivui which catalyze the direct hydrogenation of CO2 to formic acid with exceptional high rates, the hydrogen-dependent CO2 reductases (HDCRs). Since these enzymes are promising biocatalysts for the capturing of CO2 and the storage of molecular hydrogen in form of formic acid, we designed a whole-cell approach for T. kivui to take advantage of using whole cells from a thermophilic organism as H2/CO2 storage platform. Additionally, T. kivui cells were used as microbial cell factories for the production of formic acid from syngas.
Results
This study demonstrates the efficient whole-cell biocatalysis for the conversion of H2 + CO2 to formic acid in the presence of bicarbonate by T. kivui. Interestingly, the addition of KHCO3 not only stimulated formate formation dramatically but it also completely abolished unwanted side product formation (acetate) under these conditions and bicarbonate was shown to inhibit the membrane-bound ATP synthase. Cell suspensions reached specific formate production rates of 234 mmol g −1protein h−1 (152 mmol g −1CDW h−1), the highest rates ever reported in closed-batch conditions. The volumetric formate production rate was 270 mmol L−1 h−1 at 4 mg mL−1. Additionally, this study is the first demonstration that syngas can be converted exclusively to formate using an acetogenic bacterium and high titers up to 130 mM of formate were reached.
Conclusions
The thermophilic acetogenic bacterium T. kivui is an efficient biocatalyst which makes this organism a promising candidate for future biotechnological applications in hydrogen storage, CO2 capturing and syngas conversion to formate.
Similar content being viewed by others
Background
Carbon dioxide and syngas are considered as “renewable options” in biotechnological applications, especially in times of global climate change and gradual increase of atmospheric CO2 [1, 2]. Among the organisms able to reduce CO2, strictly anaerobic, acetogenic bacteria have gained much attraction in recent years [3,4,5] because they can use H2 and CO as reductant for CO2 reduction. The use of acetogenic bacteria to produce ethanol from syngas (H2, CO, CO2) is already realized on an industrial scale [6, 7]. The first step in acetogenic CO2 reduction is the reduction of CO2 to formic acid (Fig. 1). Acetogens are phylogenetically very diverse and employ different enzymes for this reaction [8, 9]. Typically, they have NADP- or ferredoxin-dependent formate dehydrogenases [10,11,12], whereas Acetobacterium woodii and Thermoanaerobacter kivui have a different enzyme, a hydrogen-dependent CO2 reductase (HDCR) [13, 14]. This enzyme has a formate dehydrogenase module and a [FeFe]-hydrogenase module that are connected by two small FeS-containing proteins. In contrast to formate dehydrogenases, these enzymes can use molecular hydrogen directly as reductant for CO2, without the need for external soluble cofactors. Interestingly, the enzyme also accepts electrons from CO (via ferredoxin) [14], making it a catalyst for the conversion of syngas to formic acid. The HDCR not only reduces CO2 with remarkable catalytic activities but also oxidizes H2 and, thus, can be used to kill two birds with one stone [14, 15]. Apart from CO2 reduction, it can be used to store hydrogen gas in a liquid, non-toxic product, formic acid or its base, formate, a so-called liquid organic hydrogen carrier (LOHC) [16, 17]. The equilibrium constant for the conversion of CO2 + H2 to formic acid is close to one and, therefore, it is an ideal biocatalyst for the storage of H2. All other enzymes known, including the membrane-bound formate hydrogen lyase of Escherichia coli have a strong bias towards formate oxidation and reduce CO2 only under harsh conditions with low activities [18, 19].
Model of the biochemistry and bioenergetics of acetogenesis from H2 + CO2 in T. kivui. The bioenergetics and biochemistry of acetogenesis from H2 + CO2 by T. kivui are shown. CODH/ACS, CO dehydrogenase/acetyl-CoA synthase; Ech, energy-conserving hydrogenase; HDCR, hydrogen-dependent CO2 reductase; hydrogenase, electron bifurcation hydrogenase; THF, tetrahydrofolic acid; HCO-THF, formyl-THF; HC-THF, methenyl-THF; H2C-THF, methylene-THF; H3C-THF, methyl-THF; CoFeSP, corrinoid iron–sulfur protein; Fd2−, reduced ferredoxin; * reduction of methylene-THF might occur using an electron donor with a similar redox potential as NADH
The isolated HDCR from A. woodii and T. kivui require strictly anoxic conditions which makes an application rather difficult. Using A. woodii, we have overcome this problem by establishing an efficient whole-cell system to convert H2 + CO2 to formic acid and vice versa [14, 15]. This system makes use of the ATP-dependent further conversion of formate in acetogens (Fig. 1). By lowering the cellular ATP content, formate is no longer reduced to acetate and stoichiometrically produced from H2 + CO2. However, A. woodii cannot grow on syngas or CO [20, 21] and resting cells produced only little formate from syngas and high amounts of acetate were still produced as unwanted side product [14]. In contrast, the HDCR containing thermophile T. kivui can grow in mineral medium on CO or syngas [22, 23]. Therefore, we started out to analyze hydrogenation of CO2 in a whole-cell system of T. kivui with the aim to increase productivity (due to its thermophilic nature) and to establish an efficient whole-cell biocatalyst for hydrogen storage and formate production from syngas.
Results
Formate production by T. kivui cells
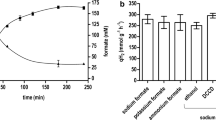
To analyze the potential use of whole cells of T. kivui as microbial cell factories for the efficient conversion of H2 + CO2 to formate, the organism was grown in complex medium with pyruvate as substrate and resting cells were prepared. As expected, the addition of H2 + CO2 to the cell suspension resulted in the production of acetate as the major end product with a specific acetate production rate of 19 mmol gprotein−1 h−1 (12 mmol gCDW−1 h−1) (Fig. 2a). Formate was only produced in low amounts at the beginning of the experiment and was consumed afterwards. This is expected since formate is an intermediate in the WLP. As seen before with A. woodii, formate accumulation requires inhibition of further formate metabolism [14]. This can be achieved by reducing the energy status of the cell (Fig. 1). Hence, formate can no longer be activated due to a lack of ATP. One possibility to uncouple the energy metabolism of cells is by using ionophores. Depending on the ionophores used, there was a variation in the formate/acetate ratio after incubation with H2 + CO2 as substrate (Fig. 2b). In contrast to A. woodii, whose energy metabolism is strictly Na+ dependent [24, 25], the Na+ ionophore ETH2120 had almost no effect on product formation in T. kivui and the dominant compound was acetate. 9.1 mM acetate was produced but only 2.3 mM formate. This is consistent with previous experiments and the assumption that H+ instead of Na+ is used as the coupling ion for the primary bioenergetics in T. kivui [26, 27]. Thus, a more favorable formate to acetate ratio of 1.7 was achieved using the protonophore 3,3,4,5-tetrachlorosalicylanilide (TCS). A four times higher formate yield was detected using the ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD). Since acetate was still produced, the membrane potential seemed to be not fully diminished by the ionophores used in this study.
Effect of ionophores/uncoupling agents and pH on product formation by resting cells of T. kivui. Cells were grown with 0.1 M pyruvate, harvested in the end-exponential growth phase and suspended in buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH 7.0 or 25 mM MES, 25 mM MOPS, 25 mM HEPES, 25 mM EPPS, 25 mM CHES, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH as indicated) to a final concentration of 1 mg/mL. a Cells were incubated without or b with ionophores/uncoupling agents or (c) at the corresponding pH for 10 min at 60 °C. The experiment was started by replacing the gas phase with H2 + CO2 (80:20%, 2 × 105 Pa) and the product formation was determined after 40 (b) or 90 min (c). Squares and white bars, acetic acid; triangles and grey bars, formic acid
Interestingly, a change in pH had a dramatic effect on the product yields (Fig. 2c). At pH 6.0, there was no formate produced, but formate production increased with increasing pH. At the same time, acetate production decreased, but to a lesser extent. This led to an inversion of the formate/acetate ratio from 0.01 at pH 6.0 to 1.7 at pH 10.
For further experiments, we added bicarbonate to resting cells to increase the available amount of CO2 in solution and to achieve higher formate yields. At 50 mM bicarbonate, the acetate formation rate was slightly increased by 24% and, more important, the transient formation of formate was also increased by 319% (Fig. 3). At 300 mM bicarbonate acetate formation was completely abolished and formate production was drastically stimulated: The formate production rate was 220 mmol g −1protein h−1 (143 mmol g −1CDW h−1) and the final formate concentration reached 126 mM after 90 min.
Effect of bicarbonate on product formation from H2 + CO2. a Resting cells of T. kivui (1 mg/mL) were incubated in anoxic buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM resazurin, pH 7.0) with H2 + CO2 (80:20%, 2 × 105 Pa). b 50 mM or c 300 mM KHCO3 were added to the cell suspensions before the experiment was started by replacing the gas phase. Squares, acetic acid; triangles, formic acid
Inhibitory effect of bicarbonate on ATP synthesis
To analyze the effect of bicarbonate on the energy metabolism of T. kivui in detail, the cellular ATP content of resting cells was measured in the presence or absence of bicarbonate (Fig. 4a). Therefore, cells were incubated in buffer with H2 + CO2 as substrate, increasing bicarbonate concentrations were added and the ATP content was measured over time. As seen in Fig. 4a, the ATP content dropped immediately to zero if 300 mM bicarbonate was present in the cell suspensions. At 50 mM bicarbonate, there was also a decrease in the intracellular ATP content, but only by 62%. Next, we investigated the effect of bicarbonate on the activity of the membrane-bound ATPase in T. kivui (Fig. 4b). After the preparation of membranes, ATP hydrolysis was measured in the presence or absence of bicarbonate. Indeed, ATP hydrolysis as catalyzed by membranes (138 mU/mg) was inhibited by 81% by 300 mM NaHCO3. The same was observed with KHCO3. Additionally, we examined the ability of ATP synthesis by cell suspensions of T. kivui with an artificial ∆pH over the membrane as driving force (Additional file 1: Figure S1). In this experiment, resting cells were incubated in the presence or absence of 300 mM KHCO3 and then HCl was added to induce a ∆pH across the membrane. At a ∆pH of 6, ATP was synthesized to 3.2 nmol mg −1protein . In contrast, when cells were incubated with 300 mM KHCO3, ATP was only synthesized to 1.1 nmol mg −1protein . In accordance with the ATP hydrolysis experiments, only 34% of the ATP was synthesized in the presence of bicarbonate. Overall, these experiments could be interpreted to mean that the ATP synthase is inhibited by bicarbonate.
Effect of bicarbonate on the ATP content of resting cells and ATP hydrolysis catalyzed by membranes of T. kivui. a Resting cells of T. kivui (1 mg/mL) were incubated in anoxic buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH 7,0) with H2 + CO2 (80:20%, 2 × 105 Pa) in the absence or presence of KHCO3 and the ATP content of cells were determined. Squares, without bicarbonate; triangles, 50 mM KHCO3; diamonds, 300 mM KHCO3. b Membranes from T. kivui were incubated for 3 min in the presence (300 mM KHCO3 or 300 mM NaHCO3) or absence of bicarbonate in buffer (100 mM Tris/HCl, 20 mM MgSO4, 20 mM KCl, pH 7.0) and the ATP hydrolysis of membranes was determined
A possible pH effect by the addition of bicarbonate to the cell suspension was excluded. Therefore, the pH was adjusted in the control experiments with KOH to the same pH as in cell suspensions with additional 300 mM bicarbonate. The change in pH from 7.0 to 8.2 by the addition of KOH did not result in the same formate production. Only 14 mM of formate was formed after 90 min (data not shown).
Characterization of hydrogen-dependent CO2 reduction by whole cells
After the discovery that bicarbonate completely inhibits further downstream processing of formate, formate production from H2 + CO2 was studied in detail in the presence of 300 mM KHCO3. The cells showed highest specific formate production rates of 220 mmol g −1protein h−1 (143 mmol g −1CDW h−1) at a temperature of 60 °C (Fig. 5a). Nevertheless, even at moderate reaction temperature of 30 °C, there was still a catalytic activity of 58 mmol g −1protein h−1 (38 mmol g −1CDW h−1). Moreover, an increase of the specific formate production rate up to 234 mmol g −1protein h−1 (152 mmol g −1CDW h−1) was observed at a cell concentration of 0.5 mg mL−1 (Fig. 5b). Increasing cell densities resulted in a linear increase of the volumetric formate production rates up to 270 mmol L−1 h−1 at 4 mg mL−1. Simultaneously, the specific rates decreased.
Characterization of hydrogen-dependent CO2 reduction by whole cells of T. kivui. a Resting cells of T. kivui (1 mg/mL) were incubated in anoxic buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH 7.0) with H2 + CO2 (80:20%, 2 × 105 Pa) in the presence of 300 mM KHCO3. Shown is the temperature profile for formate production by whole cells at the temperature indicated. b The influence of the cell density on formate production by resting cells was determined by applying a final concentration of 0.5–4 mg mL−1 in anoxic serum bottles at a temperature of 60 °C. 300 mM KHCO3 was added to the cell suspension and the experiments were started by replacing the gas phase with H2 + CO2 (80:20%, 2 × 105 Pa). The initial formate production rates (squares) and the volumetric production rates (triangle) were plotted against the cell density used in the experiment
Thermoanaerobacter kivui is a promising organism for industrial applications, since it can grow on syngas/CO in mineral medium without the requirement for yeast extract and additional vitamins [22, 28]. Therefore, we investigated the specific formate production rate of resting cells that were grown on mineral medium with pyruvate or glucose as growth substrate (Additional file 1: Figure S2). No differences in the specific formate production rates were observed if the complex medium was replaced by defined mineral medium in the cultivation process. Glucose-grown cells (in mineral medium) showed a slight decrease of 33% in the specific formate production rate compared to pyruvate grown cells.
Syngas conversion to formate
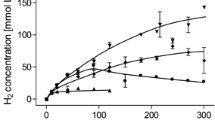
Syngas is an increasingly considered “green” option for the production of chemicals and biofuels [1] and T. kivui was already shown to grow on CO or syngas [22]. To analyze whether syngas is converted to formate, cells were grown on 50% CO and cell suspensions were prepared. A syngas mixture of H2 (26%), CO2 (11%) and CO (63%) was used as substrate. The gas consumption in the head space of the serum bottles was monitored by gas chromatography. In the absence of bicarbonate, resting cells converted syngas to acetate (Fig. 6a, b). Notably, the CO concentration decreased by 99 mM. At the same time, H2 and CO2 increased by only 26 and 74 mM, indicating that CO and H2 were used as reductant for CO2. If additional bicarbonate was added to the cell suspension, the product spectrum changed and mainly formate was produced in high titers up to 130 mM (Fig. 6c, d). The specific formate production rate was 8 mmol g −1protein h−1 (5 mmol g −1CDW h−1). CO was almost completely used up but the hydrogen level remained almost the same. This indicates that H2 is not oxidized in the presence of CO and an alternative electron donor seems to be used for the reduction of CO2 to formate. Additionally, a clear increase in the CO2 concentration was detectable, provoked by the interconversion of HCO3− to CO2. This is the first demonstration that syngas can be converted exclusively to formate by an acetogenic bacterium. T. kivui cells which were not adapted on CO, instead grown heterotrophic with pyruvate as substrate, showed only a little formation of acetate and almost no formate was produced in the presence of bicarbonate.
Formate production from syngas using whole cells of T. kivui. Cells were grown on 50% CO in complex medium, harvested in the end-exponential growth phase and suspended in buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH 7,0) to a final concentration of 1 mg/mL in anoxic serum bottles. The experiment was started by replacing the gas phase with a mixture of H2 + CO2 + CO (26:11:63%, 2 × 105 Pa) a, b in the absence of bicarbonate and c, d in the presence of 300 mM KHCO3. The product formation in liquid (a), c and the gas consumption in the head space (b, d) is shown over time. Squares, acetic acid; triangles, formic acid; diamonds, CO; triangles down, CO2; circles, H2
Formate production in closed-batch fermentation
Next, we wanted to establish a production platform for formate in closed-batch fermentation (Fig. 7). Here, T. kivui cells were grown in defined mineral medium with 28 mM glucose as substrate (tD = 3.2 h) to an optical density of ~ 0.3. Then, bicarbonate, H2 + CO2 or a combination of both were added. The addition of bicarbonate led to an immediate growth arrest and stop of acetate formation. By adding H2 + CO2, the optical density did not increase but cells produced more acetate. Formate was not produced overall. Now, when bicarbonate and H2 + CO2 were added, growth as well as acetate formation was completely abolished, but cells started to produce formate. The specific rate of formate production was 96 mmol g −1protein h−1 (62 mmol g −1CDW h−1). Finally, up to 50 mM formate was produced in the cultivation broth.
Closed-batch fermentation for hydrogen-dependent CO2 reduction to formic acid. aT. kivui was grown on 28 mM glucose in a defined mineral medium in a shaking water bath at 66 °C. At the time point indicated b 300 mM KHCO3, c H2 + CO2 (80:20%, 2 × 105 Pa) or d H2 + CO2 (80:20%, 2 × 105 Pa) + 300 mM KHCO3 were added to the growing culture. The optical density of the culture was between 0.3 and 0.4. Squares, OD600; diamonds, acetic acid; triangles, formic acid
Discussion
Resting cells of T. kivui were proven in this study as highly efficient whole-cell biocatalysts for the direct hydrogenation of CO2 to formate with remarkable catalytic activities. In addition, we showed the first whole-cell approach for the exclusive conversion of syngas to formate using an acetogenic bacterium. The recently identified hydrogen-dependent CO2 reductase (HDCR) [13] is the key enzyme in whole cell of T. kivui used as microbial cell factories for hydrogen storage, CO2 capturing and syngas conversion to formate.
Since the K value for Eq. 1
is close to one, the chemical equilibrium can be easily controlled by small variations in pH, pressure and substrate/product concentrations. High concentrations of formate will favor the backwards reaction. An alkaline environment serves as proton scavenger and therefore pulls the reaction to the product side. The favored formate formation from H2 + CO2 in a more alkaline environment was also observed in a whole-cell system for hydrogen-dependent CO2 reduction based on E. coli [29]. By the addition of bicarbonate to resting cells, the available amount of CO2 in solution was increased and the reaction was pushed towards product formation. Since formate dehydrogenases of the Mo/W-bis PGD family are known to use only CO2 and not bicarbonate as substrate [12], we inspected the genome of T. kivui to identify a putative carbonic anhydrase (CA) which catalyzes the rapid interconversion of HCO3− and CO2, and found one gene annotated as putative carbonic anhydrase/acetyltransferase (TKV_c11400). Consistent with this, cell extracts of T. kivui had specific CA activity of 0.17 U/mg [30].
Bicarbonate was identified here as an inhibitor of ATP synthesis. The inhibition of bacterial F1F0 ATP synthases by bicarbonate is not a common feature, but the effect was already described in literature [31, 32]. The effect of different anions like sulfite, azide and bicarbonate on the ATPase activity of membrane-bound F1F0 is known for decades but a detailed understanding of the mechanism of action of the activating anions is still missing and a matter of controversy [33,34,35,36]. In our study potassium bicarbonate could be replaced by sodium bicarbonate, indeed indicating an inhibitory effect of the anion HCO3−. Lodeyro et al. concluded in their study [32] that the anion bicarbonate competes with the binding of ADP to a low-affinity binding site instead of binding to a Pi site in the F1 subunit. They postulated that ATP hydrolysis and inhibition of ATP synthesis was affected by bicarbonate by modulating the relative affinities of the catalytic site for ATP and ADP. Since anions like bicarbonate and acid were shown to bind to different sites on the mitochondrial F1 subunit, further studies for the direct identification of the HCO3− binding site on the F1F0 ATP synthase of T. kivui have to be done. Purification and characterization of this enzyme could help to finally elucidate the mechanism and site of action of bicarbonate.
Whole-cell biocatalysis for the production of formate from the greenhouse gas CO2 and the energy carrier H2 was also observed in other biological systems [14, 29, 37]. Besides the acetogenic bacteria A. woodii and T. kivui, the well-known model organism E. coli was also used as a cell factory for the hydrogenation of CO2 [29]. The key enzyme in E. coli to catalyze H2 + CO2 conversion to formate is the membrane-bound formate hydrogen lyase (FHL) complex [18, 38]. But this enzyme is designed by nature to produce H2 and CO2 from formate under fermentative conditions and therefore, the catalytic rates for formate formation are pretty low and harsh conditions are required for the reaction. In a pH-controlled and highly pressurized reactor system (up to 10 bar overpressure), the specific formate production rates were 15 mmol g −1CDW h−1 [29]. This is only a small fraction of the activity of whole cells from T. kivui at moderate conditions of 30 or 60 °C with one bar overpressure. Here, the cells showed specific formate production rates of 58 mmol g −1protein h−1 (38 mmol g −1CDW h−1) and 220 mmol g −1protein h−1 (143 mmol g −1CDW h−1), respectively, qualifying T. kivui for applications at high and moderate reaction temperatures. Nevertheless, the thermophilic acetogenic bacterium T. kivui showed the highest specific formate production rates of 234 mmol g −1protein h−1 (152 mmol g −1CDW h−1) ever reported in biological systems (Table 1).
Furthermore, the volumetric formate production rates of 270 mmol L−1 h−1 at cell concentrations of 4 mg mL−1 is not an insignificant economical factor: implementing high cell densities in a later fermentation process is considered to be one of the most effective ways for enhancing the productivity [40]. Efficient cell recycling and cell retention systems with optimized conditions for the accumulation of high cell densities up to 200 g/L were already implemented in bioprocesses [41,42,43].
The fermentation of syngas into biofuels and biochemicals using acetogenic bacteria has attracted more and more interest over the last few years and some acetogens were already implemented in this process [44,45,46,47]. Since the syngas composition depends strongly on the kind of gasifier and the kind and condition of the feedstock used, there is no “universal” composition of syngas. But it was already shown that T. kivui can be adapted to a carboxydotrophic lifestyle by a stepwise adaptation on increasing CO concentrations, up to 100% CO [22]. A detailed understanding of the CO metabolism in T. kivui is still missing. Since CO is a potent inhibitor of the active site of [FeFe]-hydrogenases [48,49,50], the HDCR hydrogenase subunit should be inactive and no formate should be formed. The inhibitory effect of CO on the HDCR hydrogenase activity of A. woodii was already described [14]. However, reduced ferredoxin can serve as an alternative electron donor for the reduction of CO2 to formate in in vitro studies. This correlates with the finding that H2 was not utilized by T. kivui in the previous syngas experiment if CO was present but formate was still produced. Therefore, the two annotated CO dehydrogenases genes in the genome of T. kivui could play a key role in the oxidation of CO to CO2 with simultaneous reduction of ferredoxin, which is subsequently used by the HDCR for a ferredoxin-driven CO2 reduction to formate.
In this study, we showed the feasibility of two approaches for the efficient conversion of H2 + CO2 to formate: whole-cell biocatalysis and closed-batch bioprocess/fermentation. But the production rates as well as the finally produced formate concentration differed between the two approaches. The reasons could be diverse and are probably linked to pH, buffer capacity, feedback inhibition, etc. The applicability of growing cells as microbial cell factories has to be proven in further fermentation studies. Nevertheless, the addition of bicarbonate and H2 + CO2 can switch the growing culture to the production of formate instead of acetate. The gases H2 + CO2 can also serve in the first phase as growth substrate till the production phase is initiated. In this production phase, H2 + CO2 act as reactants for the efficient production of formate. Whether the minimized cost-intensive and time-consuming work flow in a closed-batch fermentation can rebalance the increasing downstream costs due to the accumulation of unwanted metabolic side products (e.g., acetate) in the fermentation broth during the growth phase has to be considered and individually calculated.
Conclusion
This work demonstrates an efficient whole-cell approach for the production of formate from H2 + CO2 or syngas using the thermophilic acetogen T. kivui. Bicarbonate seems to be an efficient inhibitor of the ATP synthase of this organism, thus preventing further downstream conversion of formate to acetate, resulting in high titers of the desired end product. T. kivui catalyzed the hydrogen-dependent CO2 reduction with remarkable catalytic activities at elevated and ambient temperatures. Its thermophilic nature and the autotrophic growth properties on mineral medium qualify this organism for future fermentation approaches to address the process on a larger scale and to investigate the stability of the whole-cell system.
Methods
Organism and cultivation
Thermoanaerobacter kivui LKT-1 (DSM 2030) was cultivated at 66 °C under anaerobic conditions in complex and defined mineral medium [22]. Media were prepared under anoxic conditions as described before [51, 52]. Glucose (28 mM), pyruvate (100 mM) or CO (50% CO, 40% N2 and 10% CO2 [v/v] at 2 × 105 Pa) were used as growth substrate. Cell were cultivated in 1-L flasks (Müller-Krempel, Bülach, Switzerland) containing 500 mL or 200 mL medium in the case of autotrophic cultivation. Growth was determined by measuring the optical density at 600 nm with an UV/Vis spectrophotometer.
Preparation of resting cells and cell suspension experiments
For the preparation of resting cells, T. kivui was cultivated in 1-L flasks (Müller-Krempel, Bülach, Switzerland) in the above-mentioned growth media to the late exponential growth phase. Glucose- and fructose-grown cells were harvested at an OD600 of 1.7–2.0, CO-grown cells were harvested at OD600 of 0.6. The culture was centrifuged under anoxic conditions at 11,500 g and 4 °C for 10 min and was washed twice in imidazole puffer (50 mM imidazole–HCl, 20 mM MgSO4, 20 mM KCl, 2 mM DTE, 4 µM resazurin, pH 7.0). Afterwards, the cells were resuspended, if not otherwise stated, in the same buffer to a protein concentration of 1 mg/mL and kept in gas-tight Hungate tubes. All preparation steps were performed under strictly anoxic conditions at room temperature in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI) as described [53]. The protein concentration of the cell suspension was determined according to [54] and the cells were directly used for the subsequent cell suspension experiments.
To determine the conversion of H2 + CO2 in cell suspension experiments, the 120-mL serum flasks (Glasgerätebau Ochs GmbH, Bovenden-Lenglern, Germany) contained pre-warmed buffer under a N2 atmosphere, incubated with cell suspensions for 10 min at 60 °C. Subsequently, bicarbonate (KHCO3 or NaHCO3) was added and the gas phase of the serum flasks was changed to 2 × 105 Pa H2 + CO2 (80:20 [v/v]). When syngas was the substrate, the reaction was started by replacing the head space of the serum flasks with a gas composition of 26% H2 + 11% CO2 + 63% CO [v/v] at 2 × 105 Pa. Ionophores and uncoupling agents such as 3,3,4,5-tetrachlorosalicylanilide (TCS, dissolved in EtOH), N,N,N′,N′-tetracyclohexyl-1,2-phenylenedioxydiacetamide (ETH2120, dissolved in EtOH), gramicidin (dissolved in EtOH) and N,N′-dicyclohexylcarbodiimide (DCCD, dissolved in EtOH) were added 10 min prior to the reaction start. The serum flasks contained a final volume of 10 mL buffer in all the experiments. Samples were taken and ATP [55], acetate, formate, H2, CO2 and CO were determined as described before [13, 22].
Preparation of membranes and measurement of ATP hydrolysis activity
Cells were grown in 500 mL complex medium in 1-L flasks (Glasgerätebau Ochs, Bovenden-Lenglern, Germany) with 100 mM pyruvate as carbon source to an optical density at 600 nm of 1.7–2.0. The cells were harvested under toxic conditions at 11,500g for 10 min at 4 °C, were washed twice in buffer A (50 mM imidazole–HCl, 20 mM MgSO4, 20 mM KCl, pH 7.0) and membranes were prepared as described before [26]. The protein concentration was determined as described [56] and the membranes were directly used to measure ATP hydrolysis.
For the determination of the ATP hydrolysis, membranes (200 µg) were resuspended in buffer B (100 mM Tris/HCl, 20 mM MgSO4, 20 mM KCl, pH 7.0) to a final volume of 1200 µL and incubated at 60 °C for 3 min in the presence or absence of 300 mM KHCO3. After addition of 2.5 mM Na2ATP, samples (200 µL) were taken at defined time points and the ATP content was determined as described [55].
Closed-batch fermentation
Thermoanaerobacter kivui was grown at 66 °C in 50 mL mineral medium in 120 mL serum flasks (Glasgerätebau Ochs GmbH, Bovenden-Lenglern, Germany) with 28 mM glucose as growth substrate and a gas phase of N2 + CO2 (80:20 [v/v]). At OD600 0.3–0.4 the growing cells were switched into the formate production phase by addition of 300 mM KHCO3 and by changing the gas phase to a H2 + CO2 (80:20% [v/v]) atmosphere. Samples for the product determination were taken with a syringe.
Determination of cell dry weight
For cell dry weight determination of T. kivui, three independent cultures were grown in complex medium with 0.1 M pyruvate as growth substrate. At three different optical densities in the exponential growth phase the culture was harvested (4150g, 30 min, 4 °C) in technical triplicates (3 × 50 mL). Afterwards, the cell pellet was frozen in liquid N2 and dried by lyophilisation over 24 h. The dried samples were weighted and the cell dry weight (CDW) was calculated to 0.379 mg/mL at OD600 of 1.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- LOHC:
-
Liquid organic hydrogen carrier
- HDCR:
-
Hydrogen-dependent CO2 reductase
- A. woodii :
-
Acetobacterium woodii
- T. kivui :
-
Thermoanaerobacter kivui
- CDW:
-
Cell dry weight
- FHL:
-
Formate hydrogen lyase
- E. coli :
-
Escherichia coli
- ETH2120:
-
N,N,N′,N′-Tetracyclohexyl-1,2-phenylenedioxydiacetamide
- TCS:
-
3,3,4,5-Tetrachlorosalicylanilide
- DCCD:
-
N,N′-Dicyclohexylcarbodiimide
- MES:
-
2-Morpholin-4-ylethanesulfonic acid
- MOPS:
-
3-Morpholinopropane-1-sulfonic acid
- HEPES:
-
2-[4-(2-Hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- EPPS:
-
4-(2-Hydroxyethyl)piperazine-1-propanesulfonic acid
- CHES:
-
2-(Cyclohexylamino)ethanesulfonic acid
- Tris:
-
2-Amino-2-(hydroxymethyl)propane-1,3-diol
- DTE:
-
Dithioerythritol
- HCO3− :
-
Bicarbonate
- t D :
-
Doubling time
- K :
-
Equilibrium constant
- \(\Delta G^{{0\varvec{'}}}\) :
-
Gibbs energy
- Mo/W-bis PGD:
-
Molybdenum/tungsten-bis pyranopterin guanosine dinucleotide
- CA:
-
Carbonic anhydrase
- UV/Vis:
-
Ultraviolet/visible
- OD:
-
Optical density
References
Munasinghe PC, Khanal SK. Biomass-derived syngas fermentation into biofuels: opportunities and challenges. Bioresour Technol. 2010;101:5013–22.
Mondal M, Goswami S, Ghosh A, Oinam G, Tiwari ON, Das P, Gayen K, Mandal MK, Halder GN. Production of biodiesel from microalgae through biological carbon capture: a review. 3 Biotech. 2017;7:99.
Bengelsdorf FR, Poehlein A, Linder S, Erz C, Hummel T, Hoffmeister S, Daniel R, Dürre P. Industrial acetogenic biocatalysts: a comparative metabolic and genomic analysis. Front Microbiol. 2016;7:1036.
Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, Snoeyenbos-West OL, Lovley DR. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl Environ Microbiol. 2011;77:2882–6.
Müller V. New horizons in acetogenic conversion of one-carbon substrates and biological hydrogen storage. Trends Biotechnol. 2019;37:1344–54.
Bengelsdorf FR, Straub M, Dürre P. Bacterial synthesis gas (syngas) fermentation. Environ Technol. 2013;34:1639–51.
Daniell J, Köpke M, Simpson SD. Commercial biomass syngas fermentation. Energies. 2012;5:5372–417.
Drake HL. Acetogenesis, acetogenic bacteria, and the acetyl-CoA pathway: past and current perspectives. In: Drake HL, editor. Acetogenesis. New York: Chapman and Hall; 1994. p. 3–60.
Müller V, Inkamp F, Rauwolf A, Küsel K, Drake HL. Molecular and cellular biology of acetogenic bacteria. In: Nakano M, Zuber P, editors. Strict and facultative anaerobes Medical and environmental aspects. Norfolk: Horizon Scientific Press; 2004. p. 251–81.
Wang S, Huang H, Kahnt J, Mueller AP, Köpke M, Thauer RK. NADP-specific electron-bifurcating [FeFe]-hydrogenase in a functional complex with formate dehydrogenase in Clostridium autoethanogenum grown on CO. J Bacteriol. 2013;195:4373–86.
Yamamoto I, Saiki T, Liu SM, Ljungdahl LG. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983;258:1826–32.
Maia LB, Moura JJ, Moura I. Molybdenum and tungsten-dependent formate dehydrogenases. J Biol Inorg Chem. 2015;20:287–309.
Schwarz FM, Schuchmann K, Müller V. Hydrogenation of CO2 at ambient pressure catalyzed by a highly active thermostable biocatalyst. Biotechnol Biofuels. 2018;11:237.
Schuchmann K, Müller V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science. 2013;342:1382–5.
Kottenhahn P, Schuchmann K, Müller V. Efficient whole cell biocatalyst for formate-based hydrogen production. Biotechnol Biofuels. 2018;11:93.
Preuster P, Papp C, Wasserscheid P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc Chem Res. 2017;50:74–85.
Enthaler S, von Langermann J, Schmidt T. Carbon dioxide and formic acid-the couple for environmental-friendly hydrogen storage? Energy Environ Sci. 2010;3:1207–17.
McDowall JS, Murphy BJ, Haumann M, Palmer T, Armstrong FA, Sargent F. Bacterial formate hydrogenlyase complex. Proc Natl Acad Sci USA. 2014;111:E3948–56.
Pinske C, Sargent F. Exploring the directionality of Escherichia coli formate hydrogenlyase: a membrane-bound enzyme capable of fixing carbon dioxide to organic acid. Microbiologyopen. 2016;5:721–37.
Bertsch J, Müller V. CO metabolism in the acetogen Acetobacterium woodii. Appl Environ Microbiol. 2015;81:5949–56.
Bertsch J, Müller V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol Biofuels. 2015;8:210.
Weghoff MC, Müller V. CO metabolism in the thermophilic acetogen Thermoanaerobacter kivui. Appl Environ Microbiol. 2016;82:2312–9.
Schölmerich MC, Müller V. Energy conservation by a hydrogenase-dependent chemiosmotic mechanism in an ancient metabolic pathway. Proc Natl Acad Sci USA. 2019;116:6329–34.
Biegel E, Müller V. Bacterial Na+-translocating ferredoxin:NAD+ oxidoreductase. Proc Natl Acad Sci USA. 2010;107:18138–42.
Müller V, Aufurth S, Rahlfs S. The Na+ cycle in Acetobacterium woodii: identification and characterization of a Na+-translocating F1FO-ATPase with a mixed oligomer of 8 and 16 kDa proteolipids. Biochim Biophys Acta. 2001;1505:108–20.
Hess V, Poehlein A, Weghoff MC, Daniel R, Müller V. A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genomics. 2014;15:1139.
Yang H, Drake HL. Differential effects of sodium on hydrogen- and glucose-dependent growth of the acetogenic bacterium Acetogenium kivui. Appl Environ Microbiol. 1990;56:81–6.
Leigh JA, Mayer F, Wolfe RS. Acetogenium kivui, a new thermophilic hydrogen-oxidizing, acetogenic bacterium. Arch Microbiol. 1981;129:275–80.
Roger M, Brown F, Gabrielli W, Sargent F. Efficient hydrogen-dependent carbon dioxide reduction by Escherichia coli. Curr Biol. 2018;28:140–5.
Braus-Stromeyer SA, Schnappauf G, Braus GH, Gössner AS, Drake HL. Carbonic anhydrase in Acetobacterium woodii and other acetogenic bacteria. J Bacteriol. 1997;179:7197–200.
Das A, Ljungdahl LG. Clostridium pasteurianum F1FO ATP synthase: operon, composition, and some properties. J Bacteriol. 2003;185:5527–35.
Lodeyro AF, Calcaterra NB, Roveri OA. Inhibition of steady-state mitochondrial ATP synthesis by bicarbonate, an activating anion of ATP hydrolysis. Biochim Biophys Acta. 2001;1506:236–43.
Ebel RE, Lardy HA. Stimulation of rat liver mitochondrial adenosine triphosphatase by anions. J Biol Chem. 1975;250:191–6.
Recktenwald E, Hess B. Allosteric influence of anions on mitochondrial ATPase of yeast. FEBS Lett. 1977;76:25–8.
Du ZY, Boyer PD. On the mechanism of sulfite activation of chloroplast thylakoid ATPase and the relation of ADP tightly bound at a catalytic site to the binding change mechanism. Biochemistry. 1990;29:402–7.
Hartog AF, Edel CM, Braham J, Muijsers AO, Berden JA. FSBA modifies both α- and β-subunits of F1 specifically and can be bound together with AXP at the same alpha-subunit. Biochim Biophys Acta. 1997;1318:107–22.
Alissandratos A, Kim HK, Easton CJ. Formate production through carbon dioxide hydrogenation with recombinant whole cell biocatalysts. Bioresour Technol. 2014;164:7–11.
Stephenson M, Stickland LH. Hydrogenlyases: bacterial enzymes liberating molecular hydrogen. Biochem J. 1932;26:712–24.
Mourato C, Martins M, da Silva SM, Pereira IAC. A continuous system for biocatalytic hydrogenation of CO2 to formate. Bioresour Technol. 2017;235:149–56.
Lin B, Tao Y. Whole-cell biocatalysts by design. Microb Cell Fact. 2017;16:106.
Mauerhofer LM, Pappenreiter P, Paulik C, Seifert AH, Bernacchi S, Rittmann SKR. Methods for quantification of growth and productivity in anaerobic microbiology and biotechnology. Folia Microbiol. 2019;64:321–60.
Richter K, Nottelmann S. An empiric steady state model of lactate production in continuous fermentation with total cell retention. Eng Life Sci. 2004;4:426–32.
Okabe M, Oda A, Park YS, Noguchi K, Okamoto T, Mitsui S. Continuous beer fermentation by high cell-density culture of bottom brewer’s yeast. J Ferment Bioeng. 1994;77:41–5.
Henstra AM, Sipma J, Rinzema A, Stams AJ. Microbiology of synthesis gas fermentation for biofuel production. Curr Opin Biotechnol. 2007;18:200–6.
Liew F, Martin ME, Tappel RC, Heijstra BD, Mihalcea C, Köpke M. Gas fermentation-a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front Microbiol. 2016;7:694.
Liew F, Henstra AM, Köpke M, Winzer K, Simpson SD, Minton NP. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab Eng. 2017;40:104–14.
Köpke M, Held C, Hujer S, Liesegang H, Wiezer A, Wollherr A, Ehrenreich A, Liebl W, Gottschalk G, Dürre P. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc Natl Acad Sci USA. 2010;107:13087–92.
Lubitz W, Ogata H, Rudiger O, Reijerse E. Hydrogenases. Chem Rev. 2014;114:4081–148.
Goldet G, Brandmayr C, Stripp ST, Happe T, Cavazza C, Fontecilla-Camps JC, Armstrong FA. Electrochemical kinetic investigations of the reactions of [FeFe]-hydrogenases with carbon monoxide and oxygen: comparing the importance of gas tunnels and active-site electronic/redox effects. J Am Chem Soc. 2009;131:14979–89.
Baffert C, Bertini L, Lautier T, Greco C, Sybirna K, Ezanno P, Etienne E, Soucaille P, Bertrand P, Bottin H, et al. CO disrupts the reduced H-cluster of FeFe hydrogenase. A combined DFT and protein film voltammetry study. J Am Chem Soc. 2011;133:2096–9.
Bryant MP. Commentary on the Hungate technique for culture of anaerobic bacteria. The American journal of clinical nutrition. 1972;25:1324–8.
Hungate RE. A roll tube method for cultivation of strict anaerobes. In: Norris JR, Ribbons DW, editors. Methods in microbiology, vol. 3b. New York and London: Academic Press; 1969. p. 117–32.
Heise R, Müller V, Gottschalk G. Presence of a sodium-translocating ATPase in membrane vesicles of the homoacetogenic bacterium Acetobacterium woodii. Eur J Biochem. 1992;206:553–7.
Schmidt K, Liaaen-Jensen S, Schlegel HG. Die Carotinoide der Thiorhodaceae. Arch Mikrobiol. 1963;46:117–26.
Kimmich GA, Randles J, Brand JS. Assay of picomole amounts of ATP, ADP and AMP using the luciferase enzyme system. Anal Biochem. 1975;69:187–206.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–75.
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No 741791).
Author information
Authors and Affiliations
Contributions
VM designed and supervised the research, analyzed the data and wrote the manuscript. FMS designed the research, performed the experiments, analyzed the data and wrote the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
ATP synthesis by cell suspensions of T. kivui driven by an artificial ∆pH. Cells were grown with 0.1 M pyruvate, harvested in the end-exponential growth phase and suspended in buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH 7.0). Cell suspensions (1 mg/mL) were incubated with and without KHCO3 for 10 min in buffer (25 mM Tris/HCl, 20 mM MgCl2, pH 9.0) at 60 °C. At the time point indicated (arrow), HCl was added to the cell suspensions. Shown are data from one representative experiment out of two independent replicates. Squares, without KHCO3; triangles, 300 mM KHCO3.
Additional file 2: Figure S2.
Specific formate production rates of resting cells from T. kivui grown on mineral medium. Cells were grown with 28 mM glucose or 0.1 M pyruvate in a defined mineral or complex medium, harvested in the end-exponential growth phase and suspended in buffer (50 mM Imidazole, 20 mM KCl, 20 mM MgSO4, 2 mM DTE, 4 µM Resazurin, pH 7.0) to a final concentration of 1 mg/mL in anoxic serum bottles. The bottles were incubated in a shaking water bath for 10 min at 60 °C with additional 300 mM KHCO3. The experiment was started by replacing the gas phase with H2 + CO2 (80:20%, 2 × 105 Pa). MM mineral medium, CM complex medium.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schwarz, F.M., Müller, V. Whole-cell biocatalysis for hydrogen storage and syngas conversion to formate using a thermophilic acetogen. Biotechnol Biofuels 13, 32 (2020). https://doi.org/10.1186/s13068-020-1670-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-020-1670-x