Abstract
Background
A novel series of 5-(substituted aldehyde)-7-methyl-3-oxo-N-phenyl-2-((3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)methylene)-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide analogues (1–24) was synthesized using the Biginelli condensation.
Results and discussion
The synthesized compounds were screened for their in vitro antimicrobial potential against Gram (positive and negative) bacterial and fungal strains by tube dilution technique. In the series, compound 15 exhibited significant antimicrobial activity against Candida albicans and Aspergillus niger with MIC value = 1.04 × 10−2 µM/ml and compound 2 was found to be most active antioxidant agent with IC50 value = 46.31 using DPPH assay. Anticancer activity results indicated that compound 23 displayed better anticancer activity against human breast cancer cell line (MCF-7) with GI50 value = 34.78 using SRB assay.
Conclusions
All synthesized derivatives exhibited good antimicrobial, antioxidant and anticancer activity using specific method and compared with standard drugs, especially compounds 2, 15 and 23 displayed more activity than reference drugs. Structure activity relationship demonstrated that presence of electron releasing groups of the synthesized compounds enhanced the antibacterial activity against Escherichia coli as well as antioxidant activity and electron withdrawing groups improved the antimicrobial as well as anticancer activity against human breast (MCF-7) cancer cell line.
Similar content being viewed by others
Background
Pyrimidines are obtained from the various natural resources and synthethic reaction in medicinal chemistry [1]. They are also known as m-diazine or 1,3-diazone can be considered as cyclic amine. Heterocyclic compounds are used in agricultural and medicinal reasons using biological and chemical studies. Pyrimidine derivatives play a vital role in several biological activities i.e. antihypertensive, anticancer, antimicrobial, anti-inflammatory, antifungal, analgesic, antioxidant, anticonvulsant and antiviral [2]. Antimicrobials agents are one of the most important weapons in the resistance of infection caused by bacterial strains [3]. In the past few years, increase the resistance of microorganisms toward antimicrobial agents become a serious health problem so there is a need of safe, potent and novel antimicrobial agents [4]. Pyrimidine derivatives showed most antimicrobial activity against Gram +ve and Gram –ve microorganism [5]. At that time, many antimicrobial drugs are present in the market but due to the indiscriminate use of antimicrobial agents often followed the development of resistant strains of microorganism so there is a need for the development of new class of active antimicrobial drugs with lesser or no side effects [6]. Pyrimidine agents recently attracted medicinal chemist in exploring their potential as antioxidant agents. Oxidative stress appears to play an important role in many human diseases, including cancers. The use of antioxidants in pharmacology is intensively studied, particularly for stroke and neurodegenerative diseases [7]. Antioxidants are the agents that neutralize free radicals, which scavenge reactive oxygen species may be high potent value in preventing the onset and propagation of oxidative diseases like neurovascular, autoimmune and cardiovascular diseases [8].
Cancer is one of the most serious medical problem and second leading cause of death in the world, characterized by a deregulation of the cell cycle which mainly results in a progressive loss of cellular differentiation and uncontrolled cellular growth. The current situation highlights the need for discovery and development of small molecule anticancer drugs with improved tumor selectivity, efficacy and safety remains desirable [9]. Many pyrimidine derivatives were reported to be active against various forms of cancer. Due to less effective, more side effect and lack of a broad range of anticancer agents there is a need of anticancer agents have motivated the idea of researchers toward the discovery of novel anticancer agents [10]. Owing to the pharmacological significance of pyrimidine derivatives so, we have planned to synthesize some new pyrimidine derivatives and evaluate for their antimicrobial, antioxidant and anticancer activities.
Results and discussion
Chemistry
In the research work, we have synthesized new series of 5-(substituted aldehyde)-7-methyl-3-oxo-N-phenyl-2-((3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)methylene)-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide analogues using the Biginelli condensation and synthetic steps of this series showing in Scheme 1. The physiochemical properties (molecular formula; molecular weight; melting points; percentage yield etc.) of the synthesized analogues are presented in Table 1. The chemical structures of the synthesized compounds were confirmed by 1H/13C-NMR, FT-IR, Mass spectral and elemental analysis studies. The elemental analysis results of synthesized compounds were within ±0.4% of the theoretical values.
Antimicrobial activity
The in vitro antimicrobial activity of synthesized compounds against Gram-positive bacteria: Staphylococcus aureus (MTCC 3160), Bacillus subtilis (MTCC 441), Gram-negative bacterium: Escherichia coli (MTCC 443) and fungal: Candida albicans (MTCC 227) and Aspergillus niger (MTCC 281) strains was examined by tube dilution method [11]. Norfloxacin and fluconazole used as standard for antibacterial and antifungal activities respectively. Dilutions of test and standard compounds were prepared in double strength nutrient broth for bacterial strains and sabouraud dextrose broth for fungal strains [12]. The samples were incubated at 37 ± 1 °C for 24 h (for bacterial species), at 25 ± 1 °C for 7 days (A. niger) and at 37 ± 1 °C for 48 h (C. albicans) respectively and the results were recorded in terms of MIC (the lowest concentration of test substance which inhibited the growth of microorganisms). In case of Gram positive bacteria, compounds 12 and 14 (MICsa = 2.14 × 10−2 µM/ml) having significant activity against S. aureus and compound 18 (MICbs = 0.58 × 10−2 µM/ml) exhibited most potent against B. subtilis. In case of Gram negative bacterium, compound 21 (MICec = 1.10 × 10−2 µM/ml) displayed more potent activity against E. coli. Compound 15 (MICca & an = 1.04 × 10−2 µM/ml) was found to be most potent against C. albicans and A. niger. These compounds may be taken as lead to discovery novel antimicrobial agents. The presented results are showing in Table 2.
Antioxidant activity
The antioxidant activity of the synthesized compounds was evaluated with spectrophotometrically using free radical scavenging DPPH assay. The DPPH is a stable free radical with maximal absorption at 517 nm and is reduced to a corresponding hydrazine when it reacts with hydrogen donors. When DPPH reacts with an antioxidant agent, it can donate hydrogen get reduced and deep violet colour of DPPH change to yellow, showing a considerable decreased in absorption at 517 nm. DPPH solution (3 μg/ml) was prepared in methanol (methanol: DPPH in 1:1) for blank reference. Four types of dilutions were prepared in the methanol of the synthesized derivatives and standard (ascorbic acid) in the concentration of 25, 50, 75 and 100 μg/ml and then 1 ml of each concentration was added to 1 ml of DPPH solution. The solution mixture was shaken vigorously and kept in dark place for 30 min at room temperature and absorbance was measured by UV at 517 nm [13]. Free radical DPPH inhibition in percentage (%) was calculated as follows:
where, ABlank = absorbance of the blank reaction, ASample = absorbance of the test compound
IC50 value was calculated from the graph plotted between % inhibition and synthesized compound (Figs. 1, 2, 3). Antioxidant activity demonstrated, compounds 2 and 16 exhibited excellent activity at absorbance 517 nm with IC50 values = 46.31 and 48.81 respectively and compared with ascorbic acid as standard drug. These compounds may be used as a lead for development of new antioxidant agents. The presented results are showing in Table 3.
Anticancer activity
In vitro anticancer potential of the newly synthesized 5-(substituted aldehyde)-7-methyl-3-oxo-N-phenyl-2-((3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)methylene)-1,2,3,5 tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide analogues were carried out by sulforhodamine B (SRB) assay against human breast (MCF-7) cancer cell line. All synthesized compounds submitted to screen have been tested initially at dose (10−7–10−4 M) at anticancer drug screening facility (ACDSF) at ACTREC, Tata Memorial Centre, and Mumbai. Among them, compound 23 was found to be most potent anticancer agent at dose 10−4 M against human breast (MCF-7) cancer cell line and comparable with adriamycin as standard (Tables 4, 5). Graph plotted between tested compound and standard drug presented in Fig. 4.
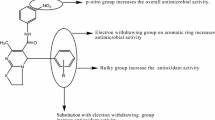
SAR (structure activity relationship) studies
From the antimicrobial, antioxidant and anticancer activities results of the synthesized 5-(substituted aldehyde)-7-methyl-3-oxo-N-phenyl-2-((3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl)methylene)-1,2,3,5-tetrahydroimidazo[1,2-a]pyrimidine-6-carboxamide analogues, the subsequent structure activity relationship can be derived in Fig. 5.
-
Presence of electron releasing groups (–OC2H5, –OH, Compound 21) on benzylidene portion improved the antibacterial activity of the synthesized compounds against E. coli.
-
Presence of electron withdrawing groups (–Br, –Cl, Compounds 12, 14, 15 and 18) on benzylidene portion improved the antimicrobial activity of the synthesized compounds against S. aureus, B. subtilis, A. niger and C. albicans.
-
Presence of electron releasing groups (trimethoxy and p-OCH3, Compounds 2 and 16) on benzylidene portion enhanced the antioxidant activity.
-
Presence of electron withdrawing group (o-Cl, Compound 23) on benzylidene portion improved the anticancer activity of the synthesized compounds against human breast (MCF-7) cancer cell line.
Experimental section
Synthesized pyrimidine derivatives followed the general procedure discussed in synthetic (Scheme 1). All reagents and solvents used in study were of both laboratory and analytical grade and procured from commercial market. Reaction steps forward was observed by thin layer chromatography making use of commercial silica gel plates. Melting points were tested in open capillary tubes method. 1H nuclear magnetic resonance (1H-NMR) spectral study demonstrated by Bruker Avance 400 NMR spectrometer in appropriate DMSO-deuterated solvents and are expressed in parts per million (δ, ppm) downfield from tetramethyl silane (internal standard). 1H-NMR data are given as multiplicity (s, singlet; d, doublet; t, triplet; m, multiplet) and number of protons. Infrared (IR) spectra were recorded on Bruker 12060280, Software: OPUS 7.2.139.1294 spectrophotometer.
General procedure for synthesized pyrimidine analogues
Step 1: intermediate-I
A mixture of 3-oxo-N-phenylbutanamide (0.02 mol), guanidine nitrate (0.030 mol) and corresponding aldehyde (0.02 mol) in the round bottom flask with 100 ml methanol and then added aluminum chloride (0.006 mol) with 4–5 drops of concentrated hydrochloric acid after that the reaction mixture was refluxed for 10–11 h. before completion of the reaction we had been checked the reaction with every 30 min by TLC plats with suitable solvent system (benzene). After completion of the reaction the reaction mixture was cooled at room temperature and poured into ice cold water with vigorous stirring, filtered and recrystallized with methanol [11].
Step 2: final analogues (1–17)
The intermediate-1 (0.02 mol, synthesized in previous step-1), sodium benzoate (4 gm), 6-(hydroxymethyl)-tetrahydro-2H-pyran-2,3,4,5-tetraol (0.02 mol), ethyl acetoacetate (15 ml), glacial acetic acid (40 ml) and monochloroacetic acid (0.030 mol) were taken in round bottom flask and refluxed with for 6–7 h (controlled temperature at 140–142 °C) before completion of the reaction, we had been checked the reaction with every 30 min by TLC plats with suitable solvent system (benzene). After completion of the reaction the reaction mixture was cooled at room temperature and poured into ice cold water to yielded solid precipitate, filtered and recrystallized with methanol.
Spectral analysis determined by
FT-IR (KBr pellets, cm −1 ) and 1 H-NMR/ 13 C-NMR (DMSO-d 6 , δ ppm), stretching = st.; pyrimidine nucleus = pn
Compound 1 (5 - (2 - Hydroxynaphthalen - 1 - yl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3060 (C–H st.), 1596 (C=C st.), 712 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3340 (N–H st., 2° amide), {1630 (N=CH st.), 1313 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1093 (C–O–C st., aryl ether), 3340 (O–H st., polyhydroxy); 1 H-NMR (DMSO-d 6 , δ ppm): 7.16–7.65 (m, 11H, Ar–H), 2.13 (s, 1H, NH), 8.03 (s, 1H, NH of 2o amide), 3.47-4.26 (m, 5H, CH of tetrahydropyran), 2.20 {s, 4H, (OH)4}. 13 C-NMR (DMSO-d 6 , δ ppm): 24.5, 51.3, 77.4, 78.3, 98.7, 115.3, 118.4, 121.5, 130.6, 146.3, 163.4, 121.3, 123.4, 122.8, 137.4, 127.1, 128.8, 133.6, 153.6, 119.3; MS ES + (ToF): m/z 572 [M++1].
Compound 2 (7 - Methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 5 - (3,4,5 - trimethoxyphenyl) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3062 (C–H st.), 1596 (C=C st.), 694 (C–C st.,) of aromatic ring}, 1630 (C = O st.), 3321 (N–H st., 2o amide), {1630 (N=CH st.), 1244 (C–N st.) of pn}, 2779 (C–H st., cyclic ether), 1126 (C–O–C st., aryl ether), 3321 (O–H st., polyhydroxy), 1244 (C–O–C st., –OCH3); 1 H-NMR (DMSO-d 6 , δ ppm): 7.45–7.49 7H, Ar–H), 7.49 (d, J = 8 Hz, 2H, Ar–H), 8.25 (s, 1H, NH of 2° amide), 4.20–4.22 (m, 5H, CH of tetrahydropyran), 2.10 {s, 4H, (OH)4}, 3.86 {s, 9H, (OCH3)3}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 72.3, 76.4, 99.5, 56.2, 60.1, 104.2, 120.3, 125.4, 128.6, 128.9, 128.0, 130.1, 137.2, 152.3, 163.2; MS ES + (ToF): m/z 596 [M++1].
Compound 3 (7 - Methyl - 5 - (4 - nitrophenyl) - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo [1,2 - a]pyrimidine - 6 - carboxamide) IR: {3073 (C–H st.), 1598 (C=C st.), 716 (C–C st.) of aromatic ring}, 1630 (C=O st., 2˚amide), 1711 (C=O st., aryl ketone), 3354 (N–H st., 2° amide), {1711 (N=CH st.), 1347 (C–N st.) of pn}, 2779 (C–H st., cyclic ether), 1107 (C–O–C st., aryl ether), 3354 (O–H st., polyhydroxy), 1347 (NO2 st., phenyl nucleus), 854 (C–N st., C6H5NO2); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28–8.09 (m, 9H, Ar–H), 1.97 (s, 1H, NH), 8.10 (s, 1H, NH of 2° amide), 3.47–4.25 (m, 5H, CH of tetrahydropyran), 2.12 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.2, 71.3, 76.2, 98.5, 59.2, 120.3, 125.4, 128.7, 128.9, 128.0, 130.1, 137.2, 149.2, 152.3, 163.1; MS ES + (ToF): m/z 551 [M++1].
Compound 4 (5 - (4 - (Diethylamino)phenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {2977 (C–H st.), 1590 (C=C st.), 708 (C–C st.) of aromatic ring}, 1650 (C=O st.,), 3283 (N–H st., 2° amide), {1650 (N=CH st., pn), 1255 (C–N st.) of pn}, 2738 (C–H st., cyclic ether), 1076 (C–O–C st., aryl ether), 3283 (O–H st., polyhydroxy), 2823 (C–H st., aliphatic chain), 1183 (C–C st., aliphatic chain); 1 H-NMR (DMSO-d 6 , δ ppm): 6.63–7.49 (m, 9H, Ar–H), 2.11 (s, 1H, NH), 8.09 (s, 1H, NH of 2° amide), 6.7 (s, 1H, ethylene), 3.45–5.39 (m, 5H, CH of tetrahydropyran), 2.19 {s, 4H, (OH)4}, 1.19 {(t, 6H, (CH3)2, 3.43 (q, 4H, (CH2)2 of (C2H5)2}; 13 C-NMR (DMSO-d 6 , δ ppm): 12.3, 21.3, 47.9, 72.3, 77.2, 98.5, 59.2, 112.7, 120.3, 121.9, 125.4, 128.5, 128.0, 128.4, 130.1, 137.2, 147.2, 152.3, 163.1; MS ES + (ToF): m/z 577 [M++1].
Compound 5 (7 - Methyl - 5 - (3 - nitrophenyl) - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3062 (C–H st.), 1597 (C=C st.), 693 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3307 (N–H st., 2° amide), {1630 (N=CH st.), 1330 (C–N st.) of pn}, 2779 (C–H st., cyclic ether), 1125 (C–O–C st., aryl ether), 3307 (O–H st., polyhydroxy), 1350 (NO2 st., phenyl nucleus), 841 (C–N st., C6H5NO2); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28–8.09 (m, 9H, Ar–H), 2.12 (s, 1H, NH), 8.10 (s, 1H, NH of 2o amide), 3.47–4.23 (m, 5H, CH of tetrahydropyran), 2.19 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.2, 72.3, 76.2, 98.5, 59.2, 120.3, 121.1, 125.4, 128.7, 128.9, 128.0, 129.1, 130.1, 133.3, 137.2, 144.2, 147.2, 152.3, 163.1; MS ES + (ToF): m/z 551 [M++1].
Compound 6 (7 - Methyl - 5 - (2 - nitrophenyl) - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {2933 (C–H st.), 1597 (C=C st.), 691 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3385 (N–H st., 2° amide), {1630 (N=CH st., pn), 1245 (C–N st.) of pn}, 2779 (C–H st., cyclic ether), 1096 (C–O–C st., aryl ether), 3385 (O–H st., polyhydroxy), 1352 (NO2 st.), 855 (C–N st., C6H5NO2); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28–7.61 (m, 9H, Ar–H), 2.08(s, 1H, NH), 8.11 (s, 1H, NH of 2° amide), 1.88(s, 3H, CH3), 3.47–4.57 (m, 5H, CH of tetrahydropyran), 2.11 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.4, 73.1, 76.2, 94.5, 120.5, 121.2, 125.4, 129.7, 127.2, 129.1, 127.1, 130.1, 131.3, 137.2, 146.2, 152.3, 162.1; MS ES + (ToF): m/z 551 [M++1].
Compound 7 (5 - (4 - (Dimethylamino)phenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3026 (C–H st.), 1559 (C=C st.), 714 (C–C st.) of aromatic ring}, 1595 (C=O st., 2° amide), 1711 (C=O st., aryl ketone), 3062 (N–H st., 2° amide), {1711 (N=CH st.), 1248 (C–N st.) of pn}, 2814 (C–H st., cyclic ether), 1070 (C–O–C st., aryl ether), 3399 (O–H st., polyhydroxy), 2934 (C–H st., aliphatic chain); 1 H-NMR (DMSO-d 6 , δ ppm): 6.65–7.62 (m, 9H, Ar–H), 2.11 (s, 1H, NH), 8.09 (s, 1H, NH of 2° amide), 6.74 (s, 1H, ethylene), 3.47–4.41 (m, 5H, CH of tetrahydropyran), 2.19 {s, 4H, (OH)4}, 3.06 {s, 6H, of (CH3)2}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.4, 41.0, 55.1, 70.1, 73.1, 76.2, 94.8, 120.5, 121.3, 124.1, 125.4, 129.0, 127.8, 127.1, 130.4, 132.6, 135.2, 147.2, 163.1; MS ES + (ToF): m/z 549 [M++1].
Compound 8 (5 - (4 - Hydroxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3064 (C–H st.), 1596 (C=C st.), 714 (C–C st.) of aromatic ring}, 1596 (C=O st., 2° amide), 1712 (C=O st., aryl ketone), 3385 (N–H st., 2° amide), {1712 (N=CH st.), 1249 (C–N st.) of pn}, 2779 (C–H st., cyclic ether), 1083 (C–O–C st., aryl ether), 3385 (O–H st., polyhydroxy), 3385 (OH st., phenyl nucleus); 1 H-NMR (DMSO-d 6 , δ ppm): 7.44–7.58 (m, 9H, Ar–H), 2.06 (s, 1H, NH), 8.07 (s, 1H, NH 2° amide), 3.45–4.96 (m, 5H, CH, tetrahydropyran), 2.16 {s, 4H, (OH)4}, 4.96 (s, 1H, Ar–OH); 13 C-NMR (DMSO-d 6 , δ ppm): 21.2, 55.1, 71.1, 73.1, 76.2, 94.3, 113.6, 120.5, 121.3, 124.1, 125.4, 128.1, 129.0, 135.2, 147.2, 152.1, 156.2, 163.1; MS ES + (ToF): m/z 522 [M++1].
Compound 9 (5 - (4 - Hydroxy - 3 - methoxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {2967 (C–H st.), 1595 (C=C st.), 713 (C–C st.) of aromatic ring}, 1595 (C=O st.,), 3422 (N–H st., 2° amide), {1595 (N=CH st.), 1249 (C–N st.) of pn}, 2832 (C–H st., cyclic ether), 1070 (C–O–C st., aryl ether), 3422 (O–H st., polyhydroxy), 3422 (OH st., phenyl nucleus), 1249 (C–O–C st., –OCH3); 1 H-NMR (DMSO-d 6 , δ ppm): 2.10 (s, 1H, NH), 5.71 (s, 1H, CH of pyrimidine), 3.46–4.85 (m, 5H, CH of tetrahydropyran), 2.18 {s, 4H, (OH)4}, 3.76 (s, 3H, OCH3); 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 55.3, 56.1, 70.1, 73.1, 76.2, 94.8, 113.6, 120.6, 116.3, 121.4, 124.4, 125.1, 129.0, 130.2, 135.9, 136.2, 143.2, 151.2, 152.7, 162.1; MS ES + (ToF): m/z 552 [M++1].
Compound 10 (5 - (2,4 - Dichlorophenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {2834 (C–H st.), 1594 (C=C st.), 703 (C–C st.) of aromatic ring}, 1594 (C=O st.,), 3380 (N–H st., 2° amide), {1594 (N=CH st.), 1350 (C–N st.) of pn}, 2735 (C–H st., cyclic ether), 1090 (C–O–C st., aryl ether), 3380 (O–H st., polyhydroxy), 758 (C–Cl st., phenyl nucleus); 1 H-NMR (DMSO-d 6 , δ ppm): 6.94–7.50 (m, 8H, Ar–H), 2.08 (s, 1H, NH), 8.10 (s, 1H, NH of 2° amide), 6.21 (s, 1H, ethylene), 3.47–5.00 (m, 5H, CH of tetrahydropyran), 2.12 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.2, 45.2, 70.0, 73.1, 76.2, 94.9, 120.6, 121.4, 124.4, 125.1, 126.2, 129.0, 130.2, 133.4, 135.2, 140.2, 146.2, 152.7, 162.1, 163.3; MS ES + (ToF): m/z 574 [M++1].
Compound 11 (5 - (2 - Methoxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3063 (C–H st.), 1595 (C=C st.), 710 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3397 (N–H st., 2° amide), {1630 (N=CH st.), 1247 (C–N st.) of pn}, 2832 (C–H st., cyclic ether), 1050 (C–O–C st., aryl ether), 3397 (O–H st., polyhydroxy), 1247 (C–O–C st., –OCH3); 1 H-NMR (DMSO-d 6 , δ ppm): 6.89–7.58 (m, 9H, Ar–H), 2.04 (s, 1H, NH), 6.88 (s, 1H, ethylene), 3.44–4.97 (m, 5H, CH of tetrahydropyran), 2.07 {s, 4H, (OH)4}, 3.70 (s, 3H, OCH3); 13 C-NMR (DMSO-d 6 , δ ppm): 21.1, 45.2, 70.3, 73.1, 76.2, 94.8, 114.1, 120.6, 121.4, 124.4, 125.1, 127.2, 128.2, 129.0, 130.2, 135.2, 140.2, 146.2, 156.7, 162.1, 163.3; MS ES + (ToF): m/z 536 [M++1].
Compound 12 (5 - (3 - Bromophenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3064 (C–H st.), 1596 (C=C st.), 712 (C–C st.) of aromatic ring}, 1596 (C=O st.,), 3386 (N–H st., 2° amide), {1596 (N=CH st.), 1253 (C–N st.) of pn}, 2832 (C–H st., cyclic ether), 1071 (C–O–C st., aryl ether), 3386 (O–H st., polyhydroxy), 510 (C–Br st.); 1 H-NMR (DMSO-d 6 , δ ppm): 7.43–7.63 (m, 9H, Ar–H), 7.63 (d, J = 8 Hz, 2H, Ar–H), 8.09 (s, 1H, NH of 2° amide), 1.97 (s, 1H, NH), 1.84 (s, 3H, CH3), 6.18 (s, 1H, CH of ethylene), 3.47–4.38 (m, 5H, CH of tetrahydropyran), 2.11 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.4, 54.8, 70.3, 73.1, 76.1, 94.7, 120.6, 121.4, 124.4, 125.1, 126.2, 129.0, 130.2, 135.2, 145.2, 146.2, 152.3, 162.1, 163.3; MS ES + (ToF): m/z 584 [M++1].
Compound 13 (5 - (3 - Methoxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3062 (C–H st.), 1595 (C=C st.), 712 (C–C st.) of aromatic ring}, 1631 (C=O st., 2° amide), 1716 (C=O st., aryl ketone), 3385 (N–H st., 2° amide), {1631 (N=CH st.), 1247 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1070 (C–O–C st., aryl ether), 3385 (O–H st., polyhydroxy), 1247 (C–O–C st., –OCH3); 1 H-NMR (DMSO-d 6 , δ ppm): 7.25–7.48 (m, 9H, Ar–H), 1.96 (s, 1H, NH), 8.0 (s, 1H, NH of 2° amide), 3.45–4.99 (m, 5H, CH of tetrahydropyran), 2.11 {s, 4H, (OH)4}, 3.76 (s, 3H, OCH3); 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 55.2, 55.8, 70.3, 73.1, 76.2, 94.9, 111.0, 197.0, 120.6, 121.5, 124.4, 125.1, 129.0, 130.2, 133.4, 135.2, 140.2, 146.2, 152.7, 162.1, 163.3; MS ES + (ToF): m/z 536 [M++1].
Compound 14 (5 - (4 - Bromophenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3058 (C–H st.), 1595 (C=C st.), 709 (C–C st.) of aromatic ring}, 1631 (C=O st., 2° amide), 1715 (C=O st., aryl ketone), 3333 (N–H st., 2° amide), {1631 (N=CH st.), 1315 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1072 (C–O–C st., aryl ether), 3333 (O–H st., polyhydroxy), 509 (C–Br st.); 1 H-NMR (DMSO-d 6 , δ ppm): 7.14–7.64 (m, 9H, Ar–H), 7.66 (d, J = 8 Hz, 2H, Ar–H), 8.1 (s, 1H, NH of 2° amide), 2.19 (s, 1H, NH), 1.82 (s, 3H, CH3), 3.47–5.00 (m, 5H, CH of tetrahydropyran), 2.13 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 55.0, 70.3, 73.1, 76.2, 94.8, 197.0, 120.6, 121.5, 124.4, 125.1, 129.0, 130.2, 131.1, 133.4, 135.7, 142.2, 146.2, 152.7, 162.1, 163.3; MS ES + (ToF): m/z 584 [M++1].
Compound 15 ( 5 - (5 - Bromo - 2 - hydroxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3062 (C–H st.), 1596 (C=C st.), 691 (C–C st.) of aromatic ring}, 1631 (C=O st., 2° amide), 1712 (C=O st., aryl ketone), 3332 (N–H st., 2° amide), {1631 (N=CH st.), 1282 (C–N st.) of pn}, 2832 (C–H st., cyclic ether), 1070 (C–O–C st., aryl ether), 3332 (O–H st., polyhydroxy), 3332 (OH st., phenyl), 543 (C–Br st.); 1 H-NMR (DMSO-d 6 , δppm): 7.29–7.63 (m, 8H, Ar–H), 7.49 (d, J = 8 Hz, 2H, Ar–H), 2.13 (s, 1H, NH), 8.1(s, 1H, NH of 2° amide), 1.71 (s, 3H, CH3), 6.6 (s, 1H of ethylene), 3.79–5.12 (m, 5H, CH of tetrahydropyran), 1.98 {s, 4H, (OH)4}, 5.07 (s, 1H, of Ar–OH); 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 44.2, 70.3, 73.4, 76.2, 94.9, 117.0, 115.3, 120.6, 121.4, 124.1, 125.1, 129.0, 130.2, 131.2, 133.4, 135.2, 146.2, 153.2, 162.1, 163.3; MS ES + (ToF): m/z 601 [M++1].
Compound 16 (5 - (4 - Methoxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3062 (C–H st.), 1595 (C=C st.), 691 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3385 (N–H st., 2° amide), {1630 (N=CH st.), 1247 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1072 (C–O–C st., aryl ether), 3385 (O–H st., polyhydroxy), 1247 (C–O–C st., –OCH3); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28–7.45 (m, 9H, Ar–H), 8.04 (s, 1H, NH of 2o amide), 4.15–4.21(m, 5H, CH of tetrahydropyran), 2.40 {s, 4H, (OH)4}, 3.44 (s, 3H, OCH3), 1.71 (s, 3H, CH3); 13 C-NMR (DMSO-d 6 , δ ppm): 21.1, 55.0, 55.8, 70.3, 73.1, 76.2, 94.5, 114.0, 120.6, 121.5, 124.4, 125.1, 128.3, 129.0, 130.2, 135.2, 146.2, 152.7, 158.1, 162.1, 163.3; MS ES + (ToF): m/z 536 [M++1].
Compound 17 (5 - (4 - Formylphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3063 (C–H st.), 1595 (C=C st.), 690 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3384 (N–H st., 2° amide), {1630 (N=CH st.), 1244 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1071 (C–O–C st., aryl ether), 3384 (O–H st., polyhydroxy), 2716 (C–H st., CHO), 1364 (C–C st., CHO group); 1 H-NMR (DMSO-d 6 , δ ppm): 7.23–7.62 (m, 9H, Ar–H), 1.97 (s, 1H, NH), 8.16 (s, 1H, NH of 2° amide), 3.47–4.99 (m, 5H, CH of tetrahydropyran), 2.12 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 55.3, 70.4, 73.1, 76.2, 94.8, 120.6, 121.4, 124.4, 125.1, 127.3, 129.0, 130.2, 134.3, 135.2, 146.2, 149.3, 152.7, 162.1, 163.3, 192.2; MS ES + (ToF): m/z 534 [M++1].
Compound 18 (5 - (3 - Chlorophenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3057 (C–H st.), 1596 (C=C st.), 689 (C–C st.) of aromatic ring}, 1666 (C=O st., 2° amide), 1717 (C=O st., aryl ketone), 3327 (N–H st., 2° amide), {1666 (N=CH st.), 1315 (C–N st.) of pn}, 2830 (C–H st., cyclic ether), 1082 (C–O–C st., aryl ether), 3327 (O–H st., polyhydroxy), 758 (C–Cl st.); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28–7.63(m, 9H, Ar–H), 7.52 (d, J = 4 Hz, 2H, Ar–H), 2.13 (s, 1H, NH), 8.11(s, 1H, NH of 2° amide), 1.85 (s, 3H, CH3), 3.47–5.00 (m, 5H, CH of tetrahydropyran), 1.97 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 55.3, 70.4, 73.1, 76.2, 94.8, 120.6, 121.4, 124.4, 125.1, 126.2, 129.0, 130.2, 135.2, 144.2, 146.3, 152.7, 162.1, 163.3; MS ES + (ToF): m/z 540 [M++1].
Compound 19 (7 - Methyl - 3 - oxo - N,5 - diphenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide): IR-{3057 (C–H st.), 1594 (C=C st.), 706 (C–C st.) of aromatic ring}, 1664 (C=O st., 2° amide), 1714 (C=O st., aryl ketone), 3335 (N–H st., 2° amide), {1664 (N=CH st.), 1315 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1073 (C–O–C st., aryl ether), 3335 (O–H st., polyhydroxy), 3335 (OH st., phenyl), 2616 (C-H st., CHO), 1364 (C–C st., C6H5CHO); 1 H-NMR (DMSO-d 6 , δ ppm): 7.48–7.64 (m, 10H, Ar–H), 1.96 (s, 1H, NH), 8.1 (s, 1H, NH of 2° amide), 1.84 (s, 3H, CH3), 3.75–4.24 (m, 5H, CH of tetrahydropyran), 2.12 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.0, 55.1, 70.3, 73.1, 76.2, 94.2, 120.5, 121.2, 124.4, 125.1, 126.2, 128.3, 129.0, 130.4, 135.2, 143.2, 146.3, 152.7, 162.1, 163.3; MS ES + (ToF): m/z 506[M++1].
Compound 20 (5 - (4 - Chlorophenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: 2958 (C–H st.), 1594 (C=C st.), 709 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3420 (N–H st., 2° amide), {1630 (N=CH st.), 1177 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1090 (C–O–C st., aryl ether), 3420 (O–H st., polyhydroxy), 775 (C–Cl st.); 1 H-NMR (DMSO-d 6 , δ ppm): 7.29–7.64 (m, 9H, Ar–H), 2.07 (s, 1H, NH), 8.0(s, 1H, NH of 2° amide), 1.83 (s, 3H, CH3), 6.08 (s, 1H of ethylene), 3.47–4.87 (m, 5H of CH of tetrahydropyran), 2.09 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.3, 55.0, 70.4, 73.1, 77.2, 94.8, 120.6, 121.4, 124.4, 125.1, 128.3, 128.5, 129.0, 130.2, 135.2, 141.4, 146.2, 152.7, 162.1, 163.1; MS ES + (ToF): m/z 540 [M++1].
Compound 21 (5 - (3 - Ethoxy - 4 - hydroxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3027 (C–H st.), 1559 (C=C st.), 710 (C–C st.) of aromatic ring}, 1594 (C=O st., 2° amide), 1713 (C=O st., aryl ketone), 3416 (N–H st., 2° amide), {1713 (N=CH st.), 1316 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1071 (C–O–C st., aryl ether), 3416 (O–H st., polyhydroxy), 3416 (OH st., phenyl nucleus), 2831 (C–H st., aliphatic chain), 1175 (C–C st., aliphatic chain); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28–7.63 (m, 8H, Ar–H), 7.50 (d, J = 8 Hz, 2H, Ar–H), 2.12 (s, 1H, NH), 8.10 (s, 1H, NH 2° amide), 1.83 (s, 3H, CH3), 3.47–4.99 (m, 5H, CH of tetrahydropyran), 1.97 {s, 4H, (OH)4}, 1.33 (dt, J = 8 Hz, 3H, CH3), 4.21 (d, J = 8 Hz, 2H, CH2); 13 C-NMR (DMSO-d 6 , δ ppm): 21.5, 65.1, 70.2, 73.4, 76.2, 77.5, 94.7, 116.3, 120.1, 121.5, 130.6, 162.3, 143.1, 146.4, 163.4, 121.3,124.4, 125.8, 135.4, 129.1, 128.8, 136.5, 137.7, 148.3; MS ES + (ToF): m/z 566 [M++1].
Compound 22 (5 - (2 - Hydroxyphenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3060 (C–H st.), 1596 (C=C st.), 691 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3333 (N–H st., 2° amide), {1630 (N=CH st.), 1294 (C–N st.) of pn}, 2832 (C–H st., cyclic ether), 1103 (C–O–C st., aryl ether), 3333 (O–H st., polyhydroxy), 3333 (OH st., phenyl nucleus); 1 H-NMR (DMSO-d 6 , δ ppm): 7.28 (m, 9H, Ar–H), 8.13 (s, 1H, NH of 2° amide), 3.47–4.24 (m, 5H, CH of tetrahydropyran), 2.19 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.2, 44.2, 70.0, 73.1, 76.2, 94.9, 115.8, 120.6, 130.4, 121.4, 122.6, 124.4, 125.1, 128.7, 129.0, 130.3, 135.2, 146.2, 152.7, 154.2, 162.4; MS ES + (ToF): m/z 522 [M++1].
Compound 23 (5 - (2 - Chlorophenyl) - 7 - methyl - 3 - oxo - N - phenyl - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {3059 (C–H st.), 1594 (C=C st.), 708 (C–C st.) of aromatic ring}, 1594 (C=O st.,), 3383 (N–H st., 2° amide), {1594 (N=CH st.), 1316 (C–N st.) of pn}, 2830 (C–H st., cyclic ether), 1071 (C–O–C st., aryl ether), 3383 (O–H st., polyhydroxy), 758 (C–Cl st.); 1 H-NMR (DMSO-d 6 , δ ppm): 7.35–7.64 (m, 9H, Ar–H), 2.13 (s, 1H, NH), 8.1 (s, 1H, NH of 2° amide), 3.47–4.69 (m, 5H, CH of tetrahydropyran), 2.13 {s, 4H, (OH)4}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.2, 45.9, 70.0, 73.1, 76.1, 94.8, 120.5, 130.4, 121.6, 122.6, 125.1, 126.1, 128.7, 129.0, 135.8, 146.2, 152.7, 162.6, 163.2; MS ES + (ToF): m/z 540 [M++1].
Compound 24 (7 - Methyl - 3 - oxo - N - phenyl - 5 - ((E) - styryl) - 2 - ((3,4,5,6 - tetrahydroxytetrahydro - 2H - pyran - 2 - yl)methylene) - 1,2,3,5 - tetrahydroimidazo[1,2 - a]pyrimidine - 6 - carboxamide) IR: {2967 (C–H st.), 1594 (C=C st.), 710 (C–C st.) of aromatic ring}, 1630 (C=O st.,), 3422 (N–H st., 2° amide), {1630 (N=CH st.), 1271 (C–N st.) of pn}, 2831 (C–H st., cyclic ether), 1070 (C–O–C st., aryl ether), 3422 (O–H st., polyhydroxy), {2831 (C–H st.), 1176 (C–C st.), 1630 (C=C st.) of aliphatic chain}; 1 H-NMR (DMSO-d 6 , δ ppm): 7.33-7.63 (m, 10H, Ar–H), 2.09 (s, 1H, NH), 8.09 (s, 1H, NH of 2° amide), 6.69 (s, 1H, ethylene), 3.48–5.04 (m, 5H, CH of tetrahydropyran), 2.1 {s, 4H, (OH)4}, 6.16 {d, 1H,(CH)2; 6.51 (d, 1H, (CH)b of aliphatic chain}; 13 C-NMR (DMSO-d 6 , δ ppm): 21.5, 73.4, 77.5, 79.3, 98.7, 122.5, 130.6, 162.3, 146.4, 163.4, 121.3, 123.4, 125.8, 137.4, 127.1, 128.8, 133.6, 136.5, 137.7 153.6, 119.3; MS ES + (ToF): m/z 532 [M++1].
Conclusions
Summarizing, we may conclude that the synthesized compounds 12, 14, 15, 18 and 21 displayed appreciable antibacterial and antifungal activities and compounds 2 and 16 exhibited excellent in vitro antioxidant activity due to the presence of electron releasing groups on benzylidene portion and anticancer activity indicated that compound 23 was found to be most active against human breast (MCF-7) cancer cell line due to the presence of electron withdrawing groups (o-Cl) on benzylidene portion. These compounds may be used as lead for the development of novel therapeutic agents.
References
Tomma JH, Khazaal MS, Al-Dujaili AH (2014) Synthesis and characterization of novel Schiff bases containing pyrimidine unit. Arab J Chem 7:157–163
Kandile NG, Mohamed MI, Zaky H, Mohamed HM (2009) Novel pyridazine derivatives: synthesis and antimicrobial activity evaluation. Eur J Med Chem 44:1989–1996
Sarkar A, Kumar KA, Dutta NK, Chakraborty P, Dastidar SG (2003) Evaluation of in vitro and in vivo antibacterial activity of dobutamine hydrochloride. Indian J Med Microbiol 21:172–178
Gold HS, Moellering RC (1996) Antimicrobial drug resistance. N Engl J Med 335:1445–1453
Sawant RL, Bansode CA, Wadekar JB (2013) In vitro anti-inflammatory potential and QSAR analysis of oxazolo/thiazolo pyrimidine derivatives. Med Chem Res 22:1884–1889
Emami S, Foroumadi A, Falahati M, Loffali E, Rajabalian S, Ebrahimi SA, Farahyar S, Shafiee A (2008) 2-Hydroxy phenacyl azoles and related azolium derivative as antifungal agents. Bioorg Med Chem Lett 18:141–146
Gursoy-Kol O, Ayazoglu E (2014) Antioxidant activities and acidic properties of some novel 4-[3,4-di-(4-nitrobenzoxy)-benzylidenamino]-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Arabian J Chem (in press)
Kotaiah Y, Harikrishana N, Nagaraju K, Rao V (2012) Synthesis and antioxidant activity of 1,3,4-oxadiazole tagged thieno[2,3-d] pyrimidine derivatives. Eur J Med Chem 58:340–345
Liu Z, Wu S, Wang Y, Li R, Wang J, Wang L, Zhao Y, Gong P (2014) Design, Synthesis and biological evaluation of novel thieno[3,2-d]pyrimidine derivatives possessing diaryl semicarbazone scaffolds as potent antitumor agents. Eur J Med Chem 87:782–793
Sigroha S, Narasimhan B, Kumar P, Khatkar A, Ramasamy K, Mani V, Mishra RK, Majeed ABA (2011) Design, synthesis, antimicrobial, anticancer evaluation, and QSAR studies of 4-(substituted benzylidene-amino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-ones. Med Chem Res (in press)
Cappuccino JG, Sherman N (1999) In microbiology-a laboratory manual, 4th edn. Addison Wesley Longman Inc, California
Pharmacopoeia of India, vol. Ӏ (2007) Controller of Publication, Ministry of Health Department, Govt. of India, New Delhi, p 37
Mukherjee DPK (2012) Pharmacological screening of herbal drugs. Quality control of herbal drugs 564
Authors’ contributions
PKV designed research and JR performed research and MS and SK analyzed the spectral data and biological data and wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
Thank to Prof. B. Narasimhan, Department of Pharmaceutical Sciences, M. D. University, Rohtak for kind support for providing chemicals.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rani, J., Saini, M., Kumar, S. et al. Design, synthesis and biological potentials of novel tetrahydroimidazo[1,2-a]pyrimidine derivatives. Chemistry Central Journal 11, 16 (2017). https://doi.org/10.1186/s13065-017-0245-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-017-0245-9