Abstract
Background
Walnut (Juglans regia L.), that belongs to the Juglandaceae family, is one of the nuts commonly found in Chinese diets. Researchers had obtained peptides from walnut protein hydrolysates, and these peptides exhibited the high antioxidant activities. The objective of this study was to develop a simple and convenient method for a facile and reproducible preparation of antioxidant peptides from walnut protein hydrolysates.
Results
Walnut proteins were extracted from walnut kernels using continuous countercurrent extraction process, and were separately hydrolyzed with six types of proteases (neutrase, papain, bromelain, alcalase, pepsin, and pancreatin). Then, hydrolysates were purified by ultrafiltration. The yields and purity of the peptides prepared using neutrase and papain were 16 and 81 % at least, respectively, higher than others, and had low molecular weight, 99 % of which were less than 1500 Da. Furthermore, the bioassay indicated that the two peptides exhibited the high antioxidant activities in the DPPH (IC50 values: 59.40 and 31.02 µg/mL, respectively), ABTS (IC50 values: 80.36 and 62.22 µg/mL, respectively), and superoxide radical scavenging assay (IC50 values: 107.47 and 80.00 µg/mL, respectively).
Conclusions
The method combines the advantages of generality, rapidity, simplicity, and is useful for the mass production of walnut peptides.

Preparation of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates
Similar content being viewed by others
Background
Oxidative stress has been suggested to be a contributory factor in development and complication of diabetes [1–3]. Antioxidants have been proven to be benefit human health because they may protect the body against molecules known as reactive oxygen species, which can attack membrane lipids, protein and DNA [4, 5]. Reactive oxygen species are atoms, molecules, or ions with unpaired electrons or open-shell configurations, such as hydroxyl radical (·OH), superoxide anion radical (O ·−2 ) [6, 7]. And their formation has been associated with many human diseases, such as heart disease [8], stroke [9], arteriosclerosis [10], diabetes [11], cancers [12], Alzheimer’s disease [13], and major disorders. Therefore, it is very important to inhibit the formation of the excessive amounts of free radicals in food products and the living body. Synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) may be added to food products to retard oxidation reactions [14, 15]. These synthetic antioxidants show stronger antioxidant activities than those of natural antioxidants, such as α-tocopherol and ascorbic acid. However, the use of these chemical compounds has begun to be restricted, because of their induction of DNA damage and their toxicity [16]. Thus, there has been a great deal of interest in finding new antioxidants from natural sources to replace synthetic antioxidants for use in food. In the recent years, many studies have reported that hydrolyzed proteins (peptides) from various animal and plant sources possess antioxidant activity [17–19]. Antioxidant activity of these peptides was enhanced by the presence of hydrophobic amino acids (proline and leucine) in the N-terminus [20], and hydrophobic amino acids can increase the accessibility of the antioxidant peptides to hydrophobic cellular targets such as the polyunsaturated chain of fatty acids of biological membranes [21].
Walnut (Juglans regia L.), that belongs to the Juglandaceae family, is one of the nuts commonly found in Chinese diets [22, 23]. It is native to the mountain ranges of Central Asia, extending from Xinjiang province of western China [24–27]. Walnut is received increasing interest as nutraceutics mainly due to the fact that their regular consumption has been reported to reduce the risk of coronary heart disease [28]. In addition, many biological activities for walnut have been reported, such as antiatherogenic, anti-inflammatory and antimutagenic properties [29–31], and antioxidant activities [32, 33]. The health benefits of walnut are usually attributed to their chemical composition. Numerous benefit compounds can be found in walnut. For example, it contains polyphenols [34], flavones [35], polysaccharides [36], aminophenols [37], minerals [38], and so on. Moreover, each ounce of walnuts offers about 17 g of fatty acid and contains about 7 g of protein. Therefore, it is considered a good source of edible oil and proteins. Recently, the use of natural protein hydrolysates has been the subject of several research works, because of their antioxidant potential [39]. Researchers had purified peptides from walnut protein hydrolysates using gel chromatography, and these peptides exhibited the highest antioxidant activities and had angiotensin I-converting enzyme (ACE) inhibitor activity [40, 41]. Every method had its own advantages and disadvantages, so all of these led to our interesting in investigating a large-scale production suitable for walnut peptides.
In the present work, we developed a facile and reproducible preparation of antioxidant peptides from walnut protein hydrolysates. Furthermore, the antioxidant effects of walnut peptides against different free radicals were investigated.
Results and discussion
Preparation of WPIs by continuous countercurrent extraction (CCCE) process
CCCE of soluble from biomass materials (such as pulp, sugarcane, fruits, seeds, and pretreated lignocellulose) can be accomplished in a variety of commercial equipment [42]. Nowadays, CCCE process is commonly used for large-scale single product plants like in oilseed industry. The process is a simple and efficient continuous extraction, with respect to yield, energy efficiency and level of sanitation [43]. Therefore, our focus is on CCCE process used in the food-processing industry because these systems are most effective in reducing water requirements.
In the present work, we obtained walnut protein isolates (WPIs) by using CCCE process, and normal process was also used. The comparison of the two methods, CCCE process versus normal process, is summarized in Table 1.
As shown in Table 1, both methods were able to extract WPIs efficiently, and the yields and purity for proteins extracted from walnuts were comparable. The protein yield and purity for CCCE process were 30.2 and 82.5 %, respectively, while for normal process were 31.0 and 81.8 %, respectively. However, the volume of water required for normal process was one time more than that for CCCE process. Thus, we did not need so much time to concentrate the protein solutions for CCCE process, which led to energy savings. These findings indicated that CCCE process could reduce production costs greatly, and it was available for WPIs extraction.
Proteolytic hydrolysis
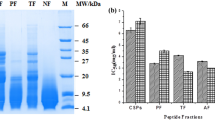
To determine whether the proteases were related to the yields, purity, and activity of peptides, WPIs were separately hydrolyzed by various proteases including neutrase, papain, bromelain, alcalase, pepsin, and pancreatin. Based on the assessment of peptide yields, we studied hydrolysis time and the protease preparation-to-WP ratios on a weight basis (Fig. 1), and the optimum conditions for enzymatic hydrolysis are summarized in Table 2.
Purification of peptides from WPHs
Many studies showed that the biological activity of peptides are related to their molecular weight (MW) [44]. Small-size peptides often present an intense biological activity [45]. Therefore, it seems interesting to select purified fractions of peptides of close MW in order to better target their action. Recently, ultrafiltration with high molecular weight cut-off (MWCO) can be used for the separation between peptides and non-hydrolyzed proteins [46].
In the present work, WPIs were separately hydrolyzed with neutrase, papain, bromelain, alcalase, pepsin, pancreatin at optimal conditions. The residue of walnut protein hydrolysates (WPHs) was removed completely using a PVDF flat microporous membrane with MWCO of 200 kDa. An ultrafiltration membrane with MWCO of 2 kDa was used to separate the WPHs into two fractions, WPH-a (MW < 2 kDa) and WPH-b (MW > 2 kDa). WPH-a was collected and concentrated. And then it was spray-dried.
As we know, trichloroacetic acid (TCA) is one of the commonly used protein precipitants [47]. Low molecular weight peptides (small acid-soluble proteins, SASPs) including free amino acids can be dissolved in 15 % TCA (GB 22492-2008 standard in China). The contents of SASPs and free amino acids can be determined by Kieldahl method and using an amino acid analyzer, respectively. The peptide content was calculated according the following formula:
where X was the content of peptides (%), X 1 was the content of SASPs (%), and X 2 was the content of free amino acids (%).
Thus, the crude proteins (CP) and ASPs contents of walnut peptides (WPs) were determined by Kieldahl method, and the contents of free amino acids were detected using an amino acid analyzer. The results are summarized in Tables 3 and 4.
As shown in Table 3, the yields of peptides obtained from WPHs by the six proteases were ranging from 8 to 18 %. The three proteases (neutrase, papain, and pancreatin) seemed to be much more efficient. Namely, their effectiveness was better than that of others, with peptide yields of 16.2, 16.5, and 17.4 %, respectively. Also, the WPIs were difficult to be hydrolyzed by alcalase, with yield not exceeding 10 %. CP contents of the six peptides were no less than 80 %, which indicated that the six proteases had no obvious impact on protein content (about 80 %). The peptide produced by pepsin (WPs-Pep) had low ASPs content (59.33 %), which revealed that walnut proteins were difficult to be broken down into small-size peptides by pepsin. In contrast, the ASAPs contents of peptides prepared by neutrase (WPs-Neu) and papain (WPs-Pap) were 87.16 and 91.99 %, respectively. The data suggested that the two proteases were very efficient.
The total contents of free amino acids of the two peptides were 6.14 and 7.56 %, respectively. Thus, their purity was very good: 81.0 and 84.4 %, respectively. However, the total contents of free amino acids in other peptides prepared by bromelain (WPs-Bro), alcalase (WPs-Alc), and pancreatin (WPs-Pan) were exceeding 15 %, which led to low peptide contents. Table 4 shows the contents of free amino acids in the six WPs. Sixteen free amino acids (Asp, Thr, Ser, Glu, Pro, Gly Ala, Val, Met, Ile, Leu, Tyr, Phe, His, Lys, Arg) were found in WPs-Pap and Bro. Pro was not found in WPs-Neu, Alc, Pep, and Pan. Lys was not detected in WPs-Neu and Pep. Ile and Gly also were not found in WPs-Pep. Phe and Arg contents in WPs-Neu, Pap, Bro, Alc and Pan were very high. The contents of Phe in WPs-Neu and Pap were 1.79 and 2.09 %, respectively, while for Arg, the contents were 0.99 and 1.62 %, respectively. This disparity may be due to the different proteases. Likewise, the kind of protease had a significant impact on the contents of amino acids.
All in all, the two types of proteases (neutrase and papain) could hydrolyze WPIs efficiently, which should be selected for further use to prepare WPs. The yield and purity of WPs were 16 and 81 % at least, respectively. This method provided a simple and convenient route for the large-scale preparation of WPs, and it showed huge in practical applications.
Molecular weight distribution of WPs
In this study, WPs-Neu and Pap were selected to analyze molecular weight distributions. To study the molecular weight distributions of peptides, sized exclusion chromatography with an HPLC system was used (Fig. 2). And the results are summarized in Table 5.
As shown in Table 5, The chromatographic data indicated both peptides were nearly all composed of lower molecular weight peptides. Both peptides had high quantities (99.10 and 99.37 %) of peptides below 1500 Da with major molecular weight located at 200–1500 Da (60 % at least). The results obtained indicated that enzymatic hydrolysis followed by membrane separation was effective in producing walnut peptides and in removing large peptides or undigested proteins.
As far as we know, hydrolytic process of proteins by proteases could generate molecules ranging from individual amino acids to peptides of various sizes and peptide length was thought to be closely related to biological activities. It was reported that low molecular weight peptides had high solubility, low viscosity, and low allergenicity [45, 48]. These peptides are better candidates than longer peptides to play a physiological role in vivo as they are less susceptible to undergo gastrointestinal hydrolysis [49]. And short peptides may be absorbed easily and transported from the intestinal lumen into the blood circulation more efficiently than either amino acids or intact proteins [50]. Additionally, many studies have shown that peptides with low molecular weights exhibit potent ACE inhibitory activity [51]. Thus, the high low molecular weight peptide content could be expected to be beneficial.
Antioxidant activity
To determine whether WPs could exert significant antioxidant activity, WPs-Neu and Pap were selected to evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid (ABTS), and superoxide radical radical scavenging capacity assay.
DPPH scavenging activity of peptides
DPPH radical scavenging assay has been widely used to evaluate the antioxidant capacity [52], which is stable due to its resonance stability and special blockade of benzene rings [53]. The purple chromogen radical DPPH is reduced by antioxidant compounds to the corresponding pale yellow hydrazine [54]. The activities of WPs-Neu and Pap were evaluated, with gallic acid (GA) as positive control. As shown in Fig. 3a, the scavenging activities of DPPH radical by the two WPs increased with increasing concentration. At a concentration of 100 µg/mL, the activities of WPs-Neu and Pap were 72.29 and 86.02 %, respectively. And the IC50 values of the two pepties were 59.40 and 31.02 µg/mL, respectively, higher than that of GA (IC50: 11.25 µg/mL). It should be noted that the scavenging activity of WPs-Pap was higher than that of WPs-Neu. Therefore, the results indicated that WPs-Pap had strong DPPH radical scavenging activity.
ABTS radical scavenging activity of peptides
The peroxidase substrate ABTS, forming a relatively stable radical (ABTS·) upon one-electron oxidation, has become a popular substrate for estimation of total antioxidant capacity [55]. ABTS radical assay is an excellent tool for determining the antioxidative activity, in which the radical is quenched to form ABTS radical complex [56]. Meanwhile, it is more sensitive to determine antioxidative capacities of protein hydrolysates samples, because it can determine their capacities at lower inhibition concentrations. ABTS radical scavenging properties of WPs-Neu and Pap are present in Fig. 3b. With increasing concentration, the two peptides showed increased ABTS radical scavenging activities, and their scavenging rates were 66.41 and 76.14 %, respectively. The IC50 value of WPs-Neu was 80.36 µg/mL, while for WPs-Pap, the IC50 value was 62.22 µg/mL. These values suggested that WPs-Pap had higher scavenging activity than that of WPs-Neu, consistent with the results for DPPH radical scavenging assay.
Superoxide radical scavenging activity of peptides
The superoxide anion radical is the most common free radical generated in vivo. Superoxide anion, derived from dissolved oxygen by a phenazine methosulphate (PMS)-NADH coupling reaction, reduces nitroblue tetrazolium (NBT) [57]. The decrease in absorbance at 560 nm in the presence of antioxidants indicates the consumption of superoxide anions. Figure 3c shows percentage inhibiton of superoxide anion radical generation for different amounts of WPs-Neu, compared with the same concentration of WPs-Pap. It can be seen from Fig. 3c that the two peptides showed dose dependent activity. The scavenging ratios of WPs-Neu and Pap at 100 µg/mL were 48.66 and 55.13 %, respectively, and the IC50 values were 107.47 and 80.00 µg/mL, respectively. These results indicated WPs-Pap is a good scavenger of the superoxide radical.
Conclusions
In this study, we developed a simple and convenient method for the large-scale preparation of WPs. Walnut proteins were obtained using CCCE process, and separately hydrolyzed with neutrase, papain, bromelain, alcalase, pepsin, pancreatin at optimal conditions. The peptides were further purified from protein hydrolysates through using an ultrafiltration membrane with MWCO of 2 kDa. Our data indicated that two types of proteases (neutrase and papain) could hydrolyze WPIs efficiently, which should be selected for further use to prepare WPs. The yield and purity of WPs prepared using the two proteases were 16 and 81 % at least, respectively, and the peptides had high quantities (99 % at least) of peptides below 1500 Da with major molecular weight located at 200–1500 Da. In addition, the antioxidant effects of the two walnut peptides were tested using DPPH, ABTS and superoxide radical scavenging capacity assays. The results revealed that both possessed excellent antioxidant activities. Therefore, this study may be of high interest for the food industry, and the method showed huge in practical applications.
Experimental
Reagents and chemicals
Walnuts (Juglans regia L.) were purchased from a local market in Xinjiang province, China. Neutrase (powder, ≥600 units/mg solid) and papain (powder, ≥1000 units/mg solid) were procured from Guangxi Pangbo Biothech Co., Ltd. Reagents of analytical grade (sodium hydroxide, hydrochloric acid, trifluoroacetic acid, trichloroacetic acid) were obtained from Sinopharm Chemical Regent Co., Ltd., and used without further purification unless otherwise noted. Acetonitrile (HPLC grade) was obtained from Merck Millipore Corp. Ultrapure water from a Milli-Q water purification system was filtered through a 0.22 µm membrane filter before use.
Preparation of WPIs
Walnut kernels were defatted using cold-pressing technology. The WPIs were obtained using CCCE process.
-
1.
The defatted flour A (200 g) was dispersed in 3000 mL of sodium hydroxide solution (pH 9.5), and extracted at 40 °C. After being stirred for 1 h, the mixture was centrifuged at 1500×g for 10 min to get residue A and supernatant A. The residue A was extracted with sodium hydroxide solution again, and then was centrifuged to yield supernatant B.
-
2.
The defatted flour B (200 g) was dispersed in supernatant A, and the pH of the mixture was adjusted to 9.5. After being stirred for 1 h at 40 °C. The mixture was centrifuged at 1500×g for 10 min to get residue B and supernatant C. The residue B was extracted with sodium hydroxide solution again, and then was centrifuged to yield supernatant D.
-
3.
The defatted flour C (200 g) dispersed in supernatant B was extracted a second time. Residue C and supernatant E were obtained by centrifuging the mixture. The residue C was poured into the supernatant D, and was extracted again. The mixture was centrifuged to obtained supernatant F. At last, the supernatant C, E, and F were combined, and its pH was adjusted to 4.5. After 30 min, the supernatant was discarded to get WPIs.
Preparation of WPHs
WPIs were dissolved in about 3000 mL of water at a total volume of 5000 mL to obtain a protein concentration of 3 %, and hydrolyzed with neutrase (5 g) or papain (10 g). Temperature and pH conditions were adjusted to 50 °C and 7.0, respectively. Agitation was maintained at a constant of 300 rpm. The pH was kept constant using 0.5 M sodium hydroxide solution. After 5 h, neutrase or papain was heat-deactivated at 95 °C for 10 min in a water bath. The mixture was centrifuged at 1500×g for 20 min at 20 °C, and residue was discarded to obtain WPHs.
Purification of WPs
The residue was further removed from WPHs using a PVDF flat microporous membrane with MWCO of 200 kDa. Then, WPHs were further purified through an ultrafiltration membrane with MWCO of 2 kDa, and concentrated using evaporator under vacuum at 60 °C to afford about 1000 mL of WPHs, which were spray-dried to obtain WPs.
Determination of walnut peptide content
Determination of SASPs content
1 g of WPs was weighed and dispersed in a 50 mL volumetric flask with a moderate amount of 15 % TCA under ultrasonic conditions, and then diluted to scale. The dispersions were separated into supernatant and precipitate with a suction filter [58]. The content of supernatant was then determined using Kjeldahl method, which was performed as previously described [59].
Determination of free amino acids content
The free amino acid analysis was carried out according to the method described by Zhang et al. [60].
Determination of molecular weight distribution
The molecular weight distribution was determined by gel permeation chromatography on a TSKgel G2000SWXL column (7.8 mm × 300 mm i.d., 5 µm) with a HPLC system according to the method of Gu et al. [61]. HPLC was carried out with the mobile phase (20 % acetonitrile with 0.1 % TFA, v/v) used at a flow rate of 0.5 ml/min and monitored at 220 nm at 27 °C. The standards used were tripeptide GGG (Mr 189), tetrapeptide GGTA (Mr 451), bacitracin (Mr 1422), and Insulin (Mr 5777) (Sigma Chemical Co., USA).
DPPH radical scavenging assay
All tested samples were dissolved in ethanol. 100 µL of DPPH in ethanol was added into a 96-well plate, and was mixed with the test samples (100 µL) at different concentrations. After shaken for 60 s in microplate reader, it was left in the dark at 37 °C for 30 min. The absorbance was then measured at 515 nm with a microplate reader (BIO-RAD, model 680) [62]. All experiments were carried out in triplicate. Ethanol was used as the blank control and vitamin C served as positive control. The DPPH radical scavenging activity were calculated according to the following formula.
ABTS radical scavenging assay
ABTS and potassium persulfate were dissolved in distilled water to a final concentration of 7 and 2.6 mmol/L, respectively, and mixed. The mixture allowed to stand in the dark at room temperature for 12 h before use. It was then diluted by mixing 1 mL ABTS solution with 60 mL of phosphate buffered saline (PBS) to obtain an absorbance of about 1.00 at 734 nm using a spectrophotometer. All tested samples were dissolved in PBS. 5 mL of fresh ABTS solution was mixed with 500 µL of tested samples for 2 h in a dark condition. The absorbance was then measured at 734 nm with a spectrophotometer [63]. All experiments were carried out in triplicate. PBS was used as the blank control and vitamin C served as positive control. The ABTS radical scavenging activity were calculated according to the following formula.
Superoxide radical scavenging activity
All tested samples were dissolved in Tris–HCl (16 mmol/L, pH 8.0). The superoxide radicals were generated in 5 mL of reaction mixture containing 1 mL of NBT (300 µmol/L) solution, 1 mL of NADH (468 µmol/L) solution and 3 mL of sample solution were mixed. The reaction started by adding 1 mL of phenazine methosulphate (PMS) solution (60 µmol/L) to the mixture. After 5 min, the absorbance was then measured at 558 nm with a spectrophotometer [64]. Tris–HCl was used as the blank control and vitamin C served as positive control. All experiments were carried out in triplicate. The percentage inhibition of superoxide anion generation was calculated using the following formula.
Statistical analysis
All statistical analyses were performed using SPSS 10.0, and the data were analyzed using one-way ANOVA. The mean separations were performed using the least significant difference method. Each experiment was performed in triplicate, and all experiments were run thrice and yielded similar results. Measurements from all the replicates were combined, and the treatment effects were analyzed.
Abbreviations
- ABTS:
-
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid
- ACE:
-
angiotensin I-converting enzyme
- BHA:
-
butylated hydroxyanisole
- BHT:
-
butylated hydroxytoluene
- CCCE:
-
continuous countercurrent extraction
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- GA:
-
gallic acid
- MWCO:
-
molecular weight cutoff
- NBT:
-
nitroblue tetrazolium
- PMS:
-
phenazine methosulphate
- SASPs:
-
small acid-soluble proteins
- TCA:
-
trichloroacetic acid
- WPs:
-
walnut peptides
- WPHs:
-
walnut protein hydrolysates
- WPIs:
-
walnut protein isolates
References
Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, Shankar V (2013) Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N Am J Med Sci 5:213–219
Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J (1998) Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci (Lond) 94:623–632
Lee AY, Chung SS (1999) Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J 13:23–30
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2:219–236
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4:118–126
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 14:312–322
Tribble DL (1999) Antioxidant consumption and risk of coronary heart disease: emphasis on vitamin C, vitamin E, and β-carotene a statement for healthcare professionals from the American heart association. Circulation 99:591–595
Shirley R, Ord ENJ, Work LM (2014) Oxidative stress and the use of antioxidants in stroke. Antioxidants 3:472–501
Cherubini A, Vigna GB, Zuliani G, Ruggiero C, Senin U, Fellin R (2005) Role of antioxidants in atherosclerosis: epidemiological and clinical update. Curr Pharm Des 11:2017–2032
Golbidi S, Ebadi SA, Laher I (2011) Antioxidants in the treatment of diabetes. Curr Diabetes Rev 7:106–125
Ozben T (2015) Antioxidant supplementation on cancer risk and during cancer therapy: an update. Curr Top Med Chem 15:170–178
Gilgun-Sherki Y, Melamed E, Offen D (2003) Antioxidant treatment in Alzheimer’s disease: current state. J Mol Neurosci 21:1–11
Thorat ID, Jagtap DD, Mohapatra D, Joshi DC, Sutar RF, Kapdi SS (2013) Antioxidants, their properties, uses in food products and their legal implications. Int J Food Stud 2:81–104
Wettasinghe M, Shahidi F (1999) Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chem 67:399–414
Balti R, Bougatef A, Ali NEH, Ktari N, Jellouli K, Nedjar-Arroume N, Dhulster P, Nasri M (2011) Comparative study on biochemical properties and antioxidative activity of cuttlefish (Sepia officinalis) protein hydrolysates produced by alcalase and Bacillus licheniformis nh1 proteases. J Amino Acids 2011:107179
Pokora M, Eckert E, Zambrowicz A, Bobak L, Szołtysik M, Dąbrowska A, Chrzanowska J, Polanowski A, Trziszka T (2013) Biological and functional properties of proteolytic enzyme-modified egg protein by-products. Food Sci Nutr 1:184–195
Wei JT, Chiang BH (2009) Bioactive peptide production by hydrolysis of porcine blood proteins in a continuous enzymatic membrane reactor. J Sci Food Agric 89:372–378
Rao G, Zhao M, Lin W, Wang H (2007) Antioxidative activity of tobacco leaf protein hydrolysates. Food Technol Biotechnol 45:80–84
Yousr M, Howell N (2015) Antioxidant and ACE inhibitory bioactive peptides purified from egg yolk proteins. Int J Mol Sci 16:29161–29178
Orsini Delgado MC, Nardo A, Pavlovic M, Rogniaux H, Añón MC, Tironi VA (2016) Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem 197:1160–1167
Thakur A (2011) Juglone: a therapeutic phytochemical from Juglans regia L. J Med Plants Res 5:5324–5330
Mao X, Hua Y, Chen G (2014) Amino acid composition, molecular weight distribution and gel electrophoresis of walnut (Juglans regia L.) proteins and protein fractionations. Int J Mol Sci 15:2003–2014
Pollegioni P, Woeste KE, Chiocchini F, Olimpieri I, Tortolano V, Clark J, Hemery GE, Mapelli S, Malvolti ME (2014) Landscape genetics of Persian walnut (Juglans regia L.) across its Asian range. Tree Genet Genomes 10:1027–1043
Beer R, Kaiser F, Schmidt K, Ammann B, Carraro G, Grisa E, Tinner W (2008) Vegetation history of the walnut forests in Kyrgyzstan (Central Asia): natural or anthropogenic origin? Quat Sci Rev 27:621–632
Shah TI, Sharma E, Ahmad G (2014) Juglans regia Linn: a phytopharmacological review. World J Pharm Sci 2:364–373
Hassan GA, Bilal AT, Ahmad BT, Sameena W, Irshad AN (2013) Economic and ethno-medicinal uses of Juglans regia L. in Kashmir Himalaya. Unique J Ayurvedic Herbal Med 1:64–67
Feldman EB (2002) The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr 132:1062S–1101S
Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM (2001) Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr 131:2837–2842
Papoutsi Z, Kassi E, Chinou I, Halabalaki M, Skaltsounis LA, Moutsatsou P (2008) Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br J Nutr 99:715–722
Ros E (2010) Health benefits of nut consumption. Nutrients 2:652–682
Oliveira I, Sousa A, Ferreira ICFR, Bento A, Estevinho L, Pereira JA (2008) Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 46:2326–2331
Negi AS, Luqman S, Srivastava S, Krishna V, Gupta N, Darokar MP (2011) Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm Biol 49:669–673
Fukuda T, Ito H, Yoshida T (2003) Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 63:795–801
Zhao MH, Jiang ZT, Liu T, Li R (2014) Flavonoids in Juglans regia L. leaves and evaluation of in vitro antioxidant activity via intracellular and chemical methods. Sci World J 2014:303878
Ruijun W, Shi W, Yijun X, Mengwuliji T, Lijuan Z, Yumin W (2015) Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia. Int J Biol Macromol 72:771–775
Martínez ML, Labuckas DO, Lamarque AL, Maestri DM (2010) Walnut (Juglans regia L.): genetic resources, chemistry, by-products. J Sci Food Agric 90:1959–1967
Cosmulescu S, Baciu A, Achim G, Botu M, Trandafir I (2009) Mineral composition of fruits in different walnut (Juglans regia L.) cultivars. Not Bot Hort Agrobot Cluj 37:156–160
Amarowicz R (2008) Antioxidant activity of protein hydrolysates. Eur J Lipid Sci Technol 110:489–490
Chen N, Yang H, Sun Y, Niu J, Liu S (2012) Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 38:344–349
Gu X, Hou YK, Li D, Wang JZ, Wang FJ (2015) Enzyme inhibitory peptides from walnut (Juglans regia L.) hydrolysate. Int J Food Prop 18:266–276
Kim KH, Tucker MP, Keller FA, Aden A, Nguyen QA (2001) Continuous countercurrent extraction of hemicellulose from pretreated wood residues. Appl Biochem Biotechnol 91–93:253–267
Tan KS, Spinner IH (1984) Numerical methods of solution for continuous countercurrent processes in the nonsteady state part I: model equations and development of numerical methods and algorithms. AIChE J 30:770–779
Vandanjon L, Johannsson R, Derouiniot M, Bourseau P, Jaouen P (2007) Concentration and purification of blue whiting peptide hydrolysates by membrane processes. J Food Eng 83:581–589
Guzmán F (2007) Peptide synthesis: chemical or enzymatic. Electron J Biotechn 10:279–315
Picot L, Ravallec R, Fouchereau-Péron M, Vandanjon L, Jaouen P, Chaplain-Derouiniot M, Guérard F, Chabeaud A, LeGal Y, Alvarez OM, Bergé JP, Piot JM, Batista I, Pires C, Thorkelsson G, Delannoy C, Jakobsen G, Johansson I, Bourseau P (2010) Impact of ultrafiltration and nanofiltration of an industrial fish protein hydrolysate on its bioactive properties. J Sci Food Agric 90:1819–1826
Hao R, Adoligbe C, Jiang B, Zhao X, Gui L, Qu K, Wu S, Zan L (2015) An Optimized trichloroacetic acid/acetone precipitation method for two-dimensional gel electrophoresis analysis of Qinchuan cattle Longissimus dorsi muscle containing high proportion of marbling. PLoS ONE 10:e0124723
Maeda Y, Kimura Y (2004) Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J Nutr 134:945–950
Gómez-Ruiz JA, Ramos M, Recio I (2007) Identification of novel angiotensin converting enzyme-inhibitory peptides from ovine milk proteins by CE-MS and chromatographic techniques. Electrophoresis 28:4202–4211
Zhu KX, Wang XP, Guo XN (2015) Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J Funct Foods 12:23–32
Ko SC, Kang N, Kim EA, Kang MC, Lee SH, Kang SM, Lee JB, Jeon BT, Kim SK, Park SJ, Park PJ, Jung WK, Kim D, Jeon YJ (2012) A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem 47:2005–2011
Kedare SB, Singh RP (2011) Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 48:412–422
Biswas M, Haldar PK, Ghosh AK (2010) Antioxidant and free-radical-scavenging effects of fruits of Dregea volubilis. J Nat Sci Biol Med 1:29–34
Krishnappa P, Venkatarangaiah K, Venkatesh Rajanna SKS, Gupta RKP (2014) Antioxidant and prophylactic effects of Delonix elata L., stem bark extracts, and flavonoid isolated quercetin against carbon tetrachloride-induced hepatotoxicity in rats. Biomed Res Int 2014:507851
Apak R, Güçlü K, Demirata B, Ozyürek M, Celik SE, Bektaşoğlu B, Berker KI, Ozyurt D (2007) Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 12:1496–1547
Sowndhararajan K, Kang SC (2013) Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci 20:319–325
Sundararajan R, Koduru R (2015) In vitro antioxidant activity on leaves of Samadera indica. Pharm Lett 7:372–382
Hsien DST, Lin C, Lang ER, Catsimpoolas N, Rha CK (1979) Molecular-distribution of soybean globulin peptides produced by peptic hydrolysis. Cereal Chem 56:227–231
Magomya AM, Kubmarawa D, Ndahi JA, Yebpella GG (2014) Determination of plant proteins via the kjeldahl method and amino acid analysis: a comparative study. Int J Sci Technol Res 3:68–72
Zhang H, Wang ZY, Yang X, Zhao HT, Zhang YC, Dong AJ, Jing J, Wang J (2014) Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an amino acid analyzer and ICP-MS with micro-wave digestion. Food Chem 147:189–194
Gu RZ, Li CY, Liu WY, Yi WX, Cai MY (2011) Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res Int 44:1536–1540
Sahu RK, Kar M, Routray R (2013) DPPH free radical scavenging activity of some leafy vegetables used by tribals of odisha, India. J Med Plants Stud 1:21–27
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Bekdeser B, Ozyürek M, Güçlü K, Apak R (2011) tert-Butylhydroquinone as a spectroscopic probe for the superoxide radical scavenging activity assay of biological samples. Anal Chem 83:5652–5660
Authors’ contributions
M-CL and S-JY performed the experiments, analyzed the data and wrote the paper. DH and J-PY performed the experiments. M-CL, YL, and C-HH planned and analyzed the data, and C-JW planned the experiments, wrote the paper and give final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge Prof. Lin Huang-Ching from Institute of Pharmacy, Taiwan National Defense Medical Center for his kind suggestions.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ming-Chuan Liu and Sheng-Jie Yang contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, MC., Yang, SJ., Hong, D. et al. A simple and convenient method for the preparation of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Chemistry Central Journal 10, 39 (2016). https://doi.org/10.1186/s13065-016-0184-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-016-0184-x