Abstract
Background
Mechano heterocyclic chemistry (MCH) is a recent quickly growing technique in the synthesis of heterocycles and draws the attention of heterocyclic chemists towards the uses of grindstone technique in a solvent free green efficient synthesis of many heterocyclic systems. On the other hand, multicomponent approach has opened the door for the rapid and efficient one-step procedures to synthesize a wide range of complex targets. Azlactones have been reported to exhibit a wide range of pharmaceutical properties including immune suppressive, anticancer. Antimicrobial, antitumor, anti-inflammatory and antiviral. It also used as useful synthons in the synthesis of several small molecules, including amino acids and peptides.
Results
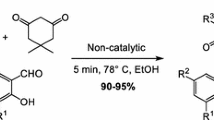
The present work describes an efficient one step green synthesis of 4-arylidene-2-phenyl-5(4H)-oxazolones (azlactones) via the multi-component synthesis by the mechanochemical grinding of glycine, benzoyl chloride, an aromatic aldehyde and fused sodium acetate in the presence of drops of acetic anhydride. This process is green, simple to handle, step and atom efficient, economical and environmentally friendly, because it does not require a reaction solvent or heating, we introduced the yield economy [YE] as a metric to assess the conversion efficiency of grinding and conventional synthetic reactions of azlactones. The structures of the newly synthesized compounds were elucidated by elemental and spectral analyses.
Conclusion
In conclusion, we have developed a simple, efficient and eco-friendly strategy for facile synthesis of azlactones. The key advantages of this strategy, over conventional approach, include its simple, solvent free conditions, as well as its facile work-up, high yield economy and environmental friendliness. It is also successful in achieving three of the green chemistry objectives of a solvent free operation, high atom economy and step efficient. Thus, combining the features of both economic and environmental advantages.

An efficient one step green synthesis of azlactones via multicomponent synthesis by a mechanochemical grinding.
Similar content being viewed by others
Background
There have been several major advances in synthetic organic chemistry during the last decade, including multicomponent [1], mechanochemical [2], green [3], combinatorial [4] and bio-organic syntheses [5]. Indeed, the development of eco-friendly, solvent-free multicomponent approaches has opened the door for the development of rapid and efficient one-step procedures to synthesize a wide range of complex targets. In contrast to multicomponent synthesis, mechanochemical synthesis has received considerable attention as a green chemistry approach for the synthesis of organic compounds because it operates under solvent-free conditions with high atom efficiency, low energy requirements and a facile work-up. Mechanochemical synthesis (i.e., the grindstone technique) is based on the idea that the grinding together of the crystals of two different reagents in a pestle and mortar leads to the formation of local heat, which mediates a reaction between these two materials. These reactions are easy to handle and are generally considered to be more economical and environmentally friendly (i.e., greener) than conventional techniques. The grinding required in these reactions to generate the necessary local heat is achieved by simply mixing the individual components, either neat or in the presence of a very small amount of liquid phase (liquid-assisted grinding), in a pestle and mortar [6, 7]. The only major limitation of this technique is that it cannot be applied to shock-sensitive materials.
Mechanochemical heterocyclic chemistry (MHC) has recently attracted considerable interest from heterocyclic chemists, who have used this technique to achieve the green synthesis of several heterocyclic systems, including pyrazolines [8], aurones [9], bis(indol-3-yl)methanes [10], 1,3,4-oxadiazoles [11], pyrimidones [12], coumarins [13, 14], flavones [14], benzodiazepines [15], 1,6-naphthyridin [16] and 1,3,4-thiadiazoles [17]. Pravin and co-workers compared the mechanochemical synthesis of pyrazolyl chalcones with a conventional synthetic method. They found that the former of these two required shorter reaction times, afforded higher yields of the desired chalcone products and proceeded smoothly at room temperature [18]. The success of the mechanochemical approach used in this case was attributed to the fact that solid-state reactions occur more efficiently and selectively than solution-phase reactions, because the molecules in a crystal lattice are arranged more tightly and regularly than those in the liquid state [19]. Based on the many benefits reported for MHC, we envisaged that this approach could be used to provide facile access to azlactones as a greener, more efficient and yield-economic strategy compared with conventional methods.
4-Arylidene-2-phenyl-5(4H)oxazolones, which are also known as azlactones, are important intermediates in the synthesis of several small molecules, including amino acids [20–23], peptides [24, 25], 2,2-disubsituted-2H-oxazol-5-ones with total region and stereo control [26]. Compounds belonging to this structural class may also be used as precursors for other heterocyclic systems [27]. Furthermore, oxazolones have been reported to exhibit a wide range of pharmaceutical properties [28], including anticancer [29], antimicrobial, antitumor [30], anti-inflammatory [31], antiviral [32] and anti-HIV [33] activities. These compounds can also be used as molecular photo switches [34] and optical sensors for pH measurements [35], as well as biosensor-coupling and photosensitive composition devices for protein analysis [36]. Based on their importance, the development of new methods for the facile and environmental friendly synthesis of azlactones is highly desired. Several methods have been reported for the synthesis of azlactones. For example, Heravi and co-workers reported the synthesis of a series of azlactones by the condensation of hippuric acid with various aromatic aldehydes in the presence of acetic anhydride under ultrasonic irradiation conditions [37]. Azlactones may also be synthesized under solvent-free conditions using Nano silica-supported tungstophosphoric acid [38] or using calcium acetate [39], aluminum oxide [40], and neutral alumina [41] under microwave irradiation conditions or organic–inorganic hybrid polyoxometalates as a catalyst [42], ytterbium (III) triflate as a catalyst [43], under free-solvent. The most commonly used route for the synthesis of Azlactones is the Erlenmeyer method [44], which involves the condensation of aldehydes with hippuric acid in the presence of sodium acetate and acetic anhydride.
It is noteworthy that all of these previously reported methods for the synthesis of azlactones start from hippuric acid [37–44], which is prepared in a separate reaction by the benzoylation of glycine, as shown in (Scheme 1).
It was envisaged that a mechanochemical approach could be used to develop a solvent-free process for the multicomponent synthesis of azlactones directly from glycine in one step.
Results and discussion
In this study, we report the development of a solvent-free mechanochemical approach for the multicomponent synthesis of a series of azlactones in one step (Scheme 2). Benzoyl chloride, glycine, various aromatic aldehydes and fused sodium acetate were mixed under mechanochemical conditions in a porcelain mortar at room temperature in the presence of few drops of acetic anhydride to afford azlactones 2a–i. These azlactones were isolated in excellent yields and with high purity. These compounds were also prepared using a conventional solution phase technique. Notably, our newly developed mechanochemical technique gave much higher yields compared with the conventional method (Table 1). This new process is simple and provides rapid, efficient and economical access to a wide range of azlactones under solvent-free and mild conditions, making it consistent with some of the key principles of green chemistry. The structures of the synthesized azlactones 2a–i were conformed based on a comparison of their m.p., mixed. m.p. TLC, IR, UV, 1H NMR and MS data with those from the literature.
We initially compared our mechanochemical approach for the synthesis of azlactones with a conventional approach in terms of their atom economy. The atom economy (AE) [45] relates to the efficiency with which the atoms in the starting materials of a reaction are incorporated into the desired product (i.e., how efficiently a particular reaction makes use of the reactant atoms). However, the AE values were the same for the mechanochemical and conventional procedures because we used two alternative reaction conditions to obtain the same target compounds.
We consequently introduced yield economy (YE) as a metric to assess the conversion efficiency of these two different approaches. The YE basically measures how much yield (%) of the desired product is obtained over a certain reaction time [i.e., \({{{\text{yield}}\left( \% \right)} \mathord{\left/ {\vphantom {{{\text{yield}}\left( \% \right)} {\text{reaction time}}}} \right. \kern-0pt} {\text{reaction time}}}(\hbox{min} )\)]. A higher YE is therefore indicative of a higher level of conversion, a much more efficient chemical process and more economical reaction. The YE of a reaction can be calculated using the following equation.
YE were used in this study to provide a decisive assessment of the yields obtained under the mechanochemical and conventional conditions (Table 1). Assessing a chemical reaction based entirely on its percentage yield can be misleading. For example, the yields for compound 2a under the mechanochemical and conventional conditions were 90 and 72 % respectively, with a difference of only 18 %. However, the YE values for the mechanochemical and conventional conditions were 22.6 and 0.6, respectively, representing a much bigger difference and highlighting the superiority of the former approach. Similar trends were observed for all of the other compounds in the series. The YE values of azlactones 2a–i are listed in Table 1.

Comparison of [\({{{\text{Y}}\left( \% \right)} \mathord{\left/ {\vphantom {{{\text{Y}}\left( \% \right)} {\text{YE}}}} \right. \kern-0pt} {\text{YE}}}\)] of solvent free Grinding technique with other solvent free literature techniques (Table 2) revealed that:
-
Yield (%) [G] of compounds 2b–c and 2e are higher than the calculated YE* of the same compounds synthesized by other solvent free techniques
-
Yield economy [G] of compounds 2a–c and 2e–g are higher than the calculated YE* of the same compounds synthesized by other solvent free techniques.
Experimental section
Methods
All of the melting points were determined in open capillary tubes on a Gallenkamp melting point apparatus (London, UK). These data have been presented as the uncorrected values. Ultraviolet (UV) spectra were recorded on a JNWAY 6505 UV/vis spectrometer (Staffordshire, UK) in dimethylformamide (DMF). IR spectra were recorded as KBr disks on a PerkinElmer RXIFTIR spectrometer (Waltham, MA, USA). 1H NMR spectra were measured on a Varian Gemini 300 MHz spectrometer (Palo Alto, CA, USA). Chemical shifts (δ) have been expressed in ppm downfield from TMS, which was used as an internal standard. 1H NMR spectra were recorded in DMSO-d 6 and the coupling constants (J) reported in Hz. Mass spectra were recorded on a Shimadzu GC–MS QP 1000 EX system (Tokyo, Japan) operating at 70 eV. All of the reactions were monitored by thin-layer chromatography (TLC) using aluminum TLC sheets coated with silica gel F254 (Merck, Darmstadt, Germany). TLC was also used to assess the purity of the synthesized compounds.
General procedure for the mechanochemical formation of azlactones 2a–i
A mixture of glycine (1.0 mmol), aromatic aldehyde (1.0 mmol), benzoyl chloride (1.0 mmol) and fused sodium acetate (1.0 mmol) was mixed in a porcelain mortar and pestle in the presence of a few drops of acetic anhydride for a few minutes (Table 1). Upon completion of the reaction, as determined by TLC, the reaction mixture turned to a yellow solid, which was washed with cold water and recrystallized from ethanol to give the desired azlactone. The structures of the azlactones were confirmed based on a comparison of their m.p., mixed. m.p., TLC, IR, UV, 1H NMR and MS data with those from the literature.
General procedure for the conventional formation of azlactones 2a-i
A mixture of N-benzoyl glycine (hippuric acid) (1.2 mmol), aromatic aldehyde (1.0 mmol), acetic anhydride (3.0 mmol) and fused sodium acetate (1.5 mmol) was heated on a hot plate to liquefaction, and the resulting mixture was then heated on a water path for 2 h. Upon completion of the reaction, as determined by TLC, the mixture was cooled to room temperature and treated with EtOH (5 ml) [27, 28, 40]. The ethanolic mixture was then held in a refrigerator at 4°C overnight, and the resulting precipitate was collected by filtration. The solid product was then washed with hot water and air-dried at room temperature for 2 h before being recrystallized from ethanol to give the corresponding azlactones 2a–i.
4-Benzylidene-2-phenyl-5(4H)-oxazolone (2a)
UV (DMF): λmax 300 (log ε = 3.95) nm. IR (KBr): 1793, 1768 (C=O), 1652 (C=N), 1594 (C=C).1H NMR (300 MHz, DMSO-d 6 ): δ 7.35 (s, 1H, CH=C), 7.33–7.75 (m, 6H, Ar–H), 8.13 (d, 2H, J = 7.5 Hz), 8.30 (d, 2H, J = 7.8 Hz). MS (ESI) m/z (%): 249 (M+, 100).
(E/Z)-4-(4-Methoxybenzylidene)-2 phenyl-5(4H)-oxazolone (2b)
UV (DMF): λmax 290 (log ε = 3.93) nm.IR (KBr): 1788, 1769 (C=O), 1653 (C=N), 1600 (C=C).1H NMR (300 MHz, DMSO-d 6 ): δ 3.88 (s, 3H, CH3), 7.11 (d, 2H, J = 9.0 Hz), 7.64 (d, 2H, J = 7.5 Hz), 7.69 (d, 1H, J = 6.9 Hz), 8.11 (d, 2H, J = 6.9 Hz), 8.30 (d, 2H, J = 9.0 Hz). For the E-isomer (71 %): 7.33 (s, 1H, CH=C), for the Z-isomer (29 %): 7.60 (s, 1H, CH=C). MS (ESI) m/z (%): 279 (M+, 88), 105 (100).
(E/Z)-4-(4-Chlorobenzylidene)-2-phenyl-5(4H)-oxazolone (2c)
UV (DMF): λmax 252 (log ε = 4.00) nm.IR (KBr): 1795, 1766 (C=O), 1653 (C=N), 1585 (C=C). 1H NMR (300 MHz, DMSO-d 6 ): δ 7.50 (d, 1H, J = 7.5 Hz), 7.61 (d, 1H, J = 8.7 Hz), 7.66 (d, 1H, J = 7.5 Hz), 7.73 (d, 1H, J = 7.5 Hz), 7.94 (d, 1H, J = 7.5 Hz), 8.14 (d, 2H, J = 7.5 Hz), 8.33 (d, 2H, J = 8.7 Hz). For the E-isomer (86 %): 7.37 (s, 1H, CH=C), for the Z-isomer (14 %): 7.47 (s, 1H, CH=C). MS (ESI) m/z (%): 285 (M+. + 2, 30), 283 (M+, 90), 105 (100).
4-(4-(Dimethylamino)benzylidene)-2-phenyl-5(4H)-oxazolone (2d)
UV (DMF): λmax 290 (log ε = 3.98) nm. IR (KBr): 1758, 1763 (C=O), 1646 (C=N), 1605, 1580 (C=C).1H NMR (300 MHz, DMSO-d 6 ): δ 3.07 (s, 6H, 2CH3), 6.83 (d, 2H, J = 9.0 Hz), 7.33 (s, 1H, CH=C), 7.58–7.66 (m, 3H), 8.06 (d, 2H, J = 6.6 Hz), 8.17 (d, 2H, J = 8.7 Hz). MS (ESI): m/z (%): 292 (M+, 91), 105 (100).
4-(4-Nitrobenzylidene)-2-phenyl-5(4H)-oxazolone (2e)
UV (DMF): λmax 252 (log ε = 4.00) nm.IR (KBr): 1750, 1686 (C=O), 1620 (C=N), 1585 (C=C). 1H NMR (300 MHz, DMSO-d 6 ): δ 7.26–7.58 [m, 6H, (5Ar–H + 1CH=C), 7.74 (d, 2H, J = 7.5 Hz), 7.88 (d, 2H, J = 7.2 Hz). MS (ESI) m/z (%): 294.15 (M+, 0.5), 105 (100).
4-(2-Chlorobenzylidene)-2-phenyl-5(4H) oxazolone (2f)
UV (DMF): λmax 300 (log ε = 3.95) nm. IR (KBr): 1794, 1772 (C=O), 1687, 1652 (C=N), 1601 (C=C). 1H NMR (300 MHz, DMSO-d 6 ): δ 7.46 (s, 1H, CH=C), 7.50 (d, 2H, J = 7.8 Hz), 7.57–7.67 (m, 3H), 7.94 (d, 2H, J = 7.2 Hz), 8.15 (d, 1H, J = 6.9 Hz), 8.88 (d, 1H, J = 8.1 Hz). MS (ESI) m/z (%): 285 (M+.+2, 7), 283 (M+, 21), 105 (100).
4-(2-Bromobenzylidene)-2-phenyl-5(4H)-oxazolone (2 g)
UV (DMF): λmax 297 (log ε = 3.96) nm.IR (KBr): 1794, 1770 (C=O), 1650 (C=N), 1583, 1552 (C=C); 1H NMR (300 MHz, DMSO-d 6 ): δ 7.40–7.51(m, 2H), 7.57–7.67 (m, 3H, (2Ar–H + 1CH=C)), 7.74 (d, 1H, J = 7.5 Hz), 7.80 (d, 1H, J = 8.1 Hz), 7.94 (d, 1H, J = 7.2 Hz), 8.14 (d, 1H, J = 7.2 Hz), 8.86 (d, 1H, J = 8.1 Hz). MS (ESI) m/z (%): 328 (M+, 5.6), 330 (M+ + 2, 4.8), 327 (27.3), 329 (26.9), 248 (59), 105 (100).
4-(3,4-Dimethoxybenzylidene)-2-phenyl-5(4H)-oxazolone (2 h)
UV (DMF): λmax 280 (log ε = 3.62) nm.IR (KBr): 1789, 1766 (C=O), 1649 (C=N), 1596, 1578 (C=C). 1H NMR (300 MHz, DMSO-d 6 ): δ 3.86 (s, 3H, OMe), 3.88 (s, 3H, OCH3), 7.13 (d, 1H, J = 8.7 Hz), 7.32 (s, 1H, CH=C), 7.60–7.73 (m, 3H), 7.81 (d, 1H, J = 9.0 Hz), 8.08–8.14 (m, 3H). MS (ESI) m/z (%): 309.15 (M+, 6.0), 105 (100).
2-Phenyl-4-(3-phenylallylidene)-5(4H)-oxazolone (2i)
UV (DMF):λmax 300 (log ε = 3.95) nm.IR (KBr): 1785, 1747 (C=O), 1640 (C=N), 1595, 1574 (C=C). 1H NMR (300 MHz, DMSO-d 6 ): δ 7.27 (d, 1H, CH=C, J = 11.4 Hz), 7.36–7.42 (m, 4H, Ar–H), 7.57–7.68 (m, 7H, (6 Ar–H + 1 CH=C)), 8.08 (d, 1H, CH=C, J = 12.0 Hz). MS (ESI) m/z (%): 275.10 (M+, 12.57), 105 (100).
Conclusion
In summary, we have developed a simple, efficient and eco-friendly method for the facile multi-component synthesis of azlactones using a solvent-free mechanochemical approach. The key advantages of this strategy over conventional approaches include its simple, solvent-free conditions, as well as its facile work-up, high yield economy and environmental friendliness.
Abbreviations
- m.p:
-
melting point
- AE:
-
atom economy
- YE:
-
yield economy
- G:
-
grinding
- Conv:
-
conventional
- TLC:
-
thin layer chromatography
References
Singh MS, Chowdhury S (2012) Recent developments in solvent-free multicomponent reactions: a perfect synergy for eco-compatible organic synthesis. RSC Adv. 2:4547–4592
James SL, Adams CJ, Bolm C, Braga D, Collier P, Friscic T, Grepioni F, Harris KDM, Hyett G, Jones W, Krebs A, Mack J, Maini L, Guy Orpen A, Parkin IP, Shearouse WC, Steed JW, Waddel DC (2012) Mechanochemistry: opportunities for new and cleaner synthesis. Chem Soc Rev 41:413–447
Li CJ, Trost BM (2008) Green chemistry for chemical synthesis. PNAS 105(36):13197–13202
Pandeya SN, Thakkar D (2005) Combinatorial chemistry: a novel method in drug discovery and its application. Indian J Chem. 44:335–348
Leonard A, Dandoy P, Danloy E, Leroux G, Meunier CF, Rooke JC, Su BL (2011) Whole-cell based hybride materials for green energy production, environmental remediation and smart cell-therapy. Chem Soc Rev 40:860–885
Trask AV, Jones W (2005) Crystal engineering of organic cocrystals by the solid-state grinding approach. Top Curr Chem 254:41–70
Bose AK, Pednekar S, Ganguly SN, Chakraborty G, Manhas MS (2004) A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’. Tetrahedron Lett 45:8351–8353
Zangade SB, Mokle SS, Shinde AT, Vibhute YB (2012) An atom Efficient, green synthesis of 2-pyrazoline derivatives under solvent-free conditions using grinding technique. Green Chem Lett Rev. 6(2):123–127
Kumar S (2014) An improves one-pot and eco-friendly synthesis of aurones under solvent-free conditions. Green Chem Lett Rev. 7(1):95–99
Talukdar D, Thakur AJ (2013) A green synthesis of symmetrical bis(indol-3-yl)methanes using phosphate-imprgenated titania catalyst under solvent free grinding conditions. Green Chem Lett Rev. 6(1):55–61
Kumar A, Makrandi JK (2011) An iodine-mediated green synthesis of 1,3,4-oxadiazoles under solvent-free conditions using grinding technique. Green Chem Lett Rev. 4(1):87–89
Khaskel A, Gogoi P, Barman P, Bandyopadhyay B (2014) Grindstone chemistry: a highly efficient and green method for synthesis of 3,4-dihydropyrimidin-2-(1H)-ones by l-tyrosine as an organocatalyst: a combined experimental and DFT study. RSC Adv. 67(4):35559–35567
Nikpassand M, Fekri LZ, Changiz N, Iman F (2014) Synthesis of new 3-cyanocoumarins with C-6 azo function using ultrasound and grinding techniques in the presence of nano Fe3O4. Lett Org Chem 11:29–34
Vhhhh ES, Matsjeh S, Mustafa M, Wahyuningsih TD (2014) Improved synthesis of 2′,6′-dihydroxy-3,4-dimethoxy chalcone by grinding technique to synthesize 5-hydroxy-3′-4′-dimethoxy flavone. Indo J Chem. 14(2):174–178
Sharma S, Jain R, Chawla C (2013) Synthesis and biological activities of some benzodiazepine derivatives. J Chem Pharm Res. 5(7):46–55
Abdel Hameed AM (2015) Rapid synthesis of 1,6-naphthyridines by grindstone chemistry. Env Chem Lett. 13:125–129
Abdel Aziem A (2015) An efficient and simple synthesis of 2,3-dihydro-1,3,4-thiadiazoles, pyrazoles and coumarins containing benzofuran moiety using both conventional and grinding methods. Int J Pharm Sci. 7(1):61–68
Kumar P, Kumar S, Husain K, Kumar A (2011) An efficient synthesis of pyrazole chalcones under solvent free conditions at room temperature. Chin Chem Lett 22:37–40
Rothenberg G, Downie AP, Raston CL, Scott JT (2001) Understanding solid/solid organic reactions. J Amer Chem Soc. 123(36):8701–8708
Alba ANR, Rios R (2011) Oxazolones in organocatalysis, new tricks for an old reagent. Chem Asian J 6:720–734
Mosey RA, Fisk JS, Tepe JJ (2008) Stereoselective syntheses of quaternary substituted α-amino acids using oxazol-5-(4H)-ones. Tetrahedron Asym. 19:2755–2762
Aleman J, Milelli A, Cabrera S, Reyes E, Jorgensen KA (2008) Asymmetric 1,4-addition of oxazolones to nitroalkanes by bifunctional cinchona alkaloid thiourea organocatalysts: synthesis of α, α-disubstituted α-amino acids. Chem Eur J 14(35):10958–10966
Balaguer AN, Companyo X, Calvet T, Font-Bardia M, Moyano A, Rios R (2009) Highly regio-and diastereoselective oxazol-5-one addition to nitrostyrenes. Eur J Org Chem 2:199–203
Gottwald K, Seebach D (1999) Ring opening with kinetic resolution of azlactones by Ti-TADDOLates. Tetrahedron 55:723–738
Donati D, Garzon-Aburbeh A, Natalini B, Marchioro C, Pellicciari R (1996) Conformationally constrained tryptophan analogs. Synthesis of (±)-(Z)-and (±)-(E)-2-amino-2,3-methano-3-(indol-3-yl)propanoic acids. Tetrahedron 52:9901–9908
Alba ANR, Valero G, Calbet T, Font-Bardia M, Moyano A, Rios R (2010) Enantioselective organocatalytic addition of azlactones to maleimides: a highly stereocontrolled entry to 2,2-disubstituted-2H-oxazol-5-ones. Chem Eur J 16:9884–9889
Croce PD, Ferraccioli R, Rosa CL (1994) Reaction of 2,4-diphenyl-4,5-dihydro-1,3-oxazol-5-one with 4-phenyl-N-tosyl-1-azabuta-1,3-diene: C=C versus C=N double bond addition. J Chem Soc Perkin Trans 1:2499–2502
Cativiela C, Fraile JM, Garcia JI, Lopez MP, Mayoral JA, Pires E (1996) Diels-alders reactions of (E)-2-phenyl-4-[(S)-2,2-dimethyl-1,3-dioxolan-4-ylmethylen]-5(4H)-oxazolone with heterogeneous catalysts. Tetrahedron Asymm. 7:2391–2394
Jat LR, Mishra R, Pathak D (2012) Synthesis and anticancer activity of 4-Benzylidene-2-phenyloxazol-5(4H)-one derivatives. J Pharm Pharm Sci. 4:378–380
Gelmi ML, Clerici F, Melis A (1997) 5(4H)-oxazolones part X: acid and base effects on the translactonization reaction of 4-(2-oxa-alkylidene)-5(4H)-oxazolones: new synthesis of 5-alklidene-3-benaoylamino-2(5H)-furanones. Tetrahedron 53:1843–1854
Salgin-Goksen U, Gokhan-Kelekci N, Goktas O, Koysal Y, Kilic E, Isik S, Aktay G, Ozalp M (2007) 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: synthesis analgesic-anti-inflammatory and antimicrobial activities. Bioorg Med Chem. 15(17):5738–5751
Perron-Sierra FM, Pierre A, Burbridge M, Guilband N (2002) Novel bicyclic oxazolone derivatives as anti-angiogenic agents. Bioorg Med Chem Lett 12:1463–1466
Witvrouw M, Pannecouque C, Clercq E, Fernandez-Alvarez E, Marco JL (1999) Inhibition of human immunodeficiency virus type (HIV-1) replication by some diversely functionalized spirocyclopropyl derivatives. Arch Pharm Pharm Med Chem. 332:163–166
Blanco-Lomas M, Campos PJ, Sampedro D (2012) Benzylidene-oxazolones as molecular photoswithches. Org Lett 14:4334–4337
Ertekin K, Alppp S, Karapire C, Yenigul B, Henden E, Icli S (2000) Fluorescence emission studies of an azlactone derivative embedded in polmer films, an optical sensor for pH measurements. J Photchem Photobiol. 137:155–161
Kojima S, Ohkawa H, Hirano T, Maki S, Niwa H, Ohashi M, Inouye S, Tsuji FI (1998) Fluorescent properties of model chromophores of tyrosine-66 substituted mutants of aequorea green fluorescent protein (GFP). Tetrahedron Lett 39:5239–5242
Heravi MRP (2009) Erlenmeyer synthesis of azlactones by sonochemical reaction in ionic liquids. J Univ Chem Tech Metallurgy. 44(1):86–90
Taki BSG, Mirkhani V, Baltork IM, Moghadam M, Tangestaninejad S, Rostami M, Khosropour AR (2013) Synthesis and characterization of nano silica supported tungstophosphoric acid: an efficient reusable heterogeneous catalyst for the synthesis of azlactones. J Inorg Organomet Polym 23:758–765
Paul S, Nanda P, Gupta R, Loupy A (2004) Calcium acetate catalyzed synthesis of 4-arylidene-2-phenyl-5(4H)-oxazolones under solvent-free conditions. Tetrahedron Lett 45:425–427
Conway PA, Devine K, Paradisi F (2009) A simple and efficient method for the synthesis of Erlenmeyer azlactones. Tetrahedron 65(15):2935–2938
Chandrasekhar S, Karri P (2007) Erlenmyer azlactone synthesis with aliphatic aldehydes under solvent-free microwave conditions. Tetrahedron Lett 48(5):785–786
Rostami M, Khosropour A, Mirkhani V, Moghadam M, Tangestaninejad S, Mohammadpoor-Baltork I (2011) Organic-inorganic hybrid polyoxometalates: efficient, heterogeneous and reusable catalysts for solvent-free synthesis of azlactones. Appl Cat A Gen 397(12):27–34
Yu C, Zhou B, Su W, Xu Z (2006) Erlenmeyer synthesis for azlactones catalyzed by Ytterbium (III)Triflate under solvent0free condition. Syn Comm 36(22):3447–3453
Erlenmeyer E (1893) Ueber die Condensation der Hippursaure mit Phthalsaureaan hydrid und mit Benzaldehyd. Annalen. 275:1–12
Sheldon RA (2000) Atom efficiency and catalysis in organic synthesis. Pure Appl Chem 72(7):1233–1246
Authors’ contributions
AFMF designed the research. AAE performed the experimental work, AAE and MMH analyzed the spectral data and shared in writing the manuscript. AFMF revised the manuscript. All correspondence on AAE. All authors read and approved the final manuscript.
Acknowledgements
Authors acknowledge Dr. James Hitchin (Synthetic organic chemist, University of Liverpool and Senior Scientific Officer for Cancer Research UK) for English Editing.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fahmy, A.F.M., El-Sayed, A.A. & Hemdan, M.M. Multicomponent synthesis of 4-arylidene-2-phenyl-5(4H)-oxazolones (azlactones) using a mechanochemical approach. Chemistry Central Journal 10, 59 (2016). https://doi.org/10.1186/s13065-016-0205-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-016-0205-9