Abstract
Statin-associated muscle symptoms are considered as obvious adverse effects of prolonged statin therapy such as myopathy, myalgia, and rhabdomyolysis. These side effects are associated with vitamin D3 deficiency and can be adjusted by amendment of serum vitamin D3 level. Green chemistry aims to decrease the harmful effects of analytical procedures. Here we have developed a green and eco-friendly HPLC method for the determination of atorvastatin calcium and vitamin D3. The two drugs were separated in less than 10 min on Symmetry column C18 (100 × 4.6 mm, 3.5 µm) using a mixture consisting of 0.1% ortho-phosphoric acid (OPA) (pH = 2.16) and ethanol as the mobile phase in gradient manner. We have used Green Analytical Procedure Index (GAPI) tools and the Analytical GREEnness Metric Approach (AGREE) for assessment of the greenness of our proposed method. The method proved linearity over concentration ranges of (5–40) and (1–8) µg/ml with low limit of detection of 0.475 and 0.041 µg/ml for atorvastatin calcium and vitamin D3 respectively. The method was successfully validated in accordance with ICH instructions and utilized for determination of the drugs of interest either in pure form or in their pharmaceuticals.
Similar content being viewed by others
Introduction
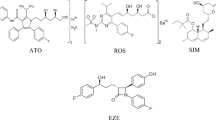
Atorvastatin calcium (Fig. 1a) is an important member of statins group which is the first line treatment of hyperlipidemia because of their effectiveness. Statins inhibit hydroxyl-methyl glutaryl Co-A reductase, a rate-controlling enzyme in cholesterol biosynthesis, reducing the cholesterol production which positively affects the rates of cardiovascular complications and general mortality in patients with coronary artery disease [1].
The vitamin D is a group of fat soluble vitamins which are vital for nearly all human body systems like immune, myocardial systems, pancreatic beta cells, neurons and so its participation in many of metabolic disorders [2]. In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). vitamin D3 or cholecalciferol (Fig. 1b) coordinates calcium regulation in the body [3] and affects impressively muscle duties and overall health especially with long use [4]. It’s very important to determine and quantify vitamin D3 levels in many cases which suffer from muscle dysfunctions [5].
A clinical trial was performed to study the role of statins on muscle work, it was found that 9.4% of the patients taking 80 mg/day atorvastatin over 6 months was developing muscle pain in comparison with 4.6% of placebo patients [6]. Statin-associated muscle symptoms (SAMS) are considered as critical adverse effects of prolonged statin therapy such as myopathy, myalgia, and rhabdomyolysis. Vitamin D has been linked with muscle health and performance. Myalgia can be controlled by adjustment of serum vitamin D level [7].
Atorvastatin calcium has been analyzed by many techniques such as HPLC [8,9,10,11,12,13,14] and spectrophotometrically [15,16,17,18,19]. While, vitamin D3 was determined by HPLC with UV detection [20,21,22,23,24] or mass spectrophotometry [25,26,27,28] and spectrophotometrically using iodine complex [29]. Atorvastatin calcium and vitamin D3 have been determined together using reversed phase-HPLC with UV detection at 265 nm using mobile phases of harmful and toxic solvents of methanol:acetonitrile (50:50) [30] and at 252 nm using mobile phase of acetonitrile:methanol ratio of 75:25 v/v, pH of 3.5 is adjusted with orthophosphoric acid [31] thus generating toxic residues and waste. Green analytical chemistry (GAC) was introduced in year 2000 to reduce or to remove the harmful effects of analytical practices. It is a challenge to increase the quality of results and improve environmental friendliness of analytical procedure. For the evaluation of the analytical greenness, we have applied Green Analytical Procedure Index (GAPI) [32] which is a new tool used for assessment of the greenness of analytical procedures. To classify the greenness of each stage of an analytical procedure, the GAPI tool applies a pictogram using a color scale from green through yellow to red depicting low, medium to high impact, respectively. GAPI estimation system fundamentally covers three categories; sampling, solvents and equipments which are subdivided into 15 parts of assessment [33].
Also, the Analytical GREEnness Metric Approach (AGREE) assessment tool has been reported recently [34] for evaluation of greenness degree of the proposed method according to the 12 main principles of green analytical chemistry in form of score from zero to one.
Here, we have developed a green and eco-friendly HPLC method for analysis of atorvastatin calcium and vitamin D3 either in pure form or in pharmaceutical formulation.
Experimental
Chemicals and reagents
Atorvastatin calcium working standard (99.4% purity) was supplied and certified by EIPICO, Egypt and its commercial product (Lipitor tablets 10 mg, Pfizer Company for pharmaceuticals, Egypt) was purchased from Egyptian market. Vitamin D3 standard (> 98% purity) was purchased from Sigma-Aldrich (Germany) and its commercial product (Breva tablets 10,000 I.U) produced by Vortex pharma company (Egypt). Ethanol (HPLC grade) and methanol (Analytical grade) were provided by Darmstadt, Merck (Germany). Phosphoric acid (Analytical grade) was purchased from Sigma Aldrich (Germany).
Instrumentation and chromatographic conditions
HPLC analyses were performed on Alliance 2695 HPLC system which composed of a quaternary gradient pump, an auto sampler, a column oven, and a photodiode array detector 2996 (Waters, USA). The separation was performed on symmetry column C18 (100–4.6 mm, 3.5 µm) (Waters, Ireland) using a mixture consisting of 0.1% ortho-phosphoric acid (OPA) pH = 2.16 and ethanol as the mobile phase in gradient manner. The mobile phase was pumped at a flow rate of 1 ml/min while the column temperature was maintained at 40 °C. Detection was monitored at wavelengths of 246 and 264 nm for atorvastatin calcium and vitamin D3 respectively. Injection volume was set as 20 µl.
Preparation of stock solutions
In a 100 ml volumetric flask, stock solutions of atorvastatin calcium, vitamin D3 (0.1 mg/ml) were prepared by dissolving 10 mg of each drug separately in methanol, sonicate for 10 min, and then the volumes were completed with methanol.
Working standard solutions were made by diluting aliquots of the stock solution with methanol to get concentrations of 1, 5, 10, 20, 30 and 40 μg/ml for atorvastatin calcium and 1, 2, 4, 6 and 8 μg/ml for vitamin D3.
Construction of calibration curves
The calibration charts were set as a relation between the peak areas and the corresponding injected concentrations of atorvastatin calcium and vitamin D3.
Application to pharmaceutical dosage form
For atorvastatin calcium and vitamin D3, 10 tablets of Lipitor® (10 mg) or Breva® (10,000 IU) respectively were powdered. weights of the powders equivalent to 0.15 gm atorvastatin calcium and 0.6 g vitamin D3 were transferred into 50 ml volumetric flasks and then dissolved in methanol. The flasks were left for 15 min in the sonicator, then filtered into dry conical flasks, completed to 50 ml with methanol and then 20 µl injected.
Method validation
The validation of this analytical method was carried in accordance with the International Conference on Harmonization (ICH) instructions that include Linearity, precision, specificity, accuracy, robustness, limit of detection and limit of quantitation [35].
Results and discussion
We have succeeded here in reaching an acceptable compromise between increasing the quality of results and improving environmental friendliness of analytical methods.
The two drugs have been separated in less than 10 min using a green mobile phase of 0.1% ortho-phosphoric acid (OPA) (pH = 2.16) and ethanol in gradient manner.
Different greenness assessment tools as GAPI and AGREE were used to assess the greenness degree of our developed method.
Optimization of HPLC conditions
Different conditions were studied and optimized to increase the resolution and sensitivity of the proposed HPLC method for the separation of atorvastatin calcium and vitamin D3. Detection was selected at wavelengths of 246 and 264 nm for atorvastatin calcium and vitamin D3 respectively because they achieved the maximum absorption for both (Fig. 2A and B).
A green mobile phase (aquatic acidic modifier: Ethanol) was used for separation in addition to two essential parameters;
-
1.
pH of separation media:
According to Henderson–Hasselbalch equation (pH = pKa + Log [A−]/[HA]); pH of the system is important factor that's calculated by values of pKa of species in our solution for adjusting pH of mobile phase to be an acidic media using non-Polar column to separate the peak of “atorvastatin calcium” making it in ionized form. The optimum pH was approximately 2.16.
-
2.
Elution strength of mobile phase (Gradient elution):
As, vitamin D3 is highly hydrophobic so, it could be separated by changing ratios of mobile phase (extremely increase in “ethanol” portion).
We have tried different mixtures of 0.1% ortho-phosphoric acid (OPA) (pH = 2.16) or 0.1% v/v formic acid (PH = 2.7) and ethanol in an isocratic or gradient elution. In case of isocratic elution, atorvastatin was early eluted in contrary to the highly retained vitamin D3 (Fig. 3). Columns used in trials included symmetry column (4.6 × 100 mm, 3.5 µm) (Waters, Ireland), XTerra column C18 (4.6 × 100 mm, 5 µm (Waters, USA).
Chromatogram of 20 µl injection of standard solutions of atorvastatin calcium (0.1 mg/ml) and vitamin D3 (0.1 mg/ml) using 0.1% orthophosphoric acid and ethanol as the mobile phase [50%:50%] at a flow rate 1 ml/min with column temperature was maintained at 40 °C and detection at 246 and 264 nm for atorvastatin calcium and vitamin D3 respectively
The best chromatographic separation was achieved using symmetry column (4.6 × 100 mm, 3.5 µm) and ethanol with 0.1% ortho phosphoric acid in gradient manner (Fig. 4, Table 1). So, gradient elution was selected for simultaneous determination of both drugs in a reasonable time with good peak symmetry and high resolution.
Chromatogram of 20 µl injection of standard solutions of atorvastatin calcium (0.1 mg/ml) and vitamin D3 (0.1 mg/ml) using 0.1% orthophosphoric acid and ethanol as the mobile phase in gradient matter at a flow rate 1 ml/min with column temperature was maintained at 40 °C and detection at 246 and 264 nm for atorvastatin calcium and vitamin D3 respectively
Method validation
Linearity
Good linearity was achieved between the peak areas of atorvastatin calcium, vitamin D3 and the corresponding concentration ranges which was confirmed by the high correlation coefficient as mentioned in Table 2.
Limit of detection and limit of quantitation
LOD and LOQ values were calculated according to the following equations, and the obtained results were shown in Table 2.
where (σ) is the standard deviation of the intercept of the regression line and (S) is the slope of the calibration curve.
Specificity and accuracy
Specificity is defined as how the proposed method can give the same drug response in presence of tablet excipients. A placebo was prepared containing excipients which could be present in the tablet formulation to prove accuracy. The obtained results, as in Table 3, showed that the method of study was highly selective to analyze atorvastatin calcium and vitamin D3 in their tablets without any effect or interference from the excipients (Fig. 5).
Also, the obtained results were compared with results of the reported method [30]. It was found that the calculated t and F values were less than the tabulated ones so there is no significant difference between the proposed method and the reported one as summarized in Table 4.
Precision
An acceptable precision of the method was proved through an intra-day and inter-day precision confirming low value of RSD% for atorvastatin calcium and vitamin D3 (< 2%) as in Table 5.
Robustness
Robustness is defined as the ability of the method to remain unchanged with small but deliberate changes in the experimental conditions such as wavelength, organic mobile phase and pH. Small changes in such conditions didn’t have any obvious effects on the optimum results produced by the proposed method as shown in Table 2.
Greenness assessment tools
Using different greenness assessment tools as GAPI and AGREE tools, our developed method has the highest greenness degree with increasing quality of results over the other reported HPLC methods [30, 31] as summarized in Table 6.
In GAPI, the sample preparation divided into; collection was on-line when the sample doesn’t need more preservation or transportation and the storage was under normal conditions in presence of green solvents like ethanol. For reagents and solvents; the amount of solvents used was 10–100 ml per run. The produced health hazards was little (score 0–1) and safety hazards of score 2–3. For instrumentation, the consumed energy was < 1.5 kWh per sample with no occupational hazards and little waste.
For AGREE, online sampling procedure was performed by HPLC system with score of 0.48. Minimal sample size was achieved (0.1 mg/ml) with score of 1. In-situ sample preparation was measured with score equals 0.66. Besides that, preparation of the sample was in less than 3 steps such as sonication and dilution leading to score of 1. Our developed method is semi-automatic, miniaturized method with score equals to 0.75.
There is no need to use derivatizing agent or toxic reagents (score = 1) and no threats with maximum safety of operator (score = 1). Moreover; waste volume was 12 ml/run (score = 0.36). Energy consumption was little (less than 1.5 kWh per sample (score = 0.5)). The proposed method was capable to determine 10 analytes per hour (score = 0.51) and can use bio-based reagents from renewable sources such as ethanol (score = 0.5).
Conclusion
The developed method provides eco-friendly approach for analysis of atorvastatin calcium and vitamin D3 that is dependant on usage of extremely green solvents such as ethanol and water. The proposed method was successfully applied for determining the investigated drugs in their commercial dosage forms. GAPI and AGREE assessment tools were used for evaluation of greenness degree confirming that the proposed method was green with high economic impact. The method was validated according to ICH recommendations. The studied validation parameters were within their acceptable ranges giving more sensitivity and applicability to the method.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Raghow R. Statins redux: a re-assessment of how statins lower plasma cholesterol. World J Diabetes. 2017;8(6):230.
Prasad P, Kochhar AJD. Interplay of vitamin D and metabolic syndrome: a review. Diabetes Metab Syndr Clin Res Rev. 2016;10(2):105–12.
Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134(2):123–39.
Shalaby M, Sakoury MMA, Harthi SM, Alshalawi FM, Alhajji MM, Alshaikh ZH, et al. Vitamin D3 for health and muscle functions of athletes. Syst Rev Pharm. 2020;11(9):851–4.
Gunton JE, Girgis CM. Vitamin D and muscle. Bone Rep. 2018;8:163–7.
Pergolizzi JV Jr, Coluzzi F, Colucci RD, Olsson H, LeQuang JA, Al-Saadi J, et al. Statins and muscle pain. Expert Rev Clin Pharmacol. 2020;13(3):299–310.
Pennisi M, Di Bartolo G, Malaguarnera G, Bella R, Lanza G, Malaguarnera M. Vitamin D serum levels in patients with statin-induced musculoskeletal pain. Dis Mark. 2019. https://doi.org/10.1155/2019/3549402.
Zarghi A, Shafaati A, Foroutan SM, Khoddam AJA. A simple and rapid HPLC method for the determination of atorvastatin in human plasma with UV detection and its application to pharmacokinetic studies. Clin Chim Acta. 2005;55(08):451–4.
Patel A, MaCwana C, Parmar V, Patel S. Simultaneous determination of atorvastatin calcium, ezetimibe, and fenofibrate in a tablet formulation by HPLC. J AOAC Int. 2012;95(2):419–23.
Londhe S, Deshmukh R, Mulgund S, Jain K. Development and validation of a reversed-phase HPLC method for simultaneous determination of aspirin, atorvastatin calcium and clopidogrel bisulphate in capsules. Indian J Pharm Sci. 2011;73(1):23.
Qutab SS, Razzaq SN, Khan IU. Simultaneous determination of atorvastatin calcium and ezetimibe in pharmaceutical formulations by liquid chromatography. J Food Drug Anal. 2007;15(2):14.
Panchal H, Suhagia B. Simultaneous analysis of atorvastatin calcium and losartan potassium in tablet dosage forms by RP-HPLC and HPTLC. Acta Chromatogr. 2010;22(2):173–87.
Bhatia NM, Gurav SB, Jadhav SD, Bhatia MS. RP-HPLC method for simultaneous estimation of atorvastatin calcium, losartan potassium, atenolol, and aspirin from tablet dosage form and plasma. J Liq Chromatogr relat Technol. 2012;35(3):428–43.
Shah D, Bhatt K, Shankar M, Mehta R, Baldania S. RP-HPLC determination of atorvastatin calcium and amlodipine besylate combination in tablets. Indian J Pharm Sci. 2006;68(6):796.
Jadhav SD, Bhatia MS, Thamake S, Pishawikar SA. Spectrophotometric methods for estimation of atorvastatin calcium form tablet dosage forms. Int J PharmTech Res. 2010;2(3):1948–53.
Wani TA, Khalil NY, Abdel-Rahman HM, Darwish IA. Novel microwell-based spectrophotometric assay for determination of atorvastatin calcium in its pharmaceutical formulations. Chem Cent J. 2011;5(1):1–8.
Sonawane SS, Shirkhedkar AA, Fursule RA, Surana S. Application of UV-spectrophotometry and RP-HPLC for simultaneous determination of atorvastatin calcium and ezetimibe in pharmaceutical dosage form. Eurasian J Anal Chem. 2006;1(1):31–41.
Thamake S, Jadhav S, Pishawikar S. Development and validation of method for simultaneous estimation of atorvastatin calcium and ramipril from capsule dosage form by first order derivative spectroscopy. Anal Chem. 2009;2(1):52–3.
Kumbhar ST, Jadhav SD, Bhatia NM, Bhatia MS. Development and validation of derivative spectrophotometric method for estimation of atorvastatin calcium and amlodipine besylate in tablet dosage form. Int J Pharm Pharm Sci. 2011;3(4):195–7.
Temova Ž, Roškar R. Stability-indicating HPLC–UV method for vitamin D3 determination in solutions, nutritional supplements and pharmaceuticals. J Chromatogr Sci. 2016;54(7):1180–6.
Brunetto M, Obando M, Gallignani M, Alarcón O, Nieto E, Salinas R, et al. HPLC determination of vitamin D3 and its metabolite in human plasma with on-line sample cleanup. Talanta. 2004;64(5):1364–70.
Sazali NH, Alshishani A, Saad B, Chew KY, Chong MM, Miskam M. Salting-out assisted liquid–liquid extraction coupled with high-performance liquid chromatography for the determination of vitamin D3 in milk samples. R Soc Open Sci. 2019;6(8):190952.
Almarri F, Haq N, Alanazi FK, Mohsin K, Alsarra IA, Aleanizy FS, et al. An environmentally benign HPLC-UV method for thermodynamic solubility measurement of vitamin D3 in various (Transcutol+ water) mixtures. J Mol Liq. 2017;242:798–806.
Babat N, Türkmen Y. Determination of serum vitamin D3 level by high performance liquid chromatography (HPLC) in patients with coronary artery ectasia. Cardiol Cardiovasc Med. 2020;4(2):97–104.
Xie W, Chavez-Eng C, Fang W, Constanzer M, Matuszewski B, Mullett W, et al. Quantitative liquid chromatographic and tandem mass spectrometric determination of vitamin D3 in human serum with derivatization: a comparison of in-tube LLE, 96-well plate LLE and in-tip SPME. J Chromatogr B. 2011;879(17–18):1457–66.
Newman MS, Brandon TR, Groves MN, Gregory WL, Kapur S, Zava DT, et al. A liquid chromatography/tandem mass spectrometry method for determination of 25-hydroxy vitamin D2 and 25-hydroxy vitamin D3 in dried blood spots: a potential adjunct to diabetes and cardiometabolic risk screening. J Diabetes Sci Technol. 2009;3(1):156–62.
Feng L, Wu L, Guo Y, Hamada N, Hashi Y, Li X, et al. Determination of vitamin D3 in daily oily supplements by a two-dimensional supercritical fluid chromatography-liquid chromatography-mass spectrometry system. J Chromatogr A. 2020;1629:461510.
Gill BD, Abernethy GA, Green RJ, Indyk HE. Analysis of vitamin D2 and vitamin D3 in fortified milk powders and infant and nutritional formulas by liquid chromatography–tandem mass spectrometry: single-laboratory validation, first action 2016.05. J AOAC Int. 2016;99(5):1321–30.
Perez AS, Matilla MG, Mendez JH. A sensitive spectrophotometric method for the determination of vitamin D3 using the charge-transfer spectra of vitamin D3-iodine complex. Anal Lett. 1993;26(4):721–31.
Patel AB, Jadav HM, Vyas AJ, Patel AI, Patel NK, Chavda JR. Specific stability indicating RP-HPLC photodiode array based method for estimation of novel combination atorvastatin calcium and vitamin D3 for the treatment of hyperlipidaemia. Anal Chem Lett. 2020;10(6):758–67.
VasavaS arita CJ. Analytical method development and validation of Atorvastatin calcium and vitamin D3 in pharmaceutical dosage form. J Crit Rev. 2020;7(16):3128–38.
Płotka-Wasylka JJT. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta. 2018;181:204–9.
Kannaiah KP, Sugumaran A, Chanduluru HK, Rathinam S. Environmental impact of greenness assessment tools in liquid chromatography—a review. Microchem J. 2021;170:106685.
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Am Chem Soc. 2020;92(14):10076–82.
Guidelines, international conference on harmonization. Geneva, Switzerland. 2005.
Acknowledgements
The authors are thankful to Dr. Hani Mohammed Hafez at AL-Esraa University College, Baghdad, Iraq for his help and support during carrying out the research work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
KM; Raising the idea, performing experiments, Writing the first Draft. MME; Project administration, supervision, review and editing. SSAE; revising the manuscript, supervision, and manuscript submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research doesn’t provide any human or animal studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maged, K., El-Henawee, M.M. & Abd El-Hay, S.S. Development and validation of an eco-friendly HPLC–UV method for determination of atorvastatin and vitamin D3 in pure form and pharmaceutical formulation. BMC Chemistry 17, 62 (2023). https://doi.org/10.1186/s13065-023-00975-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-00975-6