Abstract
The presence of minor components represents a challenging problem in spectrophotometric analysis of pharmaceuticals. If one component has a low absorptivity or present in a low concentration compared to the other components, this will hinder its quantitation by spectrophotometric methods. Continuous Wavelet Transform (CWT) as a signal processing technique was utilized to figure out a solution to such a problem. A comparative study was established between traditional derivative spectrophotometry (Numerical Differentiation, ND) and CWT to indicate the advantages and limitations of each technique and possibility of solving the problem of minor components. A mixture of ibuprofen (IBU) and phenylephrine (PHE) with its degradation products forming a ternary mixture was used for comparing the two techniques. The two techniques were applied on raw spectral data and on ratio spectra data resulting in four methods, namely ND, CWT, Derivative Ratio-Zero Crossing (DRZC) and Continuous Wavelet Transform Ratio-Zero Crossing (CWTR-ZC) methods. By comparing the results in laboratory prepared mixtures, CWT technique showed advantages in analysis of mixtures with minor components than ND. The proposed methods were validated according to the ICH guideline Q2(R1), where their linearity was established with correlation coefficient ranging from 0.9995 to 0.9999. The linearity was in the range 3–40 μg/mL for PHE in all methods, while for IBU it was 20–180 and 30–180 μg/mL in CWT and ND methods, respectively. The CWT methods were applied for quantitative determination of the drugs in their dosage form showing the ability of the methods to quantitate minor components in pharmaceutical formulations.
Similar content being viewed by others
Introduction
Spectrophotometry is one of the most commonly used techniques due to the availability of its instruments, simplicity and speed. UV–vis absorption spectroscopy is a well-established technique for rapid and accurate determination of analytes in mixtures without prior separation if the interferences between the spectra can be eliminated.
Several techniques can be used for elimination of interferences in spectroscopy such as mathematical manipulations [1,2,3], chemometrics [4,5,6] and signal processing. The most commonly used techniques are signal processing ones, of which the most popular is Derivative Spectrophotometry (DS). The first use of DS was in mass spectroscopy [7], then it was applied to UV–vis and IR spectroscopy [8]. DS is a powerful method used in analytical chemistry to eliminate background interference, resolve spectra, sharpen peaks and carry out quantitative analysis. It has a significant role in resolution of overlapped UV–vis spectra [9]. In spectral analysis, application of ND to the absorption spectra has several drawbacks such as: diminishing peak intensity, requiring smooth and scaling factor functions. Therefore, the obtained derivative spectra are deformed from the original ones, and consequently, the traditional ND may generate errors in analysis [10].
As a result, alternatives for derivative calculation were suggested to overcome these drawbacks. Least-squares procedures for the smoothing and differentiation of spectral data were introduced by Savitzky and Golay [11]. Wahbi et al. [12] applied Fourier functions for spectrophotometric quantitation of mixtures (discrete Fourier transform). Mean centering was also introduced as a processing technique for ratio spectra, and it was applied for the analysis of binary and ternary mixtures [13,14,15]. Finally, wavelet transform represents a powerful tool for signal processing that can be used for spectrophotometric analysis of mixtures [16].
The wavelet transform process performs decomposition of the spectrum into simpler, fixed building blocks at different scales and positions [16]. In the field of UV–vis spectrophotometry, Continuous wavelet transform (CWT) combined either with a zero-crossing technique or ratio spectra was used for simultaneous determination of chemical species in binary and ternary mixtures [17,18,19].
In addition to the common problem of spectral overlapping and interference that hinders the use of spectrophotometric methods in the analysis of pharmaceutical mixtures, the problem of minor components arises as a new challenge for these methods of analysis. This problem arises when the pharmaceutical formulation contains one drug in a low concentration or has very low absorptivity compared to the other components. This will make the resolution of the spectrum of such drug more difficult and will require special treatment of the dosage form during analysis to allow its quantitation such as spiking technique [20, 21]. The need for higher amounts of standards and the tedious process are the reasons why spiking is no longer the ideal solution for such a problem. New approaches emerged for solving this challenging problem such as absorptivity target concentration values [22], response correlation and advanced balance point-spectrum subtraction methods [23].
Ibuprofen (IBU), (2RS)-2-[4-(2-Methylpropyl)phenyl]propanoic acid [24] (Fig. 1), is a nonsteroidal anti-inflammatory drug that is used for treating fever and pain. It acts mainly through inhibiting the cyclooxygenase-2 enzyme [25]. Phenylephrine (PHE), (1R)-1-(3-Hydroxyphenyl)-2-(methylamino)ethanol [24] (Fig. 1), is primarily used as a decongestant, to dilate the pupil, to increase blood pressure, and to relieve hemorrhoids [26]. The combination dosage form of IBU and PHE is used to treat stuffy nose, sinus congestion, headache, fever, and minor aches and pains caused by the common cold and flu [27]. It is available in the market in the form of coated tablets under trade name Grippostad®. Each tablet of Grippostad® contains 200 mg of IBU and 5 mg of PHE, the ratio is 40:1. This ratio is a prime example of the problem of minor components.
Scientific reports showed several degradation studies using multi-component UV–vis spectroscopy and estimation of NSAIDs [28,29,30,31]. Yet, few methods were reported for assaying IBU and PHE simultaneously in their binary combination [32,33,34,35]. The two drugs were subjected to stability studies in their mixtures with other drugs [36,37,38,39,40,41,42], but no method was developed for the stability indicating assay of this binary mixture. During literature survey, IBU was found to be susceptible to strong oxidative and thermal degradation [40,41,42]. PHE was reported to be more stable toward acidic, alkaline, and thermal conditions [36,37,38,39]. PHE is also more susceptible to oxidative conditions producing 2 degradation products [37]. PHE photodegradation may produce epinephrine [43], which has stronger agonist effect on adrenergic receptors [44]. This fact indicates that possible degradation of PHE may cause severe side effects to patients. Degradation of PHE may also cause physical instability as it may be accompanied by a change in color even in light-protected media [45]. Acetylated degradation products of PHE in tablets containing aspirin were detected and characterized [46]. Phenolic cyclization is a possible decomposition pathway for PHE to form tetrahydroisoquinolines [47], which are known to induce Parkinson’s disease [48].
From these facts, it’s proven that degradation of PHE is a major health concern in pharmaceutical formulation’s development in comparison to IBU. Therefore, PHE degradation is the focus in our study.
In this work, ND and CWT techniques were applied on raw data (ND and CWT methods) and on ratio spectra data (Derivative Ratio-Zero Crossing “DRZC” and Continuous Wavelet Transform-Zero Crossing “CWTR-ZC” methods) and the methods were compared regarding their ability to solve the problem of minor components in spectrophotometric analysis of pharmaceuticals. A mixture of IBU and PHE with PHE degradation products (PHEdeg) was used to present the comparison.
Experimental
Chemicals and materials
Methanol of HPLC grade was obtained from Sigma Aldrich (Darmstadt, Germany). IBU was kindly supplied by Abbott laboratories (Cairo, Egypt). PHE was kindly supplied by Amman Pharmaceutical Industries (Amman, Jordan). Their purities were found to be 99.62 ± 1.11% and 100.02 ± 1.14% for IBU and PHE, respectively, according to the BP methods [24]. Grippostad® film coated tablets manufactured by Laboratorio Stada (Barcelona, Spain). Each tablet contains 200 mg of ibuprofen and 5 mg of phenylephrine.
Instrumentation
Shimadzu dual beam UV–Vis spectrophotometer (Kyoto, Japan), UV-1650 PC model with bundle software. Processing of absorption and derivative spectra was done using version 3.7 of the UV PC personal spectroscopy program (Shimadzu, Kyoto, Japan). Scans have been performed at intervals between 200.0 nm to 400.0 nm at 0.2 nm with 1.00 cm quartz cells.
The CWT was performed using MATLAB® 9.2.0.538062 (R2017a).
Solutions
Preparation of PHE degradation product stock solution
Oxidative degradation was performed by refluxing 100.0 mg of PHE powder with 25 mL of 3% H2O2 for 3 h and protected from light and then excess H2O2 was removed by evaporation. Complete degradation was confirmed through TLC, the solution was cooled, transferred quantitatively into a 25-mL volumetric flask, and completed to volume using methanol to prepare stock solution of PHEdeg equivalent to 4 mg/mL PHE. Silica gel 60 F254 plates were used to check the disappearance of intact drug spot at Rf 0.12 and appearance of two spots of degradation products at Rf 0.45 and 0.55 using ethyl acetate–methanol-30% ammonia solution (8:2:0.1, by volume) as the developing mobile phase.
Solutions of IBU, PHE and PHEdeg
Standard stock solutions of 1.0 mg/mL of IBU and PHE were prepared from their standard powder using methanol. Stock solution of PHEdeg equivalent to 1 mg/mL PHE was prepared by dilution from previous degradation solution. Working solutions of 100 μg/mL of IBU, PHE and PHEdeg were prepared by suitable dilutions from the corresponding stock solutions.
Procedures
Spectral characteristics of ibuprofen, phenylephrine and PHEdeg
The zero-order spectra of 120 μg/mL IBU, 3 μg/mL PHE and PHEdeg equivalent to 10 µg/mL PHE were scanned using methanol as a blank in the range 200–400 nm.
Calibration curves construction
Definite volumes of IBU and PHE working standard solutions (100 µg/mL) equivalent to 200–1800 µg and 30–400 µg, respectively, were accurately transferred into two separate series of 10-mL volumetric flasks and methanol was used to complete the volume of each flask. The absorption spectra were scanned using methanol as blank.
Numerical differentiation (ND)
For IBU, fourth derivative spectra were calculated using Δλ = 8 nm and scaling factor = 1000. For PHE, second derivative spectra were calculated using Δλ = 8 nm and scaling factor = 1000. Calibration curves were constructed relating the peak amplitude of the corresponding derivative spectra at 272.4 and 275.4 nm to the corresponding concentrations of IBU and PHE, respectively.
Continuous wavelet transform (CWT)
For IBU, CWT spectra were calculated using Morlet wavelet family (morl) with scale = 30, while for PHE Gaussian-5 family (gaus-5) with scale = 100 was used. The amplitudes at 264.6 and 288.0 nm were plotted against the corresponding concentrations of IBU and PHE, respectively.
First derivative of ratio spectra (DD1)
The spectrum of PHEdeg equivalent to 30 μg/mL PHE was used as divisor for PHE. The first derivative of these ratio spectra was calculated with Δλ = 4 nm and scaling factor = 10. Calibration curve was constructed relating the peak amplitude at 270.8 nm to the corresponding concentrations of PHE.
Continuous wavelet transform ratio-zero crossing (CWTR-ZC)
For IBU, the spectrum of 40 μg/mL PHE was used as divisor, then the CWT of these spectra were calculated using morl family with scale = 20. For PHE, the spectrum of PHEdeg equivalent to 30 μg/mL PHE was used as divisor, and the CWT of these spectra were calculated using morl family with scale = 50. Calibration curves were constructed relating the peak amplitude of the corresponding spectra at 263.8 and 282.6 nm to the corresponding concentrations of IBU and PHE, respectively.
Application of the signal processing methods for the quantitation of IBU and PHE in laboratory-prepared mixtures
Different volumes of IBU, PHE and PHEdeg were taken from their working solutions into a single series of 10-mL measuring flasks, the formed mixtures contained diverse ratios of the two drugs and different percent of degradation products. Concentrations of IBU and PHE were calculated using the previous procedures.
Application of the proposed methods for the determination of IBU and PHE in Grippostad® tablets
Ten of Grippostad® film coated tablets (labelled to contain 200 mg of IBU and 5 mg PHE per tablet) were precisely weighed, the tablets were first stripped of the film before being finely powdered. A precisely weighed portion containing 200 mg of IBU and 5 mg of PHE was sonicated in 30 mL of methanol for 10 min before being filtered into a 100-mL volumetric flask. The residues were rinsed multiple times each with 10 mL of methanol, and the solution was adjusted to the mark by the same solvent. An aliquot was then diluted in order to prepare a solution containing 120 and 3 µg/mL of IBU and PHE, respectively.
Results and discussion
The problem of minor component in the studied mixture
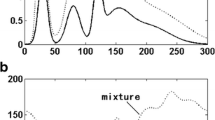
The objective of this work was to establish a comparative study between two signal processing techniques and show the advantages and weaknesses of these techniques in analysis of mixtures with minor components. A mixture of IBU and PHE was chosen for demonstration of this comparison, the two drugs are combined in Grippostad® tablets for the relief of cold symptoms. Each tablet contains 200 mg of IBU and 5 mg of PHE, this large difference in concentration (40:1) with the similar absorptivity of the two drugs as shown in Fig. 2a represent a challenge for the spectrophotometric analysis of these tablets in QC laboratories.
The main challenge is to quantify PHE in presence of the large amount and hence large absorbance of IBU as shown in Fig. 2b. PHE concentration in Fig. 2b spectrum is 1 µg/mL and shows absorbance below the accepted limit at its two λmax (217.0 and 273.0 nm). To overcome the problem of non-linearity of PHE, the first concentration of PHE that can be used is 3 µg/mL, which means in dosage form solution, IBU concentration will be 120 µg/mL. At these concentrations, IBU will be out of linearity at its λmax (220.0 nm), but at the second λmax of 264.0 nm it will be within the accepted limit of absorbance. So, to solve this problem, the dosage form will be diluted to the concentration of IBU 120 µg/mL and PHE 3 µg/mL as shown in Fig. 3.
The methods used for analyzing this mixture are ND, CWT, DRZC and CWTR-RS, all these methods depend on the presence of a zero-crossing point. So, although the concentration ratio used (120:3) will solve the problem of spiking, a new challenge will arise as the wavelength range used will be limited to the range 240–300 nm instead of 200–300 nm. This means that during the development of the four methods, searching for the zero-crossing point will be limited to 60 nm instead to 100 nm representing a new challenge. This wavelength range (240–300 nm) already shows limited absorbance features, which increase the difficulty of developing the methods.
To simulate real situations in QC laboratories the PHE oxidative degradation products (PHEdeg) were added to the mixture. PHE is proved to produce two degradation products upon exposure to stress oxidative condition [37]. PHE was degraded and a stock solution of the two degradation products (Fig. 1) was prepared, whose concentration is expressed relative to PHE concentration used for its preparation. The PHEdeg spectrum represent the two degradation products found in degradation solution of PHE, it displays severe overlap with the spectra of IBU and PHE as shown in Fig. 3.
Derivative methods (ND and CWT)
In ND method, no zero-crossing points were obtained in first order derivative for the two drugs. The second derivative was therefore calculated, where zero-crossing points were observed for determination of PHE as shown in Fig. 4a. The one that showed good linearity and was successfully used for its quantitation in laboratory prepared mixtures was 275.4 nm. In searching for a zero-crossing point for determination of IBU, third derivative was calculated and again no suitable zero-crossing points were observed. By calculating fourth order derivative spectra, zero-crossing points were obtained for IBU determination as shown in Fig. 4b. The best one regarding linearity and selectivity in laboratory prepared mixtures was 272.4 nm. For optimization of the derivative spectra, different Δλ (4, 8 and 16) with scaling factors of 10, 100 and 1000 were applied. The best parameters, regarding spectral shapes, linearity, and recovery, were Δλ = 8 and scaling factor of 1000.
a Second derivative (ND) of 3–40 µg/mL PHE (──), 20–180 µg/mL IBU (....) and PHEdeg (----) equivalent to 10–40 µg/mL PHE showing zero-crossing point for PHE determination. b Fourth derivative (ND) of 30–180 µg/mL IBU (──), 3–40 µg/mL PHE (....) and PHEdeg (----) equivalent to 10–40 µg/mL PHE showing zero-crossing point for IBU determination
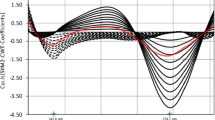
In CWT method, for obtaining zero-crossing for the two drugs, different wavelet families were applied such as Daubechies (db), Morlet (morl), Coiflets (coif), Mexican hat (mexh), Meyer (meyr), Symlets (sym), and Gaussian (gaus). The families were tested in their different orders and with changing scale parameter. Zero-crossing points with the best linearity and recovery in laboratory prepared mixtures were obtained at 264.6 and 288.0 nm with family morl and gaus-5 for IBU and PHE, respectively (Fig. 5). The scale parameter was optimized for both families and morl family was best scaled at 30, while gaus-5 family was scaled at 100.
a Continuous Wavelet Transform (morl) of 20–180 µg/mL IBU (──), 3–40 µg/mL PHE (....) and PHEdeg (----) equivalent to 10–40 µg/mL PHE showing zero-crossing point for IBU determination. b Continuous Wavelet Transform (gaus-5) of 3–40 µg/mL PHE (──), 20–180 µg/mL IBU (....) and PHEdeg (----) equivalent to 10–40 µg/mL PHE showing zero-crossing point for PHE determination
Derivative ratio methods (DRZC and CWTR-ZC)
For DRZC and CWTR-ZC methods, the choice of optimum divisor is a main factor to optimize the method. For determination of IBU, different divisor spectra of PHE and PHEdeg were tested and the best one regarding shape, zero-crossing points, linearity, and recoveries in laboratory prepared mixtures was the spectrum of 40 µg/mL PHE (IBU/PHE 40). While for determination of PHE, different divisor spectra of IBU and PHEdeg were tested and the best one was the spectrum of PHEdeg equivalent to 30 µg/mL PHE (PHE/PHEdeg 30).
For DRZC, after obtaining the best ratio spectra, first order derivatives for IBU and PHE were calculated using Δλ = 4 with a scaling factor of 10. The first derivative of ratio spectra (IBU/PHE 40) showed no possible zero-crossing points for IBU determination, the points observed showed bad recoveries in almost all the laboratory prepared mixtures. For PHE determination, zero-crossing points were observed in the first order derivative of ratio spectra (PHE/PHEdeg 30). The best wavelength regarding linearity and recovery was 270.8 nm (Fig. 6).
For CWTR-ZC, several wavelet families and scales were applied on the ratio spectra IBU/PHE 40 and PHE/PHEdeg 30. For IBU determination, morl family with scale parameter of 20 was used on IBU/PHE 40 ratio spectra, and several zero-crossing points were observed and the best one regarding linearity and recoveries was 263.8 nm (Fig. 7a). For PHE, morl family with scale parameter of 50 was used on PHE/PHEdeg 30 ratio spectra, with several zero-crossing points obtained, the best wavelength was 282.6 nm (Fig. 7b).
a Continuous Wavelet Transform (morl) of the ratio spectra (using 40 µg/mL PHE as divisor) of 20–180 µg/mL IBU (──) and PHEdeg (----) equivalent to 10–40 µg/ml PHE showing zero-crossing point for IBU determination. b Continuous Wavelet Transform (morl) of the ratio spectra (using PHEdeg equivalent to 30 µg/mL PHE as a divisor) of 3–40 µg/mL PHE (──) and 20–180 µg/mL IBU (....) showing zero-crossing point for PHE determination
Comparative study
The two derivative methods (ND and CWT) showed the same performance regarding linearity and recoveries in laboratory prepared mixtures for PHE as shown in Table 1, while differences appeared between them in case of IBU. CWT showed better sensitivity regarding IBU and the linearity range extended from 20 to 180 µg/mL instead of 30–180 µg/mL in case of ND. This may be credited to the fact that CWT has amplification property that enhances the signal in contrast to ND which diminishes the signals, especially in higher orders as the fourth order in case of IBU. This fact was highlighted in previous studies on CWT [49, 50]. Another difference appears in mixture no. 12, as IBU recovery was very bad, which can be attributed to the small absorbance of IBU (30 µg/mL) in contrast to PHEdeg (25 µg/mL). This is due to the small absorbance of PHEdeg at 272.4 nm, which was not affecting recovery of IBU when present in high concentration compared to PHEdeg. This result shows the advantage of CWT over ND, as CWT gives variety of families and orders that make finding a true zero-crossing point possible in contrast to the simple algorithm of ND with only 4 derivatives orders to look for the zero-crossing points.
Again, the two methods (DRZC and CWTR-ZC) showed the same performance regarding PHE in linearity and recoveries in laboratory prepared mixtures as shown in Table 1, while major differences appeared in case of IBU. DRZC method failed to find a suitable zero-crossing point for IBU, due to the limited options in its derivative calculation and the reduced wavelength range used (240–300 nm). On the contrast, CWT algorithm can use several wavelet families and orders to calculate the first order derivatives, which increase the possibility of finding zero-crossing points in limited range [51].
From the previous results, we can conclude that CWT technique represents a better option for solving the problem of minor components. It allows us to find the zero-crossing points between drugs in limited wavelength ranges, that will be obtained in several situations to solve the problem of minor components. This can be highlighted in the results for IBU, where ND failed to find zero-crossing points in ratio spectra data (DRZC method), while on raw data (ND method), 1st, 2nd and 3rd order derivatives failed to record a suitable zero-crossing point. In ND, we had to calculate the 4th order derivative to find this zero-crossing point. This has diminished the signal and decreased the sensitivity of the method compared to CWT, which allowed a zero-crossing point directly and having the property of amplification, preserved the signal and increased the sensitivity of the method.
Validation and application to pharmaceutical formulation
The methods were validated according to ICH guideline Q2(R1) [52]. Accuracy was assessed using three concentrations of each drug (80, 110, 140 μg/mL for IBU and 15, 25, 35 μg/mL for PHE) with three replicates for each concentration and mean recoveries were calculated which ranged from 100.20% to 100.59% showing acceptable accuracy (Table 2). Repeatability and intermediate precision were measured three times on the same day and on different three days, respectively, using three concentrations of each drug (90, 120, 150 μg/mL for IBU and 10, 20, 30 μg/mL for PHE). RSD of the responses were calculated and did not exceed 2% as shown in Table 2, proving that the method was precise.
The linearity of the methods was assessed using at least 6 points calibration for each method and correlation coefficients ranging from 0.9995 to 0.9999 proved the linearity of the proposed methods. The linearity was in the range 3–40 μg/mL for PHE in all methods, while for IBU it was 20–180 and 30–180 μg/mL in CWT and ND methods, respectively. Specificity of the methods was evaluated by calculating the mean R% ± SD of the drugs in their laboratory prepared mixtures to measure their ability to quantitate the analytes with sufficient discrimination. The results in Table 2 show the methods were specific for each drug in presence of the other drug and PHEdeg.
The robustness of the methods was measured using the new approach introduced in previous work [50], where the recoveries were calculated at ± 0.2 nm from the zero-crossing points. From robustness results, we can observe better RSD% in case of wavelengths in broad horizontal bands on the spectrum as PHE in ND and DRZC methods (Figs. 4a and 6), in contrast to a steep portion of the spectrum as IBU in ND and CWTR-ZC (Fig. 4b and Fig. 7a).
The CWT algorithm showed better performance in derivative and derivative ratio methods, so it was used successfully for determination of the two drugs in Grippostad® tablets as shown in Table 3. The results of analysis of IBU and PHE by pharmacopeial methods in pure powder were statistically compared with CWT and CWTR-ZC methods and showed no significant difference as shown in Table 4.
Conclusion
Four signal processing methods (ND, CWT, DRZC and CWTR-ZC) were developed and compared in the analysis of the ternary mixture of IBU, PHE and PHEdeg. The comparative study performed suggested that methods based on CWT algorithm show better alternative to ND in quantitation of pharmaceutical mixtures with minor components. They allow higher flexibility in calculating the transformed spectra giving more opportunities to find zero-crossing points in limited wavelength range usually associated with minor components. The CWT methods were successfully applied for the analysis of the two drugs in their pharmaceutical formulation, suggesting the suitability and validity for application of these methods in quality control laboratories to solve the problem of minor components in spectrophotometric analysis.
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CWT:
-
Continuous wavelet transform
- CWTR-ZC:
-
Continuous wavelet transform ratio-zero crossing
- DRZC:
-
Derivative ratio-zero crossing
- DS:
-
Derivative spectrophotometry
- gaus-5:
-
Gaussian-5 wavelet family
- IBU:
-
Ibuprofen
- ICH:
-
International Council for Harmonisation
- morl:
-
Morlet wavelet family
- ND:
-
Numerical differentiation
- PHE:
-
Phenylephrine
- PHEdeg:
-
PHE degradation products
References
Elghobashy M, Badran U, Salem M, Kelani K. Stability indicating spectrophotometric and chromatographic methods for the determination of azelastine hydrochloride in presence of its alkaline degradant. Anal Chem: Indian J. 2014;14:135–42.
Darwish HW, Hassan SA, Salem MY, El-Zeany BA. Development and validation of H-point standard addition method applied for the analysis of binary mixture of amlodipine and atorvastatin. Int J Pharma Bio Sci. 2013;4(2):230–43.
Ahmed MK, Michael AM, Hassan SA, Abbas SS. Different spectrophotometric methods manipulating ratio spectra for the assay of hydrocortisone acetate and clioquinol in their topical preparation. Eur J Chem. 2021;12(3):265–72.
Darwish HW, Hassan SA, Salem MY, El-Zeany BA. Advanced stability indicating chemometric methods for quantitation of amlodipine and atorvastatin in their quinary mixture with acidic degradation products. Spectrochim Acta A. 2016;154:58–66.
Basha MA, Abdelrahman MK, Bebawy L, Mostafa A, Hassan SA. A comparative Study of two analytical techniques for the simultaneous determination of Amprolium HCl and Ethopabate from combined dosage form and in presence of their alkaline degradation. Spectrochim Acta A. 2020;243: 118756.
Kelani KM, Rezk MR, Monir HH, ElSherbiny MS, Eid SM. FTIR combined with chemometric tools (fingerprinting spectroscopy) in comparison to HPLC: which strategy offers more opportunities as a green analytical chemistry technique for pharmaceutical analysis. Anal Methods. 2020;12(48):5893–907.
Dymond E. On the measurement of the critical potentials of gases. Math Proc Cambridge Philos Soc. 1924;22(3):405–8.
Hammond V, Price W. Derivative spectroscopy: theoretical aspects. J Opt Soc Am. 1953;43:924–30.
Ojeda CB, Rojas FS. Recent applications in derivative ultraviolet/visible absorption spectrophotometry: 2009–2011: a review. Microchem J. 2013;106:1–16.
Dinç E, Baleanu D. A new fractional wavelet approach for the simultaneous determination of ampicillin sodium and sulbactam sodium in a binary mixture. Spectrochim Acta A. 2006;63(3):631–8.
Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36(8):1627–39.
Wahbi A, Abdine H, Korany M. The use of Fourier functions to eliminate interferences in spectrophotometric analysis (theory and application). Pharmazie. 1978;33(5):278–82.
Elzanfaly ES, Hassan SA, Salem MY, El-Zeany BA. Different signal processing techniques of ratio spectra for spectrophotometric resolution of binary mixture of bisoprolol and hydrochlorothiazide; a comparative study. Spectrochim Acta A. 2015;140:334–43.
Hassan SA, Elzanfaly ES, Salem MY, El-Zeany BA. Mean centering of double divisor ratio spectra, a novel spectrophotometric method for analysis of ternary mixtures. Spectrochim Acta A. 2016;153:132–42.
Kelani KM, Nassar AMW, Omran GA, Morshedy S, Talaat W. Comparative study of extension area based methods for spectrophotometric determination of desmopressin acetate in the presence of its acid-induced degradation products. BMC Chem. 2022;16(1):1–10.
Leung A, Chau F, Gao J. Wavelet transform: a method for derivative calculation in analytical chemistry. Anal Chem. 1998;70(24):5222–9.
Ibrahim MM, Elzanfaly ES, El-Zeiny MB, Ramadan NK, Kelani KM. Spectrophotometric determination of meclizine hydrochloride and pyridoxine hydrochloride in laboratory prepared mixtures and in their pharmaceutical preparation. Spectrochim Acta A. 2017;178:234–8.
Sohrabi MR, Mirzabeygi V, Davallo M. Use of continuous wavelet transform approach for simultaneous quantitative determination of multicomponent mixture by UV–Vis spectrophotometry. Spectrochim Acta A. 2018;201:306–14.
Kelani KM, Gad AG, Fayez YM, Mahmoud AM, Abdel-Raoof AM. Three developed spectrophotometric methods for determination of a mixture of ofloxacin and ornidazole; application of greenness assessment tools. BMC Chem. 2023;17(1):16.
Zaazaa H, Elzanfaly E, Soudi A, Salem M. Spectrophotometric method for the determination of two coformulated drugs with highly different concentrations. Application on vildagliptin and metformin hydrochloride. J Appl Spectrosc. 2016;83(1):137–40.
Kelani KM, Emara MS, Madkour AW, Batakoushy HA, Tony RM. The simultaneous measurement of quaternary mixture in over-the-counter cold medications using sequential spectrophotometric resolution approach enhanced with in-lab sample enrichment. BMC Chem. 2023;17(1):24.
Abdalla OM, Abdel-Megied AM, Saad AS, Soliman SS. Simultaneous spectrophotometric determination of compounds having relatively disparate absorbance and concentration ranges; application to antidiabetic formulation of linagliptin and metformin. Spectrochim Acta A. 2018;203:112–7.
Lotfy HM, Mohamed D, Elshahed MS. Different mathematical processing of absorption, ratio and derivative spectra for quantification of mixtures containing minor component: an application to the analysis of the recently co-formulated antidiabetic drugs; canagliflozin and metformin. Spectrochim Acta A. 2018;189:100–9.
BP. British Pharmacopoeia, London: Medicines and Healthcare Products Regulatory Agency (MHRA), 2019.
Sweetman SC. Martindale: the complete drug reference, 2005.
Esteve-Taboada JJ, Águila-Carrasco D, Antonio J, Bernal-Molina P, Ferrer-Blasco T, López-Gil N, Montés-Micó R. Effect of phenylephrine on the accommodative system. J Ophthalmol. 2016;2016.
Farrer F. Making sense of active ingredients in combination cold and flu medicines. Prof Nurs Today. 2010;14(2):8–12.
Prajapati P, Patel D, Shah S. Design of experiments (DoE)-based enhanced quality by design approach to hydrolytic degradation kinetic study of capecitabine by eco-friendly stability-indicating UV-visible spectrophotometry. Am J PharmTech Res. 2020;10(6):115–33.
Prajapati PB, Jayswal KV, Shah SA. DoE and risk-based DMAIC principle for implementation of enhanced analytical quality by design approach to multipurpose-chromatography method for simultaneous estimation of multiple fixed-dose combination products of aspirin. J AOAC Int. 2021;104(5):1430–41.
Prajapati P, Shah H, Shah SA. Implementation of QRM and DoE-based quality by design approach to VEER chromatography method for simultaneous estimation of multiple combined dosage forms of paracetamol. J Pharm Innov. 2022;17(1):2–18.
Prajapati P, Tamboli J, Surati P, Mishra A. Risk assessment-based enhanced analytical quality-by-design approach to eco-friendly and economical multicomponent spectrophotometric methods for simultaneous estimation of montelukast sodium and bilastine. J AOAC Int. 2021;104(5):1453–63.
Patel M, Patel B, Parmar S. Simultaneous estimation of ibuprofen and phenylephrine hydrochloride in bulk and combined dosage form by first derivative UV spectrophotometry method. J Spectrosc. 2013;2013.
Salem YA, Hammouda ME, El-Enin MAA, El-Ashry SM. Application of derivative emission fluorescence spectroscopy for determination of ibuprofen and phenylephrine simultaneously in tablets and biological fluids. Spectrochim Acta A. 2019;210:387–97.
Ragab MA, Abdel-Hay MH, Ahmed HM, Mohyeldin SM. Determination of ibuprofen and phenylephrine in tablets by high-performance thin layer chromatography and in plasma by high-performance liquid chromatography with diode array detection. J Chromatogr Sci. 2019;57(7):592–9.
Vemula VRB, Sharma PK. Gradient high performance liquid chromatography method development and validation for simultaneous determination of phenylephrine and ibuprofen in tablet dosage form. Trop J Pharm Res. 2014;13(6):967–74.
Ragab MA, El Yazbi FA, Hassan EM, Khamis EF, Hamdy MM. Stability studies of over the counter quaternary mixture containing phenylephrine hydrochloride, Chlorpheniramine maleate, paracetamol and caffeine using different chromatographic methods. Anal Chem Lett. 2018;8(3):331–47.
Rezk MR, Fayed AS, Marzouk HM, Abbas SS. Chromatographic determination of cyclopentolate hydrochloride and phenylephrine hydrochloride in the presence of their potential degradation products. J AOAC Int. 2017;100(2):434–44.
Patel BS, Nayak PP, Parmar RR, Shah DA. Development and validation of stability indicating HPLC method for simultaneous estimation of ebastine and phenylephrine hydrochloride in pharmaceutical dosage. Pharm Anal Qual Assur. 2014;3:1–7.
Patel KB, Thula KC, Maheshwari DG. Stability indicating HPLC Method for simultaneous estimation of Ciprofloxacin and Phenylephrine in pharmaceutical dosage form. Pharmacophore. 2014;5(2):262–72.
Caviglioli G, Valeria P, Brunella P, Sergio C, Attilia A, Gaetano B. Identification of degradation products of ibuprofen arising from oxidative and thermal treatments. J Pharm Biomed Anal. 2002;30(3):499–509.
Ahirrao V, Pawar R. Simultaneous quantification of famotidine and ibuprofen in pharmaceutical dosage by using validated stability indicating LC method. Res J Pharm Sci. 2013;2319:555X.
Farmer S, Anderson P, Burns P, Velagaleti R. Forced degradation of ibuprofen in bulk drug and tablets. Pharm Technol. 2002;28(42):13.
Chafetz L, Chow LH. Photochemical hydroxylation of phenylephrine to epinephrine (adrenaline). J Pharm Biomed Anal. 1988;6(5):511–4.
Whalen K, Feild C, Radhakrishnan R. Lippincott Illustrated Reviews: Pharmacology. 7th ed. New York: Wolters Kluwer; 2019.
Al Taii R, Stanford J, Sugden J. Some aspects of the photolysis of aqueous solutions of phenylephrine hydrochloride. Pharm Acta Helv. 1982;57(2):56–60.
Troup A, Mitchner H. Degradation of phenylephrine hydrochloride in tablet formulations containing aspirin. J Pharm Sci. 1964;53(4):375–9.
Chafetz L, Turdiu R. Phenolic cyclization of epinephrine, metaproterenol, metaraminol, phenylephrine, and terbutaline with formaldehyde. Pharm Res. 1987;4:158–61.
Kotake Y, Tasaki Y, Makino Y, Ohta S, Hirobe M. 1-Benzyl-1, 2, 3, 4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF. J Neurochem. 1995;65(6):2633–8.
Elzanfaly ES, Hassan SA, Salem MY, El-Zeany BA. Continuous Wavelet Transform, a powerful alternative to derivative spectrophotometry in analysis of binary and ternary mixtures: a comparative study. Spectrochim Acta A. 2015;151:945–55.
Abdel-Gawad SA, Arab HH, Hassan SA. Signal processing techniques for the spectrophotometric quantitation of binary mixture of dapagliflozin and saxagliptin: a comparative study. Trop J Pharm Res. 2021;20(7):1489–96.
Hassan SA, Abdel-Gawad SA. Application of wavelet and Fuorier transforms as powerful alternatives for derivative spectrophotometry in analysis of binary mixtures: a comparative study. Spectrochim Acta A. 2018;191:365–71.
Validation of analytical procedures: text and methodology Q2(R1), ICH, 2005
Anscombe FJ. Rejection of outliers. Technometrics. 1960;2(2):123–46.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SAH: Conceptualization, Methodology, Writing—Original Draft. RAF: Methodology, Investigation, Writing—Original Draft. YMF: Visualization, Supervision. KMK: Visualization, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hassan, S.A., Fekry, R.A., Fayez, Y.M. et al. Continuous wavelet transform for solving the problem of minor components in quantitation of pharmaceuticals: a case study on the mixture of ibuprofen and phenylephrine with its degradation products. BMC Chemistry 17, 140 (2023). https://doi.org/10.1186/s13065-023-01059-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-01059-1