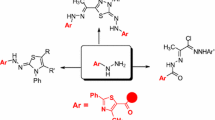
Abstract
Background
Thiazoles and 1,3,4-thiadiazoles have been reported to possess various pharmacological activities.
Results
A novel series of thiazoles carrying 1,3,4-thiadiazole core were designed and prepared via the reaction of the 2-(4-methyl-2-phenylthiazole-5-carbonyl)-N-phenylhydrazinecarbo-thioamide with the appropriate hydrazonoyl chlorides. The structures of the newly synthesized compounds were confirmed based on elemental and spectral analysis as well as their alternative syntheses. The cytotoxic potency of the newly synthesized thiadiazoles was evaluated by their growth inhibitory potency in liver HepG2 cancer cell line. Also, the structure activity relationship was studied.
Conclusions
All the newly synthesized compounds were evaluated for their anticancer activity against liver carcinoma cell line (HepG2) using MTT assay. The results revealed that the compounds 12d, 12c, 6g, 18b, 6c, and 6f (IC50 = 0.82, 0.91, 1.06, 1.25, 1.29 and 1.88 µM, respectively) had good antitumor activity against liver carcinoma cell line (HepG2) when compared with the standard drug Doxorubicin (IC50 = 0.72 µM).

A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety.
Similar content being viewed by others
Background
Cancer is the most common life-threatening disease representing a major health problem for many decades. The clinical application of chemotherapy still considered as a major compartment in treating cancer, however, it is often limited by the severity of the side effects and the development of tumor cell resistance to these cytotoxic agents. Clinical administration of high doses of anticancer drugs to overcome resistance leads to severe toxicities [1]. Therefore, the development of novel effective anticancer drugs and strategies is eagerly being pursued.
Also, it was reported that liver cancer is one from the top ten human cancers worldwide and among the top five of cancers in terms of mortality [2, 3]. A literature survey revealed that thiazole derivatives had many biological activities as antihypertension [4], antifungal [5], antimicrobial [6, 7], anti-inflammatory [8], antioxidant [9], antitubercular [10], and anticancer [11–14]. Moreover, 1,3,4-thiadiazole derivatives had many biological activities such as antibacterial, antifungal, antituberculosis, anti-hepatitis B viral, antileishmanial, anti-inflammatory, analgesic, CNS depressant, antioxidant, antidiabetic, molluscicidal, antihypertensive, diuretic, analgesic, antimicrobial, antitubercular, anticonvulsant and anticancer [15–24]. These important biological activities encouraged several researchers to find out different methods for synthesis of new thiadiazoles using different synthons, such as thiosemicarbazides, thiocarbazides, dithiocarbazates, thioacylhydrazines, acyl hydrazines, and bithioureas [25]. As a part of our research projects to synthesize new bioactive compounds [26–34], we intended in this research to synthesize a new series of thiazoles carrying 1,3,4-thiadiazole moiety in order to study their anticancer activity against liver carcinoma cell line (HepG2).
Results and discussion
Chemistry
2-(4-Methyl-2-phenylthiazole-5-carbonyl)-N-phenylhydrazinecarbothioamide (3) [35] was prepared via reaction of 4-methyl-2-phenylthiazole-5-carbohydrazide (2) with phenyl isothiocyanate in ethanol (EtOH) as depicted in Scheme 1.
The presence of the thioamide hydrazine moiety as a side chain in compound 3 prompted us to utilize it for constructing 1,3,4-thiadiazole ring through its reaction with many hydrazonoyl chlorides. Thus, treatment of compound 3 with the appropriate hydrazonoyl chlorides 4a–g [36] led to the formation of the respective 1,3,4-thiadiazoles 6a–g, rather than thiadiazines 7a–g or 1,3-thiazoles 8a–g (Scheme 1). The elemental analysis together with the spectral data are consistent with the proposed structure 6. The IR spectra of products 6 showed in each case the presence of two absorption bands around 1700, 1650 cm−1 for the two carbonyl groups, in addition to another band near v 3350 cm−1 for the NH function. The 1HNMR spectra of 6 showed in each case the presence of broad singlet signals assigned for the NH proton near δ 11.19 ppm, in addition to the expected signals for the two CH3, and the aryl protons. Also, the mass spectrum of each of products 6 revealed the presence of a molecular ion peak (see materials and methods). A suggested mechanism for the synthesis of 1,3,4-thiadiazole derivatives 6 is outlined in Scheme 1.
To explain the synthesis of 1,3,4-thiadiazole 6a–g, we assumed that the reaction started with S-alkylation to afford the non-isolable intermediate 5 followed by intramolecular cyclization and elimination of aniline molecule to give the respective thiadiazole derivatives 6a–g (Scheme 1). The structure of 6 was proved chemically via an alternative method (Scheme 1). Thus, the reaction of 5-(4-methyl-2-phenylthiazol-5-yl)-1,3,4-oxadiazole-2(3H)-thione (9) [37] with 4a in ethanol in the presence of triethylamine under reflux led to the formation of a product which is identical in all respects (mp, mixed mp, and IR) with compound 6a.
Next, to test of the biological activities of a vast array of these compounds, we reacted compound 3 with the appropriate hydrazonoyl chlorides 10a–d [38], under the same experimental conditions, which gave the corresponding 1,3,4-thiadiazole derivatives 12a–d (Scheme 2). The IR and 1H-NMR spectra of 12a taken as an example of the prepared series revealed the presence of the ester group and the disappearance of the hydrazone-NH function. Also, the mass spectrum of the reaction products 12a–d showed, in each case, a peak corresponding to their molecular ions. The structure assigned for product 12 was further evidenced via an alternative method. Thus, the reaction of ethyl 4-methyl-2-phenyl thiazole-5-carboxylate (1) with 1,3,4-thiadiazole 15 [37] in ethanol under reflux, gave a product which was typical in all respects (mp, mixed mp, and IR) with that obtained from the reaction of 3 with 10a (Scheme 2). To account for the formation of the product 12, it was suggested that the reaction of compound 3 with hydrazonoyl chloride 10 initially gave the intermediate 11, which underwent nucleophilic addition, followed by cyclization via losing of aniline molecule (route a) to give the final product 12. The other routes (b) and (c) outlined in Scheme 2 were excluded since they led to the formation of products 13 and 14, which were completely different in all respects (IR, 1H-NMR, mass spectra) from products 12.
Moreover, the reaction of compound 3 with hydrazonoyl halide of type 16 was studied. Thus refluxing compound 3 with the hydrazonoyl chloride 16a or 16b [38] under the same experimental conditions, afforded the corresponding 1,3,4-thiadiazole derivatives 18a, b (Scheme 3). The 1H-NMR spectrum of compound 18a revealed two D2O-exchangeable signals at δ 10.18 and 11.72 corresponding to two NH protons, in addition to an aromatic multiplet in the region 7.02–7.78 ppm. Also, its mass spectrum revealed a molecular ion peak at m/z = 512. In addition, compound 18a was proved chemically via an alternative method from the reaction of compound 9 with 16a which gave a product identical in all respects (mp, mixed mp, and IR) with compound 18a.
Cytotoxic activity
The Literature survey showed that many 1,3-thiazole and 1,3,4-thiadiazole derivatives have antitumor activity with excellent IG50 and IC50 as depicted in Fig. 1 [38–44].
In view of these facts, the antitumor activity of the synthesized compounds was determined against liver carcinoma cell line HepG2. Doxorubicin was used as a reference standard. Data generated were used to plot a dose–response curve of which the concentration (μM) of test compounds required to kill 50% of the cell population (IC50) was determined. Cytotoxic activity was expressed as the mean IC50 of three independent experiments. The results depicted in Table 1.

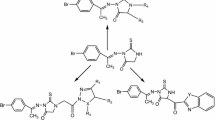
The results of Table 1 revealed that most of the tested compounds showed a great variable activity compared to reference drug. The order of their antitumor activity and the influence of the substituents were shown in Fig. 2.
From the results of Table 1 and Fig. 2, we can suggest the following points.
-
The ester group (CO2Et) at position 2 of the thiadiazole ring is necessary to have higher antitumor activity than the acetyl and the N-phenyl carboxamide (CONHPh) groups.
-
The presence of chlorine group (electron-withdrawing group) at the position 2, 4 or 4 in the aryl moiety of the thiadiazole ring increased the cytotoxic activity.
-
Chlorine at positions 2, 4 or 4 in the aryl moiety had high cytotoxic activity than halogen at position 3.
-
The compounds containing chlorine had high cytotoxic activity than the compounds containing bromine.
-
The presence of electron-donating groups such as methyl or methoxy at the position 4 in the aryl moiety as in the compounds 12b, 6b and 6d decreased the cytotoxic activity.
Experimental
Chemistry
General
Melting points were measured on an Electrothermal IA 9000 series digital melting point apparatus (Bibby Sci. Lim. Stone, Staffordshire, UK). IR spectra were measured on PyeUnicam SP 3300 and Shimadzu FTIR 8101 PC infrared spectrophotometers (Shimadzu, Tokyo, Japan) in potassium bromide discs. NMR spectra were measured on a Varian Mercury VX-300 NMR spectrometer (Varian, Inc., Karlsruhe, Germany) operating at 300 MHz (1H-NMR) and run in deuterated dimethylsulfoxide (DMSO-d 6 ). Chemical shifts were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer (Tokyo, Japan) at 70 eV. Elemental analyses were measured by using a German made Elementar vario LIII CHNS analyzer. Antitumor activity of the products was measured at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt. 2-(4-Methyl-2-phenylthiazole-5-carbonyl)-N-phenylhydrazinecarbo-thioamide (3) [37], 5-(4-methyl-2-phenylthiazol-5-yl)-1,3,4-oxadiazole-2(3H)-thione (9) [37], hydrazonoyl halides 4a–g, 10a–d and 16a, b [38], and ethyl 5-hydrazono-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (15) [37] were prepared as reported in the respective literature.
Synthetic procedures
Synthesis of 1,3,4-thiadiazole derivatives (6a–g, 12a–d and 18a, b)
General procedure A mixture of compound 3 (0.368 g, 1 mmol) and the appropriate hydrazonoyl chlorides 4a–g or 10a–d or 16a, b (1 mmol) in ethanol (20 mL), triethylamine (0.1 g, 1 mmol) was added. The mixture was refluxed for 4–6 h. The formed solid product was filtered, washed with methanol, dried and recrystallized from the proper solvents to afford products 6a–g, 10a–d and 18a, b, respectively. The physical constants and spectral data of the obtained products are listed below:
N′-(5-Acetyl-3-phenyl-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenyl thiazole-5-carbohydrazide (6a)
Yellow solid (73%); m.p. 163–165 °C (EtOH); IR (KBr) v 3317 (NH), 3038, 2951 (CH), 1701, 1647 (2C=O), 1593 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.44 (s, 3H, CH3CO), 2.74 (s, 3H, CH3–thiazole), 6.92–8.00 (m, 10H, ArH), 11.19 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 16.9, 24.9 (CH3), 114.8, 117.1, 120.9, 121.9, 123.4, 126.2, 128.9, 129.2, 129.4, 130.9, 138.3, 141.7, 159.4 (Ar–C and C=N), 167.9, 194.0 (C=O); MS m/z (%) 435 (M+, 10), 381 (13), 274 (56), 118 (31), 92 (100), 65 (38). Anal. Calcd. for C21H17N5O2S2 (435.52): C, 57.91; H, 3.93; N, 16.08. Found C, 57.86; H, 3.84; N, 16.00%.
N′-(5-Acetyl-3-(p-tolyl)-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenyl thiazole-5-carbohydrazide (6b)
Yellow solid (75%); m.p. 149–151 °C (EtOH); IR (KBr) v 3334 (NH), 3019, 2920 (CH), 1699, 1648 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.31 (s, 3H, CH3-Ar), 2.44 (s, 3H, CH3CO), 2.73 (s, 3H, CH3–thiazole), 6.98–7.89 (m, 9H, ArH), 11.18 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 16.0, 17.7, 19.4 (CH3), 116.0, 118.0, 120.8, 125.1, 126.7, 127.3, 128.1, 129.8, 131.9, 132.7, 138.2, 152.6, 159.4 (Ar–C and C=N), 166.5, 194.7 (C=O); MS m/z (%) 449 (M+, 45), 372 (54), 200 (27), 104 (36), 80 (100), 64 (35). Anal. Calcd. for C22H19N5O2S2 (449.55): C, 58.78; H, 4.26; N, 15.58. Found C, 58.65; H, 4.17; N, 15.46%.
N′-(5-Acetyl-3-(4-chlorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenyl-thiazole-5-carbohydrazide (6c)
Brown solid (75%); m.p. 171–173 °C (EtOH); IR (KBr) v 3325 (NH), 3013, 2926 (CH), 1698, 1655 (2C=O), 1594 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.45 (s, 3H, CH3CO), 2.76 (s, 3H, CH3–thiazole), 6.93–7.96 (m, 9H, ArH), 11.25 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 16.8, 24.9 (CH3), 115.1, 119.4, 120.2, 122.9, 123.8, 127.3, 128.3, 128.7, 129.0, 133.5, 138.3, 140.2, 157.9 (Ar–C and C=N), 167.8, 194.3 (C=O); MS m/z (%) 471 (M++2, 14), 469 (M+, 45), 396 (57), 200 (17), 80 (100), 64 (89). Anal. Calcd. for C21H16ClN5O2S2 (469.97): C, 53.67; H, 3.43; N, 14.90. Found C, 53.52; H, 3.37; N, 14.82%.
N′-(5-Acetyl-3-(4-methoxyphenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenyl-thiazole-5-carbohydrazide (6d)
Brown solid (68%); m.p. 143–145 °C (EtOH); IR (KBr) v 3328 (NH), 3031, 2923 (CH), 1697, 1653 (2C=O), 1596 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.45 (s, 3H, CH3CO), 2.75 (s, 3H, CH3–thiazole), 3.76 (s, 3H, OCH3), 6.99–7.99 (m, 9H, ArH), 11.29 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 16.5, 17.9, 54.2 (CH3), 116.2, 117.9, 120.7, 124.8, 126.3, 127.0, 127.7, 129.3, 131.9, 132.4, 137.6, 150.2, 159.0 (Ar–C and C=N), 166.2, 194.6 (C=O); MS m/z (%) 465 (M+, 39), 334 (87), 200 (63), 122 (80), 77 (100), 64 (45). Anal. Calcd. for C22H19N5O3S2 (465.55): C, 56.76; H, 4.11; N, 15.04. Found C, 56.63; H, 4.04; N, 14.95%.
N′-(5-Acetyl-3-(3-chlorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenylthiazole-5-carbohydrazide (6e)
Yellow solid (70%); m.p. 166–168 °C (EtOH); IR (KBr) v 3431(NH), 3025, 2932 (CH), 1698, 1659 (2C=O), 1593 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.44 (s, 3H, CH3CO), 2.66 (s, 3H, CH3–thiazole), 6.98–7.90 (m, 9H, ArH), 11.23 (s, br, 1H, D2O-exchangeable NH); MS m/z (%) 471 (M++2, 10), 469 (M+, 34), 334 (46), 200 (28), 132 (48), 80 (100), 64 (68). Anal. Calcd. for C21H16ClN5O2S2 (469.97): C, 53.67; H, 3.43; N, 14.90. Found C, 53.60; H, 3.36; N, 14.79%.
N′-(5-Acetyl-3-(4-bromophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenyl thiazole-5-carbohydrazide (6f)
Brown solid (73%); m.p. 160–162 °C (EtOH); IR (KBr) v 3429 (NH), 3012, 2924 (CH), 1696, 1654 (2C=O), 1594 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.44 (s, 3H, CH3CO), 2.65 (s, 3H, CH3–thiazole), 6.95–7.94 (m, 9H, ArH), 11.25 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 16.9, 24.8 (CH3), 114.8, 120.3, 122.0, 122.6, 123.8, 127.2, 127.9, 128.3, 130.2, 132.5, 136.9, 140.0, 157.5 (Ar–C and C=N), 167.6, 194.1 (C=O); MS m/z (%) 516 (51), 514 (M+, 53), 325 (76), 172 (44), 91 (80), 80 (100), 64 (47). Anal. Calcd. for C21H16BrN5O2S2 (514.42): C, 49.03; H, 3.14; N, 13.61. Found C, 48.93; H, 3.12; N, 13.53%.
N′-(5-Acetyl-3-(2,4-dichlorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-4-methyl-2-phenyl thiazole-5-carbohydrazide (6g)
Brown solid (77%); m.p. 181–183 °C (EtOH/dioxane); IR (KBr) v 3318 (NH), 3088, 2926 (CH), 1699, 1671 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.47 (s, 3H, CH3CO), 2.67 (s, 3H, CH3–thiazole), 6.97–8.07 (m, 8H, ArH), 11.19 (s, br, 1H, D2O-exchangeable NH); MS m/z (%) 504 (M+, 14), 407 (33), 161 (14), 80 (99), 64 (100). Anal. Calcd. for C21H15Cl2N5O2S2 (504.41): C, 50.00; H, 3.00; N, 13.88. Found C, 49.88; H, 2.92; N, 13.75%.
Ethyl 5-(2-(4-methyl-2-phenylthiazole-5-carbonyl)hydrazono)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (12a)
Yellow solid (71%); m.p. 137–139 °C (EtOH); IR (KBr) v 3432 (NH), 3035, 2923 (CH), 1749, 1659 (2C=O), 1597 (C = N) cm−1; 1H-NMR (DMSO-d 6) δ 1.20 (t, 3H, J = 7.1 Hz, CH2CH 3 ), 2.74 (s, 3H, CH3–thiazole), 4.21 (q, 2H, J = 7.1 Hz, CH 2 CH3),7.00–8.01 (m, 10H, ArH), 10.72 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 13.7, 16.8 (CH3), 61.2 (CH2), 115.8, 117.3, 118.4, 120.9, 122.4, 126.0, 128.5, 128.9, 130.0, 132.6, 135.6, 139.6, 159.1 (Ar–C and C=N), 163.4, 166.8 (C=O); MS m/z (%): 465 (M+, 27), 334 (50), 200 (34), 104 (40), 80 (100), 64 (37). Anal. Calcd. for C22H19N5O3S2 (465.55): C, 56.76; H, 4.11; N, 15.04. Found C, 56.69; H, 4.03; N, 15.01%.
Ethyl 5-(2-(4-methyl-2-phenylthiazole-5-carbonyl)hydrazono)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (12b)
Yellow solid (70%); m.p. 147–149 °C (EtOH); IR (KBr) v 3424 (NH), 3058, 2925 (CH), 1749, 1674 (2C=O), 1595 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 1.20 (t, 3H, J = 7.1 Hz, CH2CH 3 ), 2.26 (s, 3H, CH3–Ar), 2.76 (s, 3H, CH3–thiazole), 4.19 (q, 2H, J = 7.1 Hz, CH 2 CH3), 7.00–8.02 (m, 9H, ArH), 10.73 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 13.9, 16.8, 20.1 (CH3), 61.5 (CH2), 114.5, 115.8, 117.1, 120.9, 121.9, 126.2, 128.1, 129.6, 130.8, 131.8, 138.3, 140.0, 159.4 (Ar–C and C=N), 163.0, 166.5 (C=O); MS m/z (%) 479 (M+, 20), 367 (25), 251 (18), 80 (85), 64 (100). Anal. Calcd. for C23H21N5O3S2 (479.57): C, 57.60; H, 4.41; N, 14.60. Found C, 57.49; H, 4.33; N, 14.51%.
Ethyl 4-(4-chlorophenyl)-5-(2-(4-methyl-2-phenylthiazole-5-carbonyl) hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (12c)
Yellow solid (73%); m.p. 167–169 °C (EtOH/dioxane); IR (KBr) v 3340 (NH), 3050, 2927 (CH), 1748, 1670 (2C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 1.23 (t, 3H, J = 7.1 Hz, CH2CH 3 ), 2.75 (s, 3H, CH3–thiazole), 4.22 (q, 2H, J = 7.1 Hz, CH 2 CH3),7.02–7.96 (m, 9H, ArH), 10.77 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 13.4, 16.9 (CH3), 61.4 (CH2), 116.2, 117.0, 119.5, 120.9, 122.3, 127.2, 128.2, 129.4, 131.4, 132.2, 137.0, 139.4, 158.6 (Ar–C and C=N), 163.8, 167.2 (C=O); MS m/z (%) 501 (M++2, 13), 499 (M+, 45), 363 (39), 334 (100), 200 (35), 104 (30), 77 (50). Anal. Calcd. for C22H18ClN5O3S2 (499.99): C, 52.85; H, 3.63; N, 14.01. Found C, 52.79; H, 3.60; N, 13.87%.
Ethyl 4-(2,4-dichlorophenyl)-5-(2-(4-methyl-2-phenylthiazole-5-carbonyl) hydrazono)-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (12d)
Brown solid (75%); m.p. 173–175 °C (EtOH/dioxane); IR (KBr) v 3221 (NH), 3079, 2926 (CH), 1749, 1671 (2C=O), 1599 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 1.24 (t, 3H, J = 7.1 Hz, CH2CH 3 ), 2.77 (s, 3H, CH3-thiazole), 4.23 (q, 2H, J = 7.1 Hz, CH 2 CH3),7.08–8.13 (m, 8H, ArH), 10.77 (s, br, 1H, D2O-exchangeable NH); MS m/z (%) 534 (M+, 19), 449 (78), 223 (100), 200 (54), 104 (58), 80 (85). Anal. Calcd. for C22H17Cl2N5O3S2 (534.44): C, 49.44; H, 3.21; N, 13.10. Found C, 49.29; H, 3.16; N, 13.02%.
5-(2-(4-Methyl-2-phenylthiazole-5-carbonyl)hydrazono)-N,4-diphenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide (18a)
Brown solid (76%); m.p. 176–178 °C (EtOH/dioxane); IR (KBr) v 3427, 3343 (2NH), 1672, 1653 (2C=O), 1597 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.75 (s, 3H, CH3–thiazole), 7.02–7.78 (m, 15H, ArH), 10.18 (s, br, 1H, D2O-exchangeable NH), 11.72 (s, br, 1H, D2O-exchangeable NH); 13C-NMR (DMSO-d 6): δ 17.2 (CH3), 114.6, 117.3, 118.4, 120.9, 122.6, 122.8, 124.0, 126.5, 128.5, 129.1, 130.0, 130.6, 131.9, 132.6, 138.2, 142.1, 159.2 (Ar–C and C=N), 162.6, 166.0 (C=O); MS m/z (%) 512 (M+, 8), 401 (00), 282 (10), 150 (22), 92 (26), 65 (29). Anal. Calcd. For C26H20N6O2S2 (512.61): C, 60.92; H, 3.93; N, 16.39. Found C, 60.78; H, 3.85; N, 16.32%.
4-(2,4-Dichlorophenyl)-5-(2-(4-methyl-2-phenylthiazole-5-carbonyl) hydrazono)-N-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxamide (18b)
Brown solid (77%); m.p. 186–188 °C (Dioxane); IR (KBr) v 3429, 3337 (2NH), 1692, 1656 (2C=O), 1591 (C=N) cm−1; 1H-NMR (DMSO-d 6) δ 2.76 (s, 3H, CH3–thiazole), 7.13–7.83 (m, 13H, ArH), 10.19 (s, br, 1H, D2O-exchangeable NH), 11.77 (s, br, 1H, D2O-exchangeable NH); MS m/z (%) 581 (M+, 38), 473 (64), 334 (72), 200 (35), 119 (65), 64 (100). Anal. Calcd. for C26H18Cl2N6O2S2 (581.50): C, 53.70; H, 3.12; N, 14.45. Found C, 53.62; H, 3.03; N, 14.32%.
Alternate synthesis of thiadiazole derivatives 6a and 18a
To a mixture of 5-(4-methyl-2-phenylthiazol-5-yl)-1,3,4-oxadiazole-2(3H)-thione (9) (0.275 g, 1 mmol) and hydrazonoyl chloride 4a or 16a (1 mmol) in absolute EtOH (25 mL), was added triethylamine (0.1 g, 0.14 mL, 1 mmol). The reaction mixture was stirred at room temperature till methyl mercaptan ceased to evolve (3 h). The solvent was evaporated and the residue was treated with ice/HCl mixture. The solid product was collected by filtration, washed with EtOH, dried, and recrystallized to give the respective compounds 6a and 18a, that was identical in all respects (m.p., mixed m.p. and IR spectra) with that obtained from the reaction of 4a or 16a with 3.
Alternate synthesis of 12a
A mixture of ethyl 4-methyl-2-phenylthiazole-5-carboxylate (1) (0.247 g, 1 mmol) and ethyl 5-hydrazono-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carboxylate (15) (0.264 g, 1 mmol) was refluxed in ethanol for 4 h. The solid product that separated was filtered off, washed with water and finally recrystallized to give the corresponding product, 12a which was identical in all aspects (m.p., mixed m.p. and IR spectra) with those obtained from the reaction of 3 with 10a.
Evaluation of the antitumor activity using Viability assay
Human hepatocellular carcinoma (HepG2) cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD). The detailed procedure for the in vitro antitumor assay is presented in Additional file 1.
Conclusions
A series of novel thiazoles carrying 1,3,4-thiadiazole ring were synthesized. The structure of the newly prepared compounds was established based on both elemental analysis and spectroscopic data and by an alternative method wherever possible. All the synthesized compounds were evaluated for their anti-cancer activity against the human hepatocellular carcinoma (HepG2) cell line. The results showed that the thiazole derivatives 12d, 12c, 6g,18b, 6c and 6f having IC50 values 0.82, 0.91, 1.06, 1.25, 1.29 and 1.88 µM, respectively, were found to be the highly active compounds of the prepared series. Based on the experimental results of the antitumor activity, the structure–activity relationships were discussed.
Abbreviations
- HepG2:
-
human hepatocellular carcinoma
- SAR:
-
structure activity relationship
- EtOH:
-
ethanol
- m.p.:
-
melting point
- TEA:
-
triethylamine
- IR:
-
infra-red
- ATCC:
-
American Type Culture Collection
- TLC:
-
thin layer chromatography
References
Vijayaraghavalu S, Peetla C, Lu S, Labhasetwar V (2012) Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol Pharm 9:2730–2742
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG Jr, Connolly CJC, Doherty AM, Klutchko SR, Sircar I, Steinbaugh BA, Batley BL, Painchaud CA, Rapundalo ST, Michniewicz BM, Olson SCJ (1992) Structure–activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J Med Chem 35:2562–2572
Gomha SM, Abdel-Aziz HA (2012) Synthesis of new heterocycles derived from 3-(3-methyl-1H-indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull Korean Chem Soc 33:2985–2990
Karegoudar P, Karthikeyan MS, Prasad DJ, Mahalinga M, Holla BS, Kumari NS (2008) Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur J Med Chem 43:261–267
Cukurovali A, Yilmaz I, Gur S, Kazaz C (2006) Synthesis, antibacterial and antifungal activity of some new thiazolylhydrazone derivatives containing 3-substituted cyclobutane ring. Eur J Med Chem 41:201–207
Sharma PK, Sawhney SN, Gupta A, Singh GB, Bani S (1998) Synthesis and antiinflammatory activity of some 3-(2-thiazolyl)-1,2-benzisothiazoles. Indian J Chem 37B:376–381
Shih MH, Ying KF (2004) Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg Med Chem 12:4633–4643
Shiradkar M, Kumar GVS, Dasari V, Tatikonda S, Akula KC, Shah R (2007) Clubbed triazoles: a novel approach to antitubercular drugs. Eur J Med Chem 42:807–816
Gomha SM, Khalil KD (2012) A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 17:9335–9347
Gomha SM, Salah TA, Abdelhamid AO (2015) Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh Chem 146:149–158
Gomha SM, Riyadh SM, Abbas IM, Bauomi MA (2013) Synthetic utility of ethylidene thiosemicarbazide: synthesis and anti-cancer activity of 1,3-thiazines and thiazoles with imidazole moiety. Heterocycles 87:341–356
Gomha SM, Salah TA, Hassaneen HME, Abdel-aziz H, Khedr MA (2016) Synthesis, characterization and molecular docking of novel bioactive thiazolyl-thiazole derivatives as promising cytotoxic antitumor drug. Molecules 21:1–17
Kushwaha N, Kushwaha SKS, Rai AK (2012) Biological Activities of thiadiazole derivatives: a review. Inter J Chem Res. 4:517–531
Ak Singh, Mishra G, Jyoti K (2011) Review on biological activities of 1,3,4-thiadiazole derivatives. J App Pharm Sci 1:44–49
Siddiqui N, Ahuja P, Ahsan W, Pandeya SN, Alam MS (2009) Thiadiazoles: progress report on biological activities. J Chem Pharmaceutical Res. 1:19–30
Gomha SM, Riyadh SM (2011) Synthesis under microwave irradiation of [1,2,4] triazolo [3,4-b][1,3,4] thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 16:8244–8256
Gomha SM, Ahmed SA, Abdelhamid AO (2015) Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin- 5(1H)-one incorporating triazole moiety. Molecules 20:1357–1376
Gomha SM, Khalil KD, El-Zanate AM, Riyadh SM (2013) A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 87:1109–1120
Gomha SM, Badrey MG, Edrees MM (2016) Heterocyclisation of 2,5-diacetyl-3,4-disubstituted-thieno[2,3-b]thiophene bis-thiosemicarbazones leading to bis-thiazoles and bis-1,3,4-thiadiazoles as anti-breast cancer agents. J Chem Res 40:120–125
Zhang LJ, Yang MY, Sun ZH, Tan CX, Weng JQ, Wu HK, Liu XH (2014) Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett Drug Des Discov 11:1107–1111
Farag AM, Kheder NA, Mabkhot YM (2009) synthesis and antimicrobial evaluation of new pyrazole, thiophene, thiazole and 1,3,4-thiadiazole derivatives incorporating pyrimidine ring. Heterocycles 78:1787–1798
Kheder NA, Mabkhot YN, Farag AM (2008) synthesis and antimicrobial evaluation of some bis(thioxopyridine), bis(pyrazolo[3,4-b]pyridine), bis(thieno[2,3-b]pyridine), bis(1,3,4- thiadiazole) and bis-thiophene derivatives. Heterocycles 75:2937–2948
Shawali AS (2014) 1,3,4-Thiadiazoles of pharmacological interest: Recent trends in their synthesis via tandem 1,3-dipolar cycloaddition. J Adv Res 5:1–17
Gomha SM (2009) A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno [2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem 140:213–220
Gomha SM, Eldebss TMA, Abdulla MM, Mayhoub AS (2014) Diphenylpyrroles: novel p53 activators. Eur J Med Chem 82:472–479
Gomha SM, Badrey MG, Abdalla MM, Arafa RK (2014) Novel anti-HIV-1 NNRTIs based on a pyrazolo[4,3-d]isoxazole backbone scaffold: design, synthesis and insights into the molecular basis of action. Med Chem Commun 5:1685–1692
Eldebss TMA, Gomha SM, Abdulla MM, Arafa RK (2015) Regioselective synthesis of novel substituted pyrrole with protein kinases inhibitor activates selectively for VEGFR-2, EGFR, PKA and CHK1. Med Chem Com 6:977–987
Gomha SM, Eldebss TM, Badrey MG, Abdulla MM, Mayhoub AS (2015) Novel 4-heteroaryl-antipyrines as DPP-IV inhibitors. Chem Biol Drug Des 86:1292–1303
Mabkhot YN, Alatibi F, El-Sayed NN, Al-Showiman S, Kheder NA, Wadood A, Rauf A, Bawazeer S, Hadda T (2016) Antimicrobial activity of some novel armed thiophene derivatives and Petra/Osiris/Molinspiration (POM) analyses. Molecules 21:222
Kheder NA (2016) Hydrazonoyl chlorides as precursors for synthesis of novel bis-pyrrole derivatives. Molecules 21(326):1–9
Gomha SM, Kheder NA, Abdelhamid AO, Mabkhot YN (2016) One Pot single step synthesis and biological evaluation of some novel bis(1,3,4-thiadiazole) derivatives as potential cytotoxic agents. Molecules 21(1532):1–8
Kheder NA, Mabkhoot YN (2012) Synthesis and antimicrobial studies of some novel bis-[1,3,4]thiadiazole and bis-thiazole pendant to thieno[2,3-b]thiophene moiety. Int J Mol Sci 13:3661–3670
Tiperciuc B, Zaharia V, Colosi I, Moldovan C, Crişan O, Pîrnau A, Vlase L, Duma M, Oniga O (2012) Synthesis and evaluation of antimicrobial activity of some new hetaryl-azoles derivatives obtained from 2-aryl-4-methylthiazol-5-carbohydrazides and isonicotinic acid hydrazide. J Heterocycl Chem 49:1407–1414
Shawali S, Gomha SMA (2000) New entry for short and regioselective synthesis of [1,2,4]triazolo[4,3-b][1,2,4]-triazin-7(1H)-one. Adv Synth Catal 342:599–604
Abdelhamid AO, Zohdi HF, Rateb NM (1998) Reactions with hydrazonoyl halides XXI: reinvestigation of the reactions of hydrazonoyl bromides with 1,1-dicyanothio-acetanilide. J Chem Res (S) 3:184–185.
Qiu XL, Li G, Wu G, Zhu J, Zhou L, Chen PL, Chamberlin AR, Lee WH (2009) Synthesis and biological evaluation of a series of novel inhibitor of Nek2/Hec1 analogues. J Med Chem 52:1757–1767
Tsoua H, MacEwan G, Birnberg G, Grosu G, Bursavich MG, Bard J, Brooijmansa N, Toral-Barzab L, Hollanderb I, Mansoura TS, Ayral-Kaloustiana S, Yub K (2010) Discovery and optimization of 2-(4-substituted-pyrrolo[2,3-b]pyridin-3-yl) methylene-4-hydroxybenzofuran- 3(2H)-ones as potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20:2321–2325
Mavrova AT, Wesselinova D, Tsenov YA, Denkova P (2009) Synthesis, cytotoxicity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur J Med Chem 44:63–69
Oleson JJ, Slobada A, Troy WP, Halliday SL, Landes MJ, Angier RB, Semb J, Cyr K, Williams JH (1955) The carcinostatic activity of some 2-amino-1,3,4-thiadiazoles. J Am Chem Soc 77:6713–6714
Matysiak J, Opolski A (2006) Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorg Med Chem 14:4483–4489
Kaminskyy D, Kryshchyshyn A, Nektegayev I, Vasylenko O, Grellier P, Lesyk R (2014) Isothiocoumarin-3-carboxylic acid derivatives: synthesis, anticancer and antitrypanosomal activity evaluation. Eur J Med Chem. 75:57–66
Popsavin M, Spaić S, Svirčev M, Kojić V, Bogdanović G, Popsavin V (2012) Synthesis and in vitro antitumour screening of 2-(β-d-xylofuranosyl)thiazole-4-carboxamide and two novel tiazofurin analogues with substituted tetrahydrofurodioxol moiety as a sugar mimic. Bioorg Med Chem Lett 22:6700–6704
Authors’ contributions
SMG designed research; SMG, MRA and NAK performed research and analyzed the data; SMG, NAK, YNM and AMA wrote and approved the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors extend their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding this Prolific Research group (PRG-1437-29).
Competing interests
The authors declare that they have no competing interests.
Sample availability
Samples of the compounds are available from the authors.
Author information
Authors and Affiliations
Corresponding author
Additional file
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gomha, S.M., Kheder, N.A., Abdelaziz, M.R. et al. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety. Chemistry Central Journal 11, 25 (2017). https://doi.org/10.1186/s13065-017-0255-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-017-0255-7