Abstract
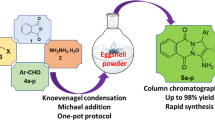
Nano-eggshell/Ti(IV) as a novel naturally based catalyst was prepared, characterized and applied for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives. The characterization of nano-eggshell/Ti(IV) was performed using Fourier Transform Infrared spectroscopy, X-ray Diffraction, Field Emission Scanning Electron Microscopy, Energy-Dispersive X-ray Spectroscopy, and Thermo Gravimetric Analysis. Dihydropyrano[2,3-c]pyrazoles were synthesized in the presence of nano-eggshell/Ti(IV) via a four component reaction of aldehydes, ethyl acetoacetate, malononitrile and hydrazine hydrate at room temperature under solvent free conditions. The principal affairs of this procedure are mild condition, short reaction times, easy work-up, high yields, reusability of the catalyst and the absence of toxic organic solvents.

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
One key-step toward green chemistry concerns on chemical transformations under solvent-free conditions [1, 2]. Solvent free conditions often have lead to decrease reaction time, increase yields and easy work-up [3, 4]. Combining this condition with multicomponent reactions (MCRs) disclosed a particular opportunity for architecting of heterocyclic molecules in short time [5, 6]. MCRs play an essential role in combinatorial chemistry due to one-pot synthesis of various complex molecules, atom economy and effectiveness compared with single step reaction [7, 8]. For economic and environmental reasons, solvent free reactions were demonstrated to be an efficient method for the synthesis of chemical product in a clean and safe conditions [9,10,11]. Dihdropyrano[2,3-c]pyrazoles (DHPPs) are important class of heterocycle componds because of their wide applications in medicinal and pharmaceutical chemistry [12]. Many of these properties are known for their anti-microbial [13], anti-inflammatory [14], anti-cancer [15], bactericidal [16], molluscicida [17], and kinase inhibitory [18] activities. In the first report, DHPP was synthesized by a reaction between 3-methyl-1-phenylpyrazolin-5-one and tetracyanoethylene [19]. Recently, DHPPs have been synthesized via the reaction of hydrazine hydrate, ethyl acetoacetate, malononitrile, and aldehydes. Some catalysts have been used to develop the above mentioned reaction such as γ-alumina [20], glycine [21], ionic liquids [22], l-proline [23], imidazole [24], I2 [25], and trietheylamine [26]. In the recent years, heterogeneous catalysts, due to the high capability for recycling and reutility, have surpassed homogeneous catalytic systems, despite their benefits such as high activity and selectivity [27]. Nowadays, nanocatalysts have been subjected of immense interest, because of their potential applications in different fields. They have several important advantages as heterogeneous catalysts including high catalytic activity, readily available, simple separation, high degree of chemical stability, and reusability [28,29,30,31].
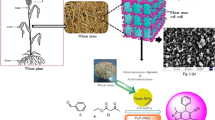
The eggshell is represented 11% of the total weight of the egg and composed predominantly of calcium carbonate (94%), organic materials (4%), calcium phosphate (1%), and magnesium carbonate (1%) [32].
In continuation of our previous works in using solid acid catalysts [33,34,35,36,37,38], herein, we reporte an efficient one-pot four-component reaction protocol for the synthesis of DHPPs in the presence of nano-eggshell/Ti(IV) (NEST) as a highly effective nanocatalyst in good to excellent yields under mild conditions (Scheme 1).
Results and discussion
Characterization of the nanocatalyst
NEST was prepared simply via addition of TiCl4 to a suspension of eggshell nanoparticles in CH2Cl2 (Scheme 2). The obtained catalyst was characterized using Fourier Transform Infrared (FT-IR) spectroscopy, X-ray Diffraction (XRD), Field Emission Scanning Electron Microscopy (FESEM), Energy-Dispersive X-ray (EDX) spectroscopy, and Thermo Gravimetric Analysis (TGA).
The FT-IR spectra of CaCO3 [39, 40], nano-eggshell, and NEST are shown in Fig. 1. Distinct absorption bands can be observed at 711, 871, and 1391 cm−1 in all compared spectra show the presence of high percentage of CaCO3 in eggshell and NEST. For NEST (Fig. 1c), in addition to the eggshell absorption bands, stretching vibrations of C–O–Ti group at 780 cm−1 (according to previously reported FT-IR about Ti(OBut)4 [41, 42]) was appeared, indicated that TiCl4 have functionalized on nano-eggshell successfully. The absorbed band at 1613 cm−1 is associated to the bending vibration of H–O–H which have shown the absorbed water on catalyst [43].
Figure 2 shows the XRD patterns of NEST, TiO2 and CaCO3 in the range of 10–70° (2θ). NEST (Fig. 2c), has shown diffraction peaks at 2θ = 23, 29, 37, 40, 43, 47, 48, 56, 57, 61 and 62°, which are quite matched with the structure of pure CaCO3. By comparison with Fig. 2a–c, we can conclude the absence of TiO2 and the presence of CaCO3 in catalyst.
Surface morphology of nano-eggshell and the synthesized NEST was observed using FESEM analysis (Fig. 3a, b). The FESEM image of NEST (Fig. 3b) indicates that morphology of the nano particles has a quasi-spherical shape. The average size of NEST was estimated about 40 nm.
The existence of expected elements in the structure of the NEST was approved by EDX analysis (Fig. 4). The EDX results have clearly confirmed the presence of C, O, Cl, Ca and Ti in the catalyst. According to this data, the weight percentages of the above-mentioned elements are 14.48, 43.13, 7.16, 29.30 and 5.94, respectively.
For thermal stability investigation of the catalyst, TGA-DTA analysis was done in a range of 45–813 °C (Fig. 5). The first decrease of weight was assigned to the catalyst moisture removal (endothermic effect at 70–130 °C, 4% weight loss). The second weight loss (16%) was occurred at 130–600 °C with an exothermic process. As the temperature increased to 800 °C, the main mass loss could be associated with the decomposition of eggshell to CO2 and CaO.
To optimize the conditions for the synthesis of the DHPPs in the presence of NEST, the condensation of 4-chlorobenzaldehyde, malononitrile, ethyl acetoacetate, and hydrazine hydrate in the molar ratio 1:1:1:2 was done under various conditions (Table 1). According to the obtained data, the best yield of 6-amino-4-(4-chlorophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (5h) was achieved using 0.06 g of NEST at room temperature under solvent-free condition (Table 1, entry 12).
After optimization of the reaction conditions for preparation of DHPPs, various aromatic and heteroaromatic aldehydes were used for expansion of this protocol. The reactions were proceeded for all used aldehydes (Table 2). The desired products were isolated in good to excellent yields in short reaction times without any byproducts.
A proposed mechanism for the synthesis of DHPPs catalyzed by NEST was shown in Scheme 3. Initially, the condensation of hydrazine hydrate (4) and ethyl acetoacetate (1) was formed intermediate (6) in the presence of NEST as a Lewis acid. The Knoevenagel condensation of malononitrile (3) with aromatic aldehyde (1) was produced the intermediate (8). Michael addition reaction of the intermediate (8) and (7) were generated intermediate (10), followed by intramolecular cyclization and tautomerization have given the DHPPs (5).
In order to investigation of the catalyst reusability, after the reaction completion, the NEST was isolated by adding acetone to reaction mixture and then filtered. The recovered catalyst was washed with dichloromethane and dried at room temperature. It was observed that the recovered nanocatalyst could be used at least four times without significant loss of its catalytic activity (Fig. 6).
The structure of recovered catalyst was studied by FT-IR (Fig. 7) and TGA-DTA (Fig. 8). The comparison between fresh and recoverable catalysts have shown no differences.
Finally, the catalytic performance of NEST was compared with that of other previously reported catalysts for the synthesis of 5a (Table 3). From the viewpoints of green chemistry and simplicity, our method is a good one.
Conclusion
In this work, we have synthesized the NEST and characterized it as a novel heterogeneous natural nanocatalyst. This catalyst was used for the synthesis of DHPPs at room temperature under solvent free condition via condensation of hydrazine hydrate, ethyl acetoacetate, malononitrile, and aromatic aldehydes. This method includes some advantages such as the solvent-free condition, good to excellent yields, room temperature, short reaction time, easy work-up and reusability of the catalyst.
Experimental section
Chemicals and apparatus
All compounds were purchased from Merck, Aldrich and Fluka chemical companies. FT-IR spectra were run on a Bruker, Equinox 55 spectrometer. A Bruker (DRX-400 Avance) NMR was used to record the 1H and 13C NMR spectra. The morphology of the particles was observed by FESEM under acceleration voltage of 120 kV. The XRD patterns were obtained on a Philips Xpert MPD diffractometer (Cu Ka, radiation, k¼ 0.154056 nm). EDS was obtained using a Phenom pro X instrument. TGA was conducted using STA 504 instrument.
Preparation of NEST
Firstly, the eggshell was heated in boiling water for 30 min, dried in oven 150 °C and powdered. Then, 1 g of prepared nano-eggshell powder was stirred for 30 min in 10 mL of dried CH2Cl2. Titanium tetrachloride (4.36 mL) was slowly added dropwise to the mixture. After stirring at room temperature for 30 min, the resulting product filtered and washed with dichloromethane three times. Finally, the obtained NEST was dried at room temperature for 3 h.
General procedure for the synthesis of DHPPs
In a 100 mL round bottom flask, a mixture of aldehyde (1 mmol), malononitrile (1 mmol), hydrazine hydrate (2 mmol), ethyl acetoacetate (1 mmol) and NEST (0.06 g) was stirred at room temperature. Progress of the reaction was monitored by TLC (n-hexane:EtOAc, 4:1). After completion of the reaction, the mixture was dissolved in acetone. Then, the catalyst was filtered off and the obtained solution was poured into cold water. The obtained solid product was filtered and purified by recrystallization from ethanol and water (4:1). The obtained NEST catalyst was then washed with EtOH, dried and reused directly for four times in other fresh reactions with negligible decreasing of the yields.
Spectroscopic data for some products
6-Amino-3-methyl-4-(3-nitrophenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 2, entry 3)
White solid. M.P. 210–211 °C FT-IR (ATR)/ῡ (cm−1): 3484, 3231, 3120, 2190, 1645, 1597, 1519, 1491, 1410, 1351, 733. 1H NMR (400 MHz, DMSO-d6)/δ (ppm): 1.82 (s, 3H), 4.89 (s, 1H), 7.08 (s, 2H), 7.64–7.70 (m, 2H), 8.04 (s, 1H), 8.13–8.15 (d, J = 8 Hz, 1H), 12.23 (s, 1H).; 13C NMR (100 MHz, DMSO-d6)/δ ppm: 161.63, 155.17, 148.36, 147.32, 136.38, 134.88, 130.47, 122.33, 121.01, 97.15, 56.59, 36.11, 10.25.
6-Amino-3-methyl-4-(4-nitrophenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 2, entry 5)
White solid. M.P. 239–242 °C. FT-IR (ATR)/ῡ (cm−1): 3475, 3227, 3106, 2195, 1646, 1592, 1513, 1399, 1348, 1163, 1109, 810, 744; 1H NMR(400 MHz, Acetone-d6)/δ ppm: 2 (s, 3H), 4.88 (s, 1H), 6.30 (br s, 2H), 7.55 (d, J = 8 Hz, 2H), 8.23 (d, J = 8 Hz, 2H), 11.43 (s, 1H). 13C NMR (100 MHz, DMSO-d6)/δ ppm: 161.62, 155.15, 152.59, 146.85, 136.36, 132.19, 129.32, 124.38, 120.98, 97.04, 56.37, 36.36, 10.22.
6-Amino-4-(4-hydroxyphenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 2, entry 9)
White solid. M.P. 222–224 °C. FT-IR (ATR)/ῡ (cm−1): 3372, 3304, 3127, 2173, 1645, 1594, 1510, 1489, 1441, 1404, 1189, 1166, 1041, 809. 1H NMR (400 MHz, Acetone-d6)/δ(ppm): 1.74 (s, 3H), 4.44 (s, 1H), 6.65 (dd, J = 7.5 Hz, J = 3.7 Hz, 2H), 6.76 (br s, 2H), 6.91 (dd, J = 7.5 Hz, J = 3.7 Hz, 2H), 9.27 (s, 1H), 12.02 (s, 1H).; 13C NMR (100 MHz, DMSO-d6)/δ ppm: 161.10, 156.49, 155.22, 135.98, 135.24, 128.92, 121.40, 115.58, 98.54, 58.21, 35.95, 10.24.
6-Amino-4-(2,4-dichlorophenyl)-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (Table 2, entry 11)
Pale yellow solid. M.P. 223–225 °C. FT-IR (ATR)/ῡ (cm−1): 3482, 3243, 3115, 2186, 1638, 1587, 1491, 1408, 1100, 1052, 866, 741.; 1H NMR (400 MHz, DMSO-d6)/δ ppm: 1.85 (s, 3H), 5.13 (s, 1H), 7.07 (s, 2H), 7.29 (d, J = 8 Hz, 1H), 7.47 (dd, J = 8.4 Hz, J = 2 Hz, 1H), 7.65 (d, J = 2.4 Hz, 1H), 12.23 (s, 1H).; 13C NMR (100 MHz, DMSO-d6)/δ ppm: 161.30, 154.88, 140.07, 135.44, 132.81, 132.10, 128.83, 128.02, 120.25, 96.32, 55.21, 33.07, 9.53.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- NEST:
-
Nano-eggshell/Ti(IV)
- MCRs:
-
Multi-component reactions
- EtOH:
-
Ethanol
- FESEM:
-
Field Emission Scanning Electron Microscope
- FT-IR:
-
Fourier Transform Infrared
- XRD:
-
X-ray diffraction
- EDX:
-
Energy-Dispersive X-ray
- TGA:
-
Thermo Gravimetric Analysis
- NMR:
-
Nuclear magnetic resonance
- TLC:
-
Thin layer chromatography
References
Choudhary G, Peddinti RK (2011) An expeditious, highly efficient, catalyst-free and solvent-free synthesis of nitroamines and nitrosulfides by Michael addition. Green Chem 13:276–282
Jain SL, Singhal S, Sain B (2007) PEG-assisted solvent and catalyst free synthesis of 3, 4-dihydropyrimidinones under mild reaction conditions. Green Chem 9:740–741
Metzger JO (1998) Solvent-free organic syntheses. Angew Chem Int Ed 37:2975–2978
Tavakolian M, Vahdati-Khajeh S, Asgari S (2019) Recent advances in solvent-free asymmetric catalysis. ChemCatChem 11:2943–2977
Rahman M, Sarkar A, Ghosh M, Majee A, Hajra A (2014) Catalytic application of task specific ionic liquid on the synthesis of benzoquinazolinone derivatives by a multicomponent reaction. Tetrahedron Lett 55:235–239
De Graaff C, Ruijter E, Orru RV (2012) Recent developments in asymmetric multicomponent reactions. Chem Soc Rev 41:3969–4009
Domling A, Wang W, Wang K (2012) Chemistry and biology of multicomponent reactions. Chem Rev 112:3083–3135
Hulme C, Chappeta S, Griffith C, Lee YS, Dietrich J (2009) An efficient solution phase synthesis of triazadibenzoazulenones: ‘designer isonitrile free’methodology enabled by microwaves. Tetrahedron Lett 50:1939–1942
Trost BM (1991) The atom economy-a search for synthetic efficiency. Science 254:1471–1477
Shen ZL, Ji SJ (2009) Alkali salt of l-proline as an efficient and practical catalyst for the cyanosilylation of a wide variety of carbonyl compounds under solvent-free conditions. Synth Commun 39:775–791
Tanaka K, Toda F (2000) Solvent-free organic synthesis. Chem Rev 100:1025–1074
Das D, Banerjee R, Mitra A (2014) Bioactive and pharmacologically important pyrano [2,3-c] pyrazoles. J Chem Pharm Res 6:108–116
Mandour A, El-Sawy E, Ebaid M, Hassan S (2012) Synthesis and potential biological activity of some novel 3-[(N-substituted indol-3-yl) methyleneamino]-6-amino-4-aryl-pyrano (2,3-c) pyrazole-5-carbonitriles and 3,6-diamino-4-(N-substituted indol-3-yl) pyrano (2,3-c) pyrazole-5-carbonitriles. Acta Pharm 62:15–30
Zaki ME, Soliman HA, Hiekal OA, Rashad AE (2006) Pyrazolopyranopyrimidines as a class of anti-inflammatory agents. Z Naturforsch C 61:1–5
Mohamed NR, Khaireldin NY, Fahmyb AF, El-Sayeda AAF (2010) Facile synthesis of fused nitrogen containing heterocycles as anticancer agents. Der Pharm Chem 2:400–417
Nasr MN, Gineinah MM (2002) Pyrido [2,3-d] pyrimidines and pyrimido [5′,4′:5,6] pyrido [2,3-d] pyrimidines as new antiviral agents: synthesis and biological activity. Arch Pharm Int J Pharm Med Chem 335:289–295
Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF (2006) Synthesis and molluscicidal activity of new cinnoline and pyrano [2,3-c] pyrazole derivatives. Arch Pharm Int J Pharm Med Chem 339:456–460
Foloppe N, Fisher LM, Howes R, Potter A, Robertson AG, Surgenor AE (2006) Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg Med chem 14:4792–4802
Junek H, Aigner H (1973) Synthesen mit nitrilen, XXXV. Reaktionen von tetracyanäthylen mit heterocyclen. Chem Ber 106:914–921
Mecadon H, Rohman MR, Rajbangshi M, Myrboh B (2011) γ-Alumina as a recyclable catalyst for the four-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano [2,3-c] pyrazole-5-carbonitriles in aqueous medium. Tetrahedron lett 52:2523–2525
Reddy MM, Jayashankara VP, Pasha MA (2010) Glycine-catalyzed efficient synthesis of pyranopyrazoles via one-pot multicomponent reaction. Synth Commun 40:2930–2934
Ebrahimi J, Mohammadi A, Pakjoo V, Bahramzade E, Habibi A (2012) Highly efficient solvent-free synthesis of pyranopyrazoles by a Brønsted-acidic ionic liquid as a green and reusable catalyst. J Chem Sci 124:1013–1017
Mecadon H, Rohman MR, Kharbangar I, Laloo BM, Kharkongor I, Rajbangshi M, Myrboh B (2011) l-Proline as an efficicent catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano [2,3-c] pyrazole-5-carbonitriles in water. Tetrahedron Lett 52:3228–3231
Siddekha A, Nizam A, Pasha MA (2011) An efficient and simple approach for the synthesis of pyranopyrazoles using imidazole (catalytic) in aqueous medium, and the vibrational spectroscopic studies on 6-amino-4-(4′-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano [2,3-c] pyrazole using density functional theory. Spectrochim Acta A 81:431–440
Reddy MB, Pasha MA (2012) One-pot, multicomponent synthesis of 4H-pyrano [2,3-c] pyrazoles in water at 25 °C. Indian J Chem Sect B 51:537–541
Litvinov YM, Shestopalov AA, Rodinovskaya LA, Shestopalov AM (2009) New convenient four-component synthesis of 6-amino-2,4-dihydropyrano [2,3-c] pyrazol-5-carbonitriles and one-pot synthesis of 6′-aminospiro [(3H)-indol-3,4′-pyrano [2,3-c] pyrazol]-(1H)-2-on-5′-carbonitriles. J Comb Chem 11:914–919
Zolfigol MA, Yarie M (2017) Fe3O4@TiO2@O2PO2(CH2)NHSO3H as a novel nanomagnetic catalyst: application to the preparation of 2-amino-4,6-diphenylnicotinonitriles via anomeric-based oxidation. Appl Organomet Chem 31:e3598
Shokouhimehr M, Piao Y, Kim J, Jang Y, Hyeon T (2007) A magnetically recyclable nanocomposite catalyst for olefin epoxidation. Angew Chem Int Ed 46:7039–7043
Kwon SG, Hyeon T (2008) Colloidal chemical synthesis and formation kinetics of uniformly sized nanocrystals of metals, oxides and chalcogenides. Acc Chem Res 41:1696–1709
Cai X, Wang H, Zhang Q, Tong J, Lei Z (2014) Magnetically recyclable core–shell Fe3O4@chitosan-Schiff base complexes as efficient catalysts for aerobic oxidation of cyclohexene under mild conditions. J Mol Catal A Chem 383:217–224
Polshettiwar V, Varma RS (2010) Green chemistry by nano-catalysis. Green Chem 12:743–754
Krishna DSR, Siddharthan A, Seshadri SK, Kumar TS (2007) A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J Mater Sci Mater Med 18:1735–1743
Mirjalili BF, Zolfigol MA, Bamoniri A, Hazar A (2005) Al(HSO4)3 as an efficient catalyst for acetalization of carbonyl compounds under heterogeneous or solvent-free conditions. J Brazil Chem Soc 16:877–880
Mirjalili BF, Hashemi MM, Sadeghi B, Emtiazi H (2009) SnCl4/SiO2: an efficient heterogeneous alternative for one-pot synthesis of β-acetamidoketones. J Chin Chem Soc 56:386–391
Safajoo N, Mirjalili BF, Bamoniri A (2019) Fe3O4@nano-cellulose/Cu(II): a bio-based and magnetically recoverable nano-catalyst for the synthesis of 4H-pyrimido [2,1-b] benzothiazole derivatives. RSC Adv 9:1278–1283
Salehi N, Mirjalili BF (2017) Synthesis of highly substituted dihydro-2-oxopyrroles using Fe3O4@nano-cellulose–OPO3H as a novel bio-based magnetic nanocatalyst. RSC Adv 7:30303–30309
Azad S, Mirjalili BF (2016) Fe3O4@nano-cellulose/TiCl: a bio-based and magnetically recoverable nano-catalyst for the synthesis of pyrimido [2,1-b] benzothiazole derivatives. RSC Adv 6:96928–96934
Mirjalili BF, Reshquiyea RZ (2015) BF3/nano-sawdust as a green, biodegradable and inexpensive catalyst for the synthesis of highly substituted dihydro-2-oxopyrroles. RSC Adv 5:15566–15571
Su C, Suarez DL (1995) Coordination of adsorbed boron: a FTIR spectroscopic study. Environ Sci Technol 29:302–311
Ahmad R, Kumar R, Haseeb S (2012) Adsorption of Cu2+ from aqueous solution onto iron oxide coated eggshell powder: evaluation of equilibrium, isotherms, kinetics, and regeneration capacity. Arab J Chem 5:353–359
Valbe R, Tarkanovskaja M, Mäeorg U, Reedo V, Hoop A, Kink I, Lõhmus A (2014) Elaboration of hybrid cotton fibers treated with an ionogel/carbon nanotube mixture using a sol-gel approach. Open Chem 13:279–286
Zhu Y, Zhang L, Gao C, Cao L (2000) The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J Mater Sci 35:4049–4054
Keller OL (1963) Identification of complex ions of niobium (V) in hydrofluoric acid solutions by Raman and infrared spectroscopy. Inorg Chem 2:783–787
Aliabadi RS, Mahmoodi NO (2016) Green and efficient synthesis of pyranopyrazoles using [bmim][OH−] as an ionic liquid catalyst in water under microwave irradiation and investigation of their antioxidant activity. RSC Adv 6:85877–85884
Moosavi-Zare AR, Zolfigol MA, Salehi-Moratab R, Noroozizadeh E (2016) Catalytic application of 1-(carboxymethyl) pyridinium iodide on the synthesis of pyranopyrazole derivatives. J Mol Catal A Chem 415:144–150
Huang X, Li Z, Wang D, Li Y (2016) Bovine serum albumin: an efficient and green biocatalyst for the one-pot four-component synthesis of pyrano [2,3-c] pyrazoles. Chin J Catal 37:1461–1467
Tameh FA, Safaei-Ghomi J, Mahmoudi-Hashemi M, Shahbazi-Alavi H (2016) One-pot multicomponent reaction synthesis of spirooxindoles promoted by guanidine-functionalized magnetic Fe3O4 nanoparticles. RSC Adv 6:74802–74811
Pore DM, Patil PB, Gaikwad DS, Hegade PG, Patil JD, Undale KA (2013) Green access to novel spiro pyranopyrazole derivatives. Tetrahedron Lett 54:5876–5878
Muramulla S, Zhao CG (2011) A new catalytic mode of the modularly designed organocatalysts (MDOs): enantioselective synthesis of dihydropyrano [2,3-c] pyrazoles. Tetrahedron Lett 52:3905–3908
Zolfigol MA, Tavasoli M, Moosavi-Zare AR, Moosavi P, Kruger HG, Shiri M, Khakyzadeh V (2013) Synthesis of pyranopyrazoles using isonicotinic acid as a dual and biological organocatalyst. RSC Adv 3:25681–25685
Vasuki G, Kumaravel K (2008) Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett 49:5636–5638
Acknowledgements
The authors would like to thank Yazd University, Yazd, Iran.
Funding
This study was financially supported by Yazd University. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
ADT and BFM designed and performed the research, analyzed the data, interpreted the results and prepared the manuscript. ADT performed the assay, conducted the optimization, purification of compounds. AB and NS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dehghani Tafti, A., Mirjalili, B.F., Bamoniri, A. et al. Rapid four-component synthesis of dihydropyrano[2,3-c]pyrazoles using nano-eggshell/Ti(IV) as a highly compatible natural based catalyst. BMC Chemistry 15, 6 (2021). https://doi.org/10.1186/s13065-021-00734-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-021-00734-5